Abstract
Dr. Gordon Amidon developed the Biopharmaceutical Classification System (BCS) to classify pharmacological compounds based on their solubility in water and intestinal permeability. His work in this area earned him the Science Award from the International Pharmaceutical Federation (FIP) in August 2006. The BCS has since been used by the U.S. Food and Drug Administration to estimate the absorption of oral medications in the intestines. It has become an indispensable tool in regulatory decision-making for drug development, allowing for the evaluation of dissolution, solubility, and intestinal permeability, which are the three primary factors affecting the absorption of oral drugs from quick-release solid oral dosage forms. This study highlights the BCS's key objectives, benefits, and classification. The article illuminates the potential new standards and divisions suggested for additional biowaivers , which are based on the fundamental physiology of the gastrointestinal tract in necessary instances. It delves into the potential uses of BCS in the fields of drug discovery, drug delivery, and drug research, while also exploring its potential expansion.
Keywords
Biopharmaceutical Classification System , dissolution, permeability, Gastrointestinal track
Introduction
The field of drug delivery technologies is still dominated by the oral route, which is the preferred method of drug administration for formulators. However, this route has limitations on bioavailability and absorption in the gastrointestinal tract environment, despite its widespread use[1,2]. When an oral dose form is used, the medication in the dosage form is released and turns into a Solution after dissolving un the surrounding gastrointestinal fluid. This process has a restricted solubility, and the medication cam only travel the cell membranes lining the gastrointestinal tract when it is in solution form. The medication enters systemic circulation at that point, and the degree of medication solubility and permeability determines oral absorption and bioavailability. The Biopharmaceutical Classification System (BCS) is a framework for classifying pharmacological compounds based on their relationship to dose and intestinal permeability in relation to their aqueous solubility[3,4]. The rate and amount of drug absorption from IR solid oral dosage forms are controlled by intestinal permeability, solubility, and dissolution when combined with the dissolution of the dosage form. According to the BCS, the solubility and permeability of the medicines allow for classification into four fundamental classes. In vivo bioequivalence research is only applicable to non- critical pharmaceutical forms and non-critical pharmacological ingredients in terms of solubility, permeability, and therapeutic range[5]. Despite being often discussed, BCS-based approaches are still hardly employed, which is mainly due to uncertainty on the parts of both pharmaceutical corporations and regulatory agencies. Significant differences across their associated assessments lead to the perception that there is a lack of consensus regarding the successful use of the BCS concept to support. By creating an annex to the guideline on Bioavailability and Bioequivalence, it is hoped to achieve an optimal and uniform use of principles among the European Union [6, 7].
BCS Classification

BCS Classes
Class I
The absorption and dissolution rates of drugs are considerably high. However, the rate of drug dissolution is the limiting factor, and if it happens too quickly, the rate of gastric emptying becomes the determining factor. It is worth noting that absorption takes place at a faster pace than excretion. Examples of drugs with high absorption and dissolution rates include Metoprolol, Diltiazem, Verapamil, and Propranolol.
Class II
Drugs have the ability to absorb significant quantities of blood, yet their dissolution occurs at a slow pace. The crucial factor that determines the rate of absorption is the in vivo drug dissolution, unless an exceedingly high dosage is administered. Class II drugs generally exhibit a slower absorption rate compared to class I drugs, and consequently, they require more time to dissolve. It is worth noting that class I and class II medications are typically not subjected to in vitro-in vivo correlation (IVIVC). Examples of such medications include Phenytoin, Diazole, Ketoconazole, Mefenamic Acid, and Nifedipine.
Class III
The permeability of membranes is the crucial factor that limits the rate of drug absorption. The extent and speed at which drugs are absorbed can differ significantly among various medications. Instead of being influenced by factors related to the dosage form, the variability in drug absorption is primarily attributed to alterations in physiology and membrane permeability. Notable examples of medications exhibiting rapid dissolution include captopril, acyclovir, neomycin B, and cimetidine.
Class IV
Class IV drugs are administered orally with effective results. Examples of such drugs include Taxon and griseofulvin.
BCS Classification of drugs

Example of some drug as per Biopharmaceutical Classification system

The system can be used to flag drugs that should not be tested clinically unless appropriate formulation strategies are employed

Aim of BCS guidelines [11, 12]
- To increase the effectiveness of drug development and the review process, we recommend a strategy for identifying expendable clinical BE tests. To predict in
- vivo performance of drug products from in vitro measurements of permeability and solubility.
- To suggest classification methods based on drug product solubility and permeability properties as well as dosage form dissolution.
- To offer a regulatory tool that will allow precise in-vitro dissolution tests to take the place of some bioequivalence studies.
- This will lower the price of developing new drugs and prevent unneeded drug exposure to healthy objects.
- To offer direction to business.
Basic Requirements of BCS [13]
- The in-vivo dissolution system must be accurately predicted
- A clearly defined rate limiting step is required for in vivo absorption.
- There must be a balance between the permeability and solubility limits.
- In-vitro techniques ought to be reliable enough for accurate classification.
Solubility Determination [14, 15, 16, 17]
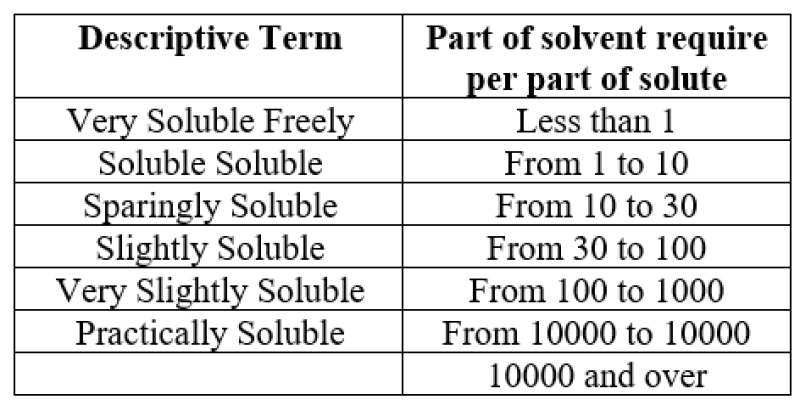
When equilibrium is reached between the excess ( un dissolved substance) and the solution at a particular temperature and pressure, the amount of substance that has passed into solution is known as the solubility of any given substance When the maximum dose strength of a drug substance or active pharmaceutical ingredient(API) dissolves in 250 millilitres or less of aqueous medium within a particular pH range, it is said to be highly soluble [5,7].The estimated volume of 250 millilitre’s is based on the average amount of water consumed, or approximately one glassful, or eight ounces, during the oral administration of the dosage form.This boundary value represents the lowest expected stomach fluid volume at the time of drug administration (a light meal or repast). The profile of the pH solubility of the drug substance is measured in an aqueous medium with a pH between 1 and 7.5, according to the US Food and Drug FDA guidelines. Guidelines from the World Health Organization (WHO) and European Medicines Academy (EMEA) There should be enough pH conditions to assess in order to precisely define the profile of pH-solubility. The quantity For a solubility determination, the pH conditions must ionization properties of the drug under test. Consideration should be given if drug degradation is seen to be influenced by the pH or composition of the buffer. The focus of drug material in particular buffers or pH settings ought to be ascertained using a verified assay technique for solubility indication that Can differentiate between the drug ingredients from their deterioration.
USP & BP Solubility Criteria
Permeability Determination [18, 19, 20, 21, 22]
The determination of permeability often involves the use of various techniques. These techniques include pharmacokinetic research with human participants, such as intestinal permeability techniques, mass balance studies, and absolute bioavailability (BA) studies. Another approach is to utilize an appropriate animal model, either through in vivo or in situ intestinal perfusion. Additionally, in vitro permeability techniques can be employed using removed intestinal tissues or appropriate epithelial cell monolayers .To evaluate the extent of drug absorption, mass balance studies utilize either radiolabeled or stable isotopes. Absolute BA research involves comparing oral BA with reference intravenous BA. Intestinal perfusion models are also utilized in these studies .For drugs that are passively transported, in vitro Techniques are recommended. An interesting alternative to intestinal tissue models is the use of in vitro systems based on the cell line of human adenocarcinoma, specifically the Caco-2 cells. These cells are employed to simulate small intestine tissue. The brush-border enzymes and microvilli, which are integral membrane proteins found in the small intestinal mucosa, are present in the differentiated cells. These cells also form fluid-filled domes, which are a characteristic feature of a permeable epithelium. Recent investigations using the Caco-2 cell line have shown that they have the ability to transport peptides, sugars, and ions. These findings have confirmed the usefulness of the Caco-2 cell line as an in vitro model for studying the intestinal tract.
Permeability Determination Method
Research on methods to improve the solubility of Gresiofulvine , a class II drug for BCS, is anticipated.
- Use of water soluble carrier[34]
The solubilisation capacity of micelles solutions of Plutonic F127 was found to be enhanced by co-forming with higher solubility. This was observed in the case of Gresiofulvin, where polymers soluble in water, such as PVP and PEG, played a significant role. Specifically, PEG 6000 and PVP K30, which are low molecular weight polymers, showed identical results in terms of solubility when compared to the Gresiofulvin curve in F127 solution. On the other hand, high molecular weight polymers like PEG 35000 and PVP K90 exhibited similarities. At a concentration of 0.5%, there was a sharp increase in the solubility of Gresiofulvin. This increase can be attributed to the interaction between F127 and PVP, which leads to the transfer of PVP's hydrophobic chains and hydrophobically bonded water, ultimately resulting in the increase in solubility of Gresiofulvin and a decrease in Gibbs energy[35]. The longer chain length of PVP K90 may explain its higher solubility increase compared to PVP K30. In another study conducted by Alden M. et al., the critical surfactant concentration for hydrophobic drug solubility in various polyethylene glycols (PEG) was investigated. Solid dispersions of Gresiofulvin in different PEG grades were created using the melting method, with or without the addition of the surfactant alkali dodecyl sulphates (MDS). It was found that PEG 3000 required the highest concentration for solubility. However, when Li+ was used as the surfactant's counter ion in PEG 3000, the lowest critical concentration for the formation of solid solutions was achieved, thereby increasing the stability of the system. Furthermore, Nozawa Y. et al. improved the solubility and dissolution of Gresiofulvin by employing a roll mixing technique and using saccharides as the dispersion carrier. It was observed that the solid dispersion of Gresiofulvin with saccharides became more stable as the molecular weight of the saccharides increased. When combined with corn-starch or amylopectin, the initial dissolution of Gresiofulvin increased by 117 times, while roll mixing with British gum resulted in a 170-fold increase compared to Gresiofulvin alone. The solid dispersion of Gresiofulvin.
- Use of surfactants or stabilizers [36 , 37 ]
Lucas M. et al. enhanced the solubility of griseofulvin by utilizing micelles solutions of mixtures of Gemini (N) and heterogemini (P) surfactants. The solvent surface tension values were determined using a Kruse 100 MK2 densitometer and the Wilhelm plate method. The values of cmc were obtained from surface tension vs. log concentration of surfactants plots. The findings suggest that anionic surfactants are superior griseofulvin solubilizes. Surfactant mixtures in micelle form exhibit superior solubilisation qualities compared to the N and P solution alone. Hisami Al-Obadiah et al. created griseofulvin binary and ternary solid dispersions with poly[N-(2-hydroxypropyl) methacrylate] (PHPMA) as a stabilizer and hydroxypropyl methylcellulose acetate succinate (HPMCAS) using spray drying. Both solid dispersion formulations showed increased stability and dissolution when compared to GF.
Hisham Al-Obaidi et al. utilized spray drying to produce solid dispersions of griseofulvin (GF) with poly[N-(2-hydroxypropyl) methacrylate] (PHPMA) as a stabilizer and hydroxypropyl methylcellulose acetate succinate (HPMCAS). In the binary solid dispersions (GF/HPMCAS, 50:50%), GF (2.5 g dissolved in 185 ml of acetone) was combined with 85 ml of distilled water. On the other hand, the ternary solid dispersion (GF/PHPMA/HPMCAS, 50:25:25%) followed the same procedure as the binary version, but with the addition of PHPMA. X-ray diffraction analysis revealed that HPMCAS alone exhibited crystallinity, whereas PHPMA demonstrated increased stability by forming an amorphous structure due to its stronger interaction with GF. Both solid dispersion formulations exhibited enhanced stability and dissolution compared to GF.
- Complextion with ?-cyclodextrin[37,39,40]
Dhanaraju M. et al. utilized the co-precipitation method to enhance the bioavailability of GF by forming an inclusion complex with ?-Cyclodextrin. The complex was prepared using ethanol as a solvent in various molar ratios of 1:1, 2:1, 3:1, and 1:2 of the drug and ?-Cyclodextrin. The dissolution rate of the 1:2 molar ratio complex was significantly higher than that of GF alone, as confirmed by UV, HPLC, and dissolution studies. Toxicity tests on Swiss Albino mice demonstrated the safety of the 1:2 molar ratio complex. In vivo research on animals and human participants revealed that the complex had a faster dissolution rate and a fourfold increase in relative bioavailability, ranging from 73 to 84%, compared to pure Griseofulvin.
The release of griseofulvin from hydrophilic aerogel formulations and liquisolid systems has been enhanced by Leopold C. et al. To create Liquisolid formulations, the mixture of carrier (Avicel®) and coating material (Aerosil) was combined with the liquid component (drug in PEG 300). However, due to the high drug dose and low solubility of the liquid vehicle, the tablet weight increased, necessitating a large amount of carrier and coating materials. As a result, it is feasible to reduce the tablet weight by substituting the coating and carrier material with Neusilin® (a highly adsorptive silicate).
Summary of research work Done by griseofulvin

- Excipient risk for BCS class I[59, 60, 61 ]
Due to their high permeability and solubility, BCS Class I pharmacological products are easily absorbed and have minimal risk of excipient alterations. Even though the stages that determine the rate of absorption include dissolution, permeation, or stomach emptying, it is still important to assess excipients that have the potential to change the pace or extent of drug absorption. For instance, there are situations where excipients enhance the absorption rate of slowly absorbed medications or regulate uptake transporters that are crucial for the drug's high permeability. In the case of BCS Class I drugs, a bio waiver can be granted if the excipients that could impact absorption are quantitatively similar (within ±10% of the excipient weight in the reference product and a cumulative difference) and qualitatively the same (same chemistry, grade, and characterization). The active absorption of cephalexin, a BCS Class I medication, is facilitated by the proton-coupled oligopeptide transporter PEPT1 across the apical membrane of enterocytes, leading to increased intestinal permeability. Variations in PEPT1 expression in vitro and in vivo (such as in rat, human duodenum, and Caco-2 cells) are associated with differences in cephalexin permeability. Therefore, excipients that have the capacity to alter PEPT1 expression or activity should be taken into account during formulation development. In general, the amount of absorption may vary by 10-15%, which could result in bioequivalence. In vitro findings suggest that non-ionic surfactants like Solito® ,polysorbate 20, and polysorbate 80 inhibit PEPT1 in transfected MDCKII cells. These surfactants are also known to increase the medication's ability to pass through the gut membrane by interfering with its integrity and function. Therefore, it is crucial to consider the overall net effect since multiple factors can influence drug absorption.
- Excipient risk of BCS class II[62, 63, 64, 65]
BCS Class III drug products are known to have poor permeability and can only be absorbed locally in certain areas of the gastrointestinal tract. This susceptibility to excipient alterations means that a bio waiver for BCS Class III drugs is permissible if all excipients, except for those used in small quantities, are qualitatively identical and quantitatively similar. This is based on the assumption that all excipients have the potential to influence medication absorption. Studies have shown that osmotic ally active excipients can change the bioavailability of BCS Class III medications. For example, sorbitol can reduce the absorption of ranitidine by boosting intestinal fluid volume, while mannitol can lower the bioavailability of cimetidine. PEG 400 can also change the absorption of ranitidine and speed up transit time. Non-ionic surfactants have been shown to inhibit intestinal transporters, which can potentially affect the absorption of certain drugs. However, the FDA guidelines do not specify how to evaluate transporter-mediated excipient-drug interactions. Although medications that cause passive permeability may not be significantly affected, there is a possibility of improved bioavailability if the inhibition of intestinal efflux transporters influences permeability. The intestinal efflux mediated by P- gp is observed in BCS Class III medications like famotidine and cimetidine. Studies have shown that P-gp inhibitors can reduce the secretion of these medications in rats during single-pass intestinal perfusion tests, and the reduction is concentration dependent. Interestingly, the in vivo permeability of these medications along the small intestine demonstrated segmental dependent intestinal absorption, which correlated with the levels of P-gp expression. Literature on verapamil suggests that site-specific inhibition of P-gp in the gut may impact the overall absorption of the medication. In vitro experiments on MDCK-MDR1 cells have shown that many surfactants and one polymer inhibit P-gp, while HEK293 cells have demonstrated modest inhibition by five dyes and one suspending agent. In Caco-2 monolayers and other cell lines, the surfactant vitamin E TPGS has been categorized as a P-gp inhibitor for drug transport. Furthermore, it has been shown to increase the oral bioavailability of the BCS Class III medication colchicine in rats.
- The bio pharmaceutics classification system's applications
Drug Delivery Technology
- Class I system
The Class I drugs are not those in which either solubility or permeability is limiting within the target regions of the GI tract. The drug release in such cases can be modulated using controlled release technology. Drugs classified as Class I do not have limiting properties in the GI tract's target regions, such as permeability or solubility. In such circumstances, the drug release can be regulated by using controlled technology. Class I drugs that are subject to controlled release technologies include a variety of products like Microcap, Micro pump , SCOT (Single Osmotic tablet system composition), Microsphere, CONSURF (drug delivery shuttle with continuous surface area), Diametric (Diffusion delayed pulsatile hydrogel system, or DPHS (controlled matrix system), Two drug absorption systems are DUREDAS (dual release) and GMHS (granulated adjusting hydrogel system), the intestinal protective drug system (IPAS), absorption system), Pharm zone (Drug Micro particle), and Multiport delivery technology), pulsatile delivery system (PPDS) pelletized, Bio erodible enhanced oral drug absorption system, or BEODAS or SODAS (System of Oral Drug Absorption Programming), ODAS (Orally absorbed drugs spheroidal), SMHS (Solubility SPDS (stabilized pellet delivery system) and modulating hydrogel system scheme).
- Class II System
This course focuses on scenarios where the absorption and bioavailability (BA) of substances are significantly affected by limitations in solubility or dissolution rate. Within this category of technologies, there are various methods employed, including high-energy state stabilization and classical micronization. These methods utilize surfactants, lyophilized fast-melt systems, emulsion or micro emulsion systems, solid dispersion, and agents that form complexes, such as cyclodextrins. Examples of tools falling under this category are Zer-Os and Soft Gel (a formulation of soft gelatine capsules). Additionally, tablet technology, Triglas, and Nano
scale carriers like osmotic systems, Nano crystals, Nano emulsion, and Nano suspension are considered promising strategies for enhancing the BA and solubility of poorly soluble active components in water.
- Class III System
Class III technologies encompass various methods to modify the site or rate of exposure, as well as the addition of functional agents to alter the metabolic functioning of enzyme systems. Within this classification, notable examples include the Telemetric Capsule, High-Frequency Capsule, Gastric Retention System, and Oral Vaccination System.
- Class IV
Class IV compounds are rarely developed for commercial purposes, with only a few exceptions. Examples of such medications include Furosemide, Ritonavir, Saquinavir, Taxol, and Cyclosporin A.
- Industrial Implementation Of BCS
In 1995, Amidon et al. developed the bio-pharmaceutics classification system (BCS) to classify drugs based on their water solubility and intestinal permeability. It has been demonstrated that the bioavailability of highly soluble and highly permeable drugs (BCS Class I) is not affected by the dissolution rate if it is rapid enough. As a result, several regulatory agencies, including the US Food and Drug Administration (FDA), now allow the use of in vitro dissolution testing to determine the bioequivalence of BCS Class I drug formulations, which is often referred to as a bio-waiver.
- The potential savings that can be achieved depend on the number and cost of bio equivalency studies that can be avoided. In order to estimate these savings, an examination was conducted on the annual number of bioequivalency studies conducted by the pharmaceutical industry. It is estimated that these studies cost between 90 and 150 million dollars.
- All the compounds studied, approximately 25% were found to have high solubility and permeability, while the remaining 41% did not have sufficient data to be categorized.
- Based on this estimation of 25%, there is a possibility to save one quarter of the annual expenses on bioequivalence studies, which amounts to 22 to 38 million dollars per year. If bioequivalence studies are a bottleneck in medication development, there may be additional indirect savings.
- Let's assume that the findings of a bioequivalence research are necessary before proceeding with the development of a chemical that has the potential for one billion dollars in annual peak sales. It is reasonable to expect that in vitro dissolution findings can be obtained six weeks prior to in vivo bioequivalence experiment findings. This time-saving corresponds to an additional 110 million dollars in potential sales from an earlier approval.
- Furthermore, since a human bioequivalence investigation is not required, clinical resources can be allocated to other areas.
- BCS Implementation of Drug development
In the early stages of pharmaceutical research and later on in the management of modifications throughout the product's lifespan, it is essential to utilize this approach.
- The medicine's physical and biological properties are better comprehended with the aid of this, enhancing fundamental understanding.
- The establishment of connections between in vitro and in vivo can be facilitated by employing selective disintegration techniques.
- By utilizing this method, it is possible to acquire an a bio waiver.
- On-going development efforts are focused on drugs that exhibit low solubility.
- Drug discovery and early development[3, 46]
Pharmaceutical product development heavily relies on bioavailability (BA) and bioequivalence (BE) studies. Currently, BE studies are being conducted for New Drug Applications (NDAs) of innovative compounds, as well as for supplemental NDAs to expand product lines and explore new medical indications. BE studies are also conducted for generic products' shortened new drug applications and for post approval modifications and scale-up. One of the initial challenges in applying the Bio pharmaceutics Classification System (BCS) criteria to novel drug substances is the lack of precise knowledge about the dose during the early stages of preformulation or formulation. Therefore, the Dose to Solubility ratio (D:S) can only be expressed as a likely range at this time. It is rare for compounds with aqueous solubility exceeding 100 mg/ml to exhibit dissolution rate-limited absorption. Instead, the maximum absorbable dose can be calculated by considering the drug's solubility and the typical volumes of gastrointestinal (GI) fluid accessible under the planned dosing conditions. When selecting the appropriate media for solubility determinations, it may be beneficial to consider the drug's physicochemical properties in relation to its solubility. For instance, measuring solubility at all pH values recommended by the BCS is unnecessary for neutral compounds in the early stages of development. However, when comparing formulations later on, it will be useful to have dissolution data for the drug product across the entire pH range of the GI tract to assess the release robustness under GI conditions. Hydrophilic drugs may have poor solubility in water and simple buffers, but the presence of bile in GI fluids can often enhance their solubility to a significant extent. Compounds with log P values may experience increases in solubility of one to two orders of magnitude. For promising compounds that are both ionisable and lipophilic, conducting extensive solubility experiments in bio relevant media will aid in characterizing their likely solubility behaviour.
- Pharmacokinetic optimization in drug research[47]
Drug absorption relies on two important bio pharmaceutics parameters: solubility and permeability. These parameters play a crucial role in lead optimization and the discovery of new drugs, as well as in pharmacokinetics related to these attributes. The bio pharmaceutical Classification System (BCS) provides an opportunity for medication development by allowing the alteration of the structure or physicochemical properties of potential leads to enhance deliverability. In the pharmaceutical sector, there is a significant interest in utilizing high throughput parallel and combinatorial synthesis techniques to create a large number of molecules for permeability and solubility screening. The ultimate goal is to optimize pharmacokinetics by modifying the molecules to exhibit BCS Class I characteristics without compromising pharmacodynamics performance. High throughput methods are necessary to ensure efficient drug delivery and pharmacokinetics right from the early stages of drug design. In order to achieve this, in silica predictions and theoretical development profiles of lipophilicity and solubility are used for structure-based optimization of biopharmaceuticals. These predictions are then verified using in vitro experimental models such as cell cultures and microliter plate assays. By characterizing biopharmaceuticals during drug design and early development, it becomes possible to identify and withdraw chemical entities that may have unsolvable developmental issues related to pharmacokinetic enhancement. Therefore, BCS plays a crucial role in optimizing the properties of new chemical entities and reducing the likelihood of their disapproval.
Extention to biopharmaceutical classification system[48,49]
-
- Six classes bio pharmaceutical classification system
Bergstrom's six BCS classes were utilized to classify solubility as either "high" or "low" and permeability as "low," "intermediate," or "high." This novel classification was determined based on the correlation between molecular surface calculations area descriptors and solubility/permeability. The study's findings indicate that employing multivariate data analysis on comprehensible molecular surface descriptors provides computational methods to predict the solubility and permeability of drugs. Notably, surface areas associated with the nonpolar component of the molecule yielded accurate solubility predictions, while surface areas representing the polar regions of the molecule yielded reliable permeability predictions.
- Regulatory Application Of BCS Classification System[50, 51, 52, 53]
BCS is extensively utilized in the pharmaceutical sector, primarily due to its inclusion in the subsequent compilation of guideline materials and guidance documents mentioned below.
- New drug applications (NDAs) and investigational new drug applications (INDs) are important in the pharmaceutical industry. If the dosage forms of a drug exhibit similar dissolution patterns in laboratory tests, biopharmaceutical classification system (BCS)-based bio waivers can be applied to the formulation that will be marketed, as long as modifications are made to the components, makeup, or manufacturing process of the formulation used in clinical trials. This strategy is only applicable to highly soluble and highly permeable drugs (BCS Class I) that have identical formulations before and after the changes. The main objective of BCS-based bio waivers is to ensure bio equivalency (BE) in research. However, it is important to note that these bio waivers do not cover research on the bioavailability (BA) of food or other pharmacokinetic studies.
- If the reference listed drug product is also rapidly dissolving and the test product shows similar dissolving profiles to the reference listed drug product, BCS-based bio waivers can be requested for rapidly dissolving immediate release (IR) test products containing highly soluble and highly permeable drug substances. When the test and reference dose forms are pharmaceutical equivalents, this method works well.
- If the dissolution rate of the post change product remains fast and the dissolution profiles of the pre- and post-change products are comparable, it is possible to request BCS-based bio waivers for significant post approval changes, such as Level 3 changes in components and composition, for a rapidly dissolving immediate release (IR) product that contains a highly soluble and highly permeable drug substance. Currently, bioequivalence studies are being conducted for novel medicine NDAs, generic product ANDAs, scale up, and post-approval modifications.
SUGGESTED IMPROVEMENT OF BCS CLASSIFICATION SYSTEM[54, 55, 56, 57,58]
1. Class-I Permeation rate limited absorption: Drugs with in-vivo K diss > in vivo K pe belong to class I regardless fa.
2. Class-II Dissolution rate limited absorption: Drugs with in-vivo K diss< in>
CONCLUSION
The in vivo pharmacokinetics of medicines can be influenced by their solubility and permeability characteristics. The BCS classification system concept provides a useful framework for predicting the in vivo performance of a medicine and developing innovative drug delivery methods that are suitable for both the drug's physiological activity and the control of its bioequivalence product, both during approval and scale-up. BCS classification system also allows for the prediction of medication elimination, absorption, transportation, and disposal. In the future, it is possible that the BCS classification system hypothesis will be increasingly utilized in the early development of new medications, particularly for analogue selection and preliminary formulation techniques. The BCS classification system serves as a guiding principle for the development of various oral medication delivery systems. It takes into account dissolution, solubility, and intestinal permeability, which are the key factors influencing the absorption of drugs from immediate-release solid dosage forms. By considering these parameters, the BCS classification system allows drug designers to modify the physiochemical or structural characteristics of the lead candidate. Apart from cost savings, the BCS classification system also reduces drug exposure to a wide range of human participants and can potentially shorten the time required for developing new therapeutic products. However, there may be concerns regarding the accuracy and practicality of the binary definitions of permeability and solubility used by the BCS classification system especially when dealing with borderline cases. The potential for misclassification exists, particularly when considering lower doses of the same substance or dose formulations with controlled release. It is also important to determine at what stage of the development process the BCS classification system concepts can be effectively implemented. Despite its advantages, BCS-based systems are still not widely adopted, possibly due to uncertainties among regulatory agencies and pharmaceutical companies. The existence of significant discrepancies in bio waiver dossiers and evaluations suggests a lack of consensus on the proper application of the BCS classification system concept to provide support.
REFERENCES
- Amid on GL, Lanners H, Shah VP, and Crison JR, “A theoretical basis for a bio pharmaceutics drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability,” Pharm. Res., 1995; 12: 413–420.
- Guidance for industry, “Waiver of in vivo bioavailability and bioequivalence studies for immediate release solid oral dosage forms based on a bio pharmaceutics classification system,” CDER/FDA, August 2000.
- bio pharmaceutics Classification System Guidance Office of Pharmaceutical Science, CDER/FDA, August 2006.
- Dress man J, Butler J, Hempen stall J, Pappas C, “The BCS: where do we go from here,” Pharmaceutical Technology., 2001; 68-76.
- Amid on GL et al, “Estimating the fraction dose absorbed from the suspensions of poorly soluble compounds in humans: a mathematical model,” Pharm Res., 1993; 10(3): 264-270.
- Dress man JB, Amid on GL, Reppas C, Shah VP, “Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms,” Pharm Res., 1998; 15(1): 11-22.
- Amid on GL, Lennernas H, Shah VP, Crison JR, “A theoretical basis for a biopharmaceutical drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability,” Pharm. Res., 1995; 12(3): 413-419.
- Nattee S, Natalie D, “In vitro-in vivo correlations,” Int. J. Generic Drugs., 2005; 250-258.
- Devane J, and Butler J, “The impact of in vitro-in vivo relationships on product development,” Pharm. Tech., 1997; 21(9): 146-159.
- Emami J, “In vitro - in vivo correlation: From theory to applications,” J. Pharm. Pharmaceut. Sci., 2006; 9(2): 169-189.
- Guidance for Industry, “Waiver of in vivo bioavailability and bioequivalence studies for immediate release solid oral dosage forms containing certain active moieties/active ingredients based on biopharmaceutics classification system,” FDA, August 1999.
- Swarbrick J, “Encyclopaedia of pharmaceutical technology”, Vol III, 3rd Edition, Pharmaceu tech inc,Informa Healthcare USA, 2007; 2049-2062.
- Chowdary KPR, Vijayasrinivas S, “Biopharmaceutical classification system,” The Indian Pharmacist, Dec 2004; 7-10.
- Gothoskar AV. Biopharmaceutical classification system of drugs [online]. Pharmaceut Rev. Availablen from: URL: http:// www. pharmainfo.net/reviews/biopharmaceutical-classification-drugs. [last cited on 2005 Feb
- Center for Drug Evaluation, USFDA. Guidance for industry: Waiver of In vivo bioavailability and bioequivalence for immediate release solid oral dosage forms based on a biopharmaceutics classification system. August 2000.
- Multisource (generic) pharmaceutical products: Guidelines on registration requirements to establish interchange ability. In: WHO Expert Committee on Specifications for Pharmaceutical Preparations, Fortieth Report. Geneva, World Health Organization. WHO Technical Report Series, No. 937, Annex 7: 2000. p. 347-390. 1
- Committee for Medicinal Products for Human Use. Guideline on the Investigation of Bioequivalence (CPMP/EWP/QWP/1401/98 Rev. 1), July 2008.
- Sinko PJ, Leesman GD, Amidon GL. Predicting fraction dose absorbed in humans using a macroscopic mass balance approach. Pharmaceutics Res 1991;8:979-88.
- Lennernäs H, Knutson L, Knutson T, Hussein A, Lesko L, Salmons on T, et al. The effect of amiloride on the in vivo effective permeability of amoxicillin in human jejunum: Experience from a regional perfusion technique. Eur J Pharm Sci 2002;15:271-7.
- Bjarnason I, Smethurst P, Fenn CG, Lee CE, Menzies IS, Levi AJ. Misoprostol reduces indomethacin-induced changes in human small intestinal permeability. Dig Dis Sci 1989;34:407-11.
- Farthing MJ. Disease-related animal models for optimising oral rehydration solution composition. Acta Paediatr 2008;78:23-30.
- Scherer D, Mooren FC, Kinne RK, Kreuter J. In Vitro permeability of PBCA nanoparticles through porcine small intestine. J Drug Target 1993;1:21-
- Yee S. In Vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man-fact or myth. Pharm Res 1997;14:763- 6.
- Grès MC, Julian B, Bourrié M, Meunier V, Roques C, Berger M, et al. Correlation between oral drug absorption in humans, and apparent drug permeability in TC-7 cells, a human epithelial intestinal cell line: Comparison with the parental Caco-2 cell line. Pharm Res 1998;15:726-33.
- Khar RK, Pandita D, “Biopharmaceutical classification system and its importance,” The Indian Pharmacist, March 2005; 25-30.
- Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutics drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res 1995;12:413-20.
- Helga M. The Biopharmaceutical Classification System (BCS) and its usage. Drugs Made in Germany 2002;45:63-5.
- Dressman J, Butler J, Hempenstall J, Reppas C. The BCS: Where do we go from here? Pharmaceut Technol 2001;25:68-76.
- Gothoskar AV. Biopharmaceutical classification system of drugs [online]. Pharmaceut Rev. Availablen from: URL: http:// www. pharmainfo.net/reviews/biopharmaceutical-classification-drugs. [last cited on 2005 Feb 12].
- Oliveira CP, Ribeiro MENP, Ricardo NMPS, Souza TVP, Moura CL, Chibundit C, Yeates SG, Nixon K , Attwood D. (2011) The effect of water- soluble
- polymers, PEG and PVP, on the solubilisation of griseofulvin in aqueous micellarsolutions of Pluronic F127. Int. J. Pharm. 421: 252–257.
- Wulff M, Alden M, Craig DQM. (1996) An investigation into the critical surfactant concentration for solid solubility of hydrophobic drug in different polyethylene glycols. Int. J. Pharm. 142: 189- 198.
- Saito M, Ujajin T, Nozawa Y, Sadzuka Y, Miyagishima A, Sonobe T. (2002)
- Preparation and dissolution characteristics of griseofulvin soliddispersions with saccharides. Int. J. Pharm. 249: 71-79.
- Lukac M, Prokipcak I, Lacko I, Devinsky F. (2011) Solubilisation of griseofulvin and rutin in aqueous micellar solutions of Gemini and heterogemini surfactants and their mixtures. Eur. J. Pharm. Sci. 44: 194–199.
- Al-Obaidi H,. Buckton G. (2009) Evaluation of Griseofulvin Binary and Ternary Solid Dispersions with HPMCAS. AAPS Pharm. Sci. Tech.:10: 1172- 1177.
- Dhanaraju MD, Kumaran KS, Baskaran T, Moorthy MSR. Enhancement of Bioavailability of Griseofulvin by Its Complexation with ?-Cyclodextrin. Drug Dev.
- Ind. Pharm. 24: 583-587. 40) Dandagi PM, Kaushik S, Telsang S. (2011) Enhancement of solubility and dissolution property of griseofulvin by nanocrystallization. Int. J. Drug Dev.
- Dandagi PM, Kaushik S, Telsang S. (2011) Enhancement of solubility and dissolution property of griseofulvin by Nano crystallization. Int. J. Drug Dev. 26 Res. 3: 180-190.
- Jadav PA, Deshmukh VT, Patil PS, Dhawale SC. (2011) Enhancement of solubility and dissolution rate of griseofulvin by microparticulate systems. Current Pharm. Res. 2(1): 432-438.
- Yadav VB, Yadav AV. (2009) Effect of Different Stabilizers and Polymers on Spherical Agglomerates of Gresiofulvine by Emulsion Solvent Diffusion (ESD) System. Int. J. Pharm. Tech. Res. 1(2): 149-150.
- Gohel MC, Mehta NR. An audit of recent inputs on biopharmaceutical classification system [online]. Pharmaceut Rev. Available from: http://www.pharmainfo.net/reviews/audit-recent-inputs- biopharmaceuticalclassification-system. [last cited on 2005 Jan 09].
- Yu LX, Amidon GL, Polli JE, Zhao H, Mehta MU, Conner DP, et al. Biopharmaceutics classification system: The scientific basis for biowaiver extension. Pharm Res 2002;19:921-5.
- Wilding I. Evolution of the Biopharmaceutics Classification System (BCS) to Modified Release (MR) formulations: What do we need to consider? Eur J Pharm Sci 1999;8:157-9.
- Devane J. Oral drug delivery technology: ddressing the solubility/permeability paradigm. Pharmaceut Technol 1998;22:68-80.
- Lennernäs H, Abrahamsson B. The use of biopharmaceutic classification of drugs in drug discovery and development: Current status and future extension. J Pharm Pharmacol 2005;57:273-85.
- Varma MV, Khandavilli S, Ashokraj Y, Jain A, Dhanikula A, Sood A, et al. Biopharmaceutical classification system: A scientific framework for pharmacokinetic optimization in drug research. Curr Drug Metab 2004;5:375- 88.
- Bergestrom CA, Lazorova L, Avdeef A, Luthman K, Artursson P. Absorption classification of oral drugs based on molecular surface properties. J Med Chem 2003;46:558-70.
- Todd PA and Heel RC, “Enalapril a review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in hypertension and congestive heart failure. Drugs,1986;31(3): 198-248.
- Beaumont K, Webster R, Gardner I and Dack K, “Design of ester prodrugs to enhance oral absorption of poorly permeable compounds: Challenges to the discovery scientist. Curr. Drug Metab, 2003; 4: 461-485.
- Bogues K, Dixon GT, Fowler P, Jenner WN, Maconochie JG, Martin LE and WilloughbyBA, “Pharmacokinetics and bioavailability of ranitidine in humans,” Br. J. Clin. Pharmacol, 1980; 73: 275-276.
- Garg DC, Weidler DJ, Baltodano N, Eshelman FN, “Pharmacokinetics of ranitidine, a new histamine H2-receptor blocker,” Br. J. Clin. Pharmacol, 1981; 29(2): 247-248.
- The European Agency for the Evaluation of Medicinal Products (EMEA), Note for Guidance on the Investigation of Bioavailability and Bioequivalence. Committee for Proprietary Medicinal Products, 2002.
- Polli JE, Yu LX, Cook JA, Amidon LA, Borchardt RT, Burnside BA, Burton PS, Chen M-L, Conner DP,Faustino PJ, Hawi AA, Hussain AS, Joshi HN, Kwei G, Lee VHL, Lesko LJ,Lipper RA, Loper AE,Nerurkar SG, Polli JW, Sanvordeker DR, Taneja R, Uppoor RS, Vattikonda CS, Wilding I and Zhang G, Summary workshopreport: Biopharmaceutics classification system- implementation challenges and extension opportunities.J. Pharm. Sci., 2005; 93(6): 1375-1381.
- Gupta E., Barends DM, Yamashita E, Lentz KA, Harmsze AM, Shah VP, Dressman JB and Lipper RA,. Review of global regulations concerning biowaivers for immediate release solid oral dosage forms. Eur. J. Pharm. Sci., 2006; 26: 315-324.
- The European Agency for the Evaluation of Medicinal Products (EMEA), Concept paper on BCS based biowaiver. Committee for Medicinal Products for Human Use, 2007.
- Barends DM, Application and experience in the EU of BCS in the review of new generics, J. Pharm.Pharmacol, 2005; 57(11): 117.
- Plöger GF, Quizon PM, Abrahamsson B, Cristofoletti R, Groot DW, Parr A, Langguth P, Polli JE, Shah VP, Tajiri T, Mehta MU, Dressman J. Biowaiver monographs for immediate release solid oral dosage forms: cephalexin monohydrate. J Pharm Sci. 2020;109(6):1846–62. https://doi.org/10.1016/j.xphs.2020.03.025.
- Midha KK, Hubbard JW, Rawson M, Gavalas L. The applica-tion of partial areas in assessment of rate and extent of absorption in bioequivalence studies of conventional release products: experimental evidence. Eur J Pharm Sci. 1994;2(5):351–63. https://doi.org/10.1016/0928-0987(94)00062-X.
- Otter M, Oswald S, Siegmund W, Keiser M. Effects of frequently used pharmaceutical excipients on the organic cation transporters 1-3 and peptide transporters 1/2 stably expressed in MDCKII cells. Eur J Pharm Biopharm: Official Journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2017;112:187–95. https://doi.org/10.1016/j.ejpb.2016.11.028.
- Chen M-L, Sadrieh N, Yu L. Impact of osmotically active excipients on bioavailability and bioequivalence of BCS class III drugs. AAPS J. 2013;15(4):1043–50. https://doi.org/10.1208/ s12248-013-9509-z.
- Yang B, Smith DE. Significance of peptide transporter 1 in the intestinal permeability of valacyclovir in wild-type and PepT1 knockout mice. Drug Metab Dispos. 2013;41(3):608–14. https://doi.org/10.1124/dmd.112.049239.
- Ganapathy ME, Huang W, Wang H, Ganapathy V, Leibach FH. Valacyclovir: a substrate for the intestinal and renal peptide transporters PEPT1 and PEPT2. Biochem Biophys Res Commun. 1998;246(2):470–5. https://doi.org/10.1006/ bbrc.1998.8628.
- Zhang W, Li Y, Zou P, Wu M, Zhang Z, Zhang T. The effects of pharmaceutical excipients on gastrointestinal tract metabolic enzymes and transporters-an Update. AAPS J. 2016;18(4):830– 43. https://doi.org/10.1208/s12248-016-9928-8.


 Kalyani J. Bhor*
Kalyani J. Bhor*
 Rashid Azeez 2
Rashid Azeez 2







 10.5281/zenodo.10603065
10.5281/zenodo.10603065