Abstract
Transdermal Drug Delivery System is controlled medicated that is set on the skin to deliver a particular Portion of medicine through the skin and into the circulatory system. It is likewise significant because of its Interesting benefit such as less absorption, more uniform plasma levels, improved bioavailability, decrease Side effect, efficacy and quality of the product. Transdermal dose structures may give clinicians a chance to Offer more therapeutic alternatives to their patients to upgrade their consideration. For thousands of years, human civilizations have applied substances to the skin as cosmetic and medicinal agents. However, it Was not until the twentieth century that the skin came to be used as a drug delivery route. In fact, Merriam Webster dates the Word “transdermal” to 1944 highlighting that it is a relatively recent concept in medical and pharmaceutical practice. Transdermal drugs are self?contained, discrete dosage form. Drug delivery through the skin to achieve a systemic effect Without producing any fluctuations in plasma concentration of the drug. Topical administration of therapeutic agents offers Many advantages over conventional oral and invasive methods of drug delivery. And also provide controlled release of the Drug for extended period of the time. Today about 74% of drugs are taken orally and are found not to be as effective as desired. To improve such characters transdermal Drug delivery system was emerged. The transdermal route of drug delivery has attracted researchers due to many biomedical Advantages associated with it. However, excellent impervious nature of skin is the greatest challenge that has to be overcome for Successfully delivering drug molecules to the systemic circulation by this route. Drug delivery through the skin to achieve a systemic Effect of a drug is commonly known as transdermal drug delivery and differs from traditional topical drug delivery. The Development of transdermal drug delivery systems is a multidisciplinary activity that encompasses fundamental feasibility studies Starting from the selection of a drug molecule to the demonstration of sufficient drug flux in an ex vivo and/or in vivo model the Fabrication of a drug delivery system that meets all the stringent needs that are specific to the drug molecule (physicochemical and Stability factors), the patient (comfort and cosmetic appeal), the manufacturer (scale-up and manufacturability), and most Important, the economy.
Keywords
Skin, transdermal drug delivery, novel transdermal drug delivery system.
Introduction
Now a day many drugs are administered orally, but they are Observed not more effective as desired so to upgrade such Character TDDS was created. Drug delivery administered By the skin and attain a systemic effect of drug is called As transdermal drug delivery system. 1 These are kind of Dosage form which includes drug transport to reasonable Epidermis and potentially dermal tissue of the skin locally Therapeutic effect. 2 While an exceptionally significant Division of the drug is transported in systemic blood Circulation. A transdermal dermal patch is characterized as A medicated adhesive patch which is set over the skin to Deliver a particular dose of medication by the skin with A foreordained rate of release to reach into the circulation System. 3
Drugs administered in the conventional dosage forms Usually produce large range in fluctuations in plasma drug Concentrations leading to undesirable toxicity or poor Effectiveness. These factors as well as other factors such As repetitive dosing and unpredictable absorption, led to The concept of the controlled drug delivery system or Therapeutic system. A dosage form that releases one or More drugs continuously in a predetermined pattern for a Fixed period of time, either systemically or to a specified Target organ is a controlled drug delivery system. The Primary objectives of controlled drug delivery are to Ensure safety and to improve efficacy of drugs as well as Patient compliance. This is achieved by better control of Plasma drug levels and less frequent dosing. Transdermal Therapeutic systems are defined as self-contained discrete Dosage forms which, when applied to the intact skin, Deliver the drug(s), through the skin, at controlled rate to The systemic circulation1, 2.
During the seventies, the newer forms of medication did not Match rapid growth of new drugs. From eighties a sort of Reverse trend is being witnessed. Research and Development Activities have become far more vigorous in the field of novel Drug delivery system, rather than in the research for newer Drugs. The enormous cost, long drawn time and uncertainty About the reward have dampened the discovery of newer Drugs. These novel drug delivery systems are developed by the Application of the concepts and techniques of controlled release Drug administration which cannot only extend the potent life of Existing drug but also minimize the scope and expenditure of Testing required for FDA approval and which make clinically Already established drugs do their therapeutic best. The goal of any drug delivery system is to provide a Therapeutic amount of drug to the proper site in the body to Achieve promptly and maintain the desired drug concentration. Many novel drug delivery systems have been developed e.g. Transdermal, Intrauterine, Intravaginal, and Implants etc. These drug delivery systems have added a new dimension of Optimizing the treatment of several disease conditions by modifying various pharmacokinetics parameters. This drug Delivery system releases the drug either by zero order kinetics Or by first order kinetics or by both simultaneously. Transdermal drug application has been well known since Ancient times. Several ancient cultures used ointments, pastes, Plasters and complex inunctions in the treatment of various Symptoms or disease.
Advantages of TDDS:
- Avoids hepatic first pass metabolism.
- Maintains constant blood levels for longer period of time.
- Improve bioavailability.
- Decrease the dose to be administered.
- Decrease side or unwanted effects.
- Decrease gastrointestinal side effects.
- Easy to discontinue in case of toxic effects.
- Increase patient compliance.
Disadvantages of TDDS :
- Cost is high.
- TDDS cannot deliver ionic drugs.
- TDDS cannot achieve high drug levels in blood/plasma.
- Cannot develop TDDS for drugs of large molecular size.
- TDDS cannot deliver drugs in a pulsatile fashion.
- Cannot develop TDDS, if drug or formulation causes Irritation to skin.
SKIN
Structure of the Skin:
Skin is most extensive and readily accessible organ in the Body. Its chief functions are concern with protection, Temperature regulation, control of water output and sensation. In an average adult it covers an area of about 1.73m2 And Receives one third of circulating blood through the body at any Given time. The potential of using intact skin as the site of Administration for dermatological preparations to elicit Pharmacological action in the skin tissue has been recognized For several years. Until the turn of the century, the skin was Thought to be impermeable. Skin is the complex organ and Allows the passage of chemicals into and across the skin. The Permeation of chemicals, toxicants and drugs are much slower Across the skin when compared to other biological membranes In the body. The understanding of this complex phenomenon Has lead to the development of transdermal drug delivery System, in which the skin serves as the site for the Administration of systemically active drugs. Following skin Permeation, the drug first reaches the systemic circulation. The Drug molecules are then transported to the target site, which Could be relatively remote from the site of administration, to Produce their therapeutic action. In discussing skin structure, we limit ourselves to those Features of the membrane which are pertinent to drug delivery; In particular, we play special attention to the stratum corneum (SC), the outermost layer wherein skin’s barrier function Principally resides.

Figure 1. Structure of Skin
Microscopically, skin comprises two main layers: the Epidermis and the Dermis (~ 0.1 and 1 mm in thickness, Respectively) (Figure 1). The dermal-epidermal junction is highly convoluted ensuring a maximal contact area. Other Anatomical features of the skin of interest are the appendageal Structure: the hair follicles, nails and sweat glands.
The epidermis is a stratified, squamous, keratinizing Epithelium. The keratinocytes comprise the major cellular Component (> 90%) and the responsible for the evolution of Barrier function. Other cells present include Melanocytes, Langerhans cells and Markel cells, none of which appears to Contribute to the physical aspects of the barrier. The stratum corneum Is usefully thought of as a “brick Wall”, with the fully differentiated corneocytes comprising the „bricks?, embedded in the „mortar? created by the intercellular Lipids. The corneocytes are flat, functionally dead cells, the Cytoplasmic space of which is predominantly keratin. When the Lamellar bodies of the upper granular cells extrude their Contents, the flattened lipid vesicles fuse “edge-to-edge” and Organize into extremely well ordered, multilamellar, bilayer Shits. A layer of lipid covalently bound to the cornified Envelope of the corneocyte has been suggested to contribute Uniquely to this exquisite organization. Particularly noteworthy Is that the intercellular lipids of the stratum corneum, in Contrast to almost all other biomembranes, include no Phospholipids, comprising rather an approximately equimolar Mixture of ceramides, cholesterol and free fatty acids. These Non-polar and somewhat rigid components of the stratum Corneum „cement? play a critical role in barrier function. On average, there are about 20 cell layers in the stratum Corneum, each of which is perhaps 0.5 µm in thickness. Yet, The architecture of the membrane is such that this very thin Structure limits, under normal conditions, the passive loss of Water across the entire skin surface to only about 250 mL per Day, a volume easily replaced in order to maintain homeostasis. The link between skin barrier and stratum corneum lipid Composition and structure has been clearly established. For Example, change in intercellular lipid composition and/or Organization typically results in a defective and more Permeable barrier. Lipid extraction with organic solvents Provokes such an effect. Skin permeability at different body Sites has been correlated with local variation in lipid content. Moreover, most convincingly, the conformational order of the Intercellular lipids of stratum corneum is correlated directly With the membrane’s permeability to water. Taken together, These observations have led to the deduction that the stratum Corneum has achieved such an excellent barrier capability by Constraining the passive diffusion of molecules to the Intercellular path (the corneocytes being simply too Impermeable to allow efficient transfer from one side of the Membrane to the other). This mechanism is tortuous and Apparently demands a diffusion path length at least an order of Magnitude greater than that of the thickness of the stratum Corneum. Current opinion, then, is that the stratum corneum is most convincingly viewed as a predominantly lipophilic Barrier (this makes perfect good sense as it was designed to Inhibit passive loss of tissue water in an aired environment), Which manifests a high degree of organization, and which Constrains permeating molecules to a long and convoluted Pathway of absorption. These characteristics, therefore, dictate The permeability of the membrane and determine the extent to Which drug of various physicochemical properties may be Expected to transport. The dermis, inner and larger (90%) skin layer, comprises Primarily connective tissue and provides supports to the Epidermis. The dermis incorporates blood and lymphatic Vesicles and nerve endings. The extensive microvasculature Network found in the dermis represents the site of resorption For drugs absorbed across the epidermis; i.e. at this point that Transdermally absorbed molecules gain entry to the systemic Circulation and access to their central target. The dermis also supports skin’s appendageal structure, Specially the hair follicles and sweat glands. The pilosebaceous Unit comprises the hair follicle, the hair shaft and sebaceous Gland. The hair follicle is an invagination of the epidermis that Extends deeper into the dermis. The lining of the lower Portion of the hair follicle is not keratinized and presumably Offers a lesser barrier to diffusion than the normal stratum Corneum. With respect drug delivery, interest in these Structures has centered upon the possibility that they may Provide “shunt” pathway across the skin, circumventing the Need to cross the full stratum corneum. While this is Completely reasonable hypothesis, it is somewhat irrelevant From the practical standpoint because the follicles occupy Relatively insignificant fraction of the total surface area available For transport (~0.1%). A similar argument can be made with Respect to the sweat glands, which cover a considerably smaller Total area than the follicles. As noted later, however, Appendageal transport may assume a much more important Role when specialized enhancing technologies are used to Increase transdermal delivery.

Figure 2. Drug permeation through skin
In addition to relationship between rate of drug delivery to The skin and maximum achievable drug permeation across the Skin, the choice of drugs to be delivered transdermally, clinical Needs and drug pharmacokinetics are some of the important Consideration in the development of transdermal drug delivery Systems (TDDS). Schematic representation of drug levels in Blood from P.O. and transdermal route of administration is Shown in Figure 2. As can be seen from Figure 2, a TDDS is Design to release drugs at a predetermined rate and Continuously, avoiding unnecessarily high peaks and Sub therapeutic troughs in plasma drug levels. Skin is structurally complex and thick membrane. Molecules moving from the environment must penetrate the Stratum corneum and any material of endogenous or Exogenous origin on its surface. They must then penetrate the Viable epidermis, the papillary dermis and the capillary walls Into the blood stream or lymph channels, whereupon they are Removed from the skin by flow of blood or lymph. To move Across the skin membrane is obviously a complex phenomenon And challenge in analysis.
Enhancement of transdermal delivery :
External stimuli, such as electrical, mechanical, or physical stimuli, are known to enhance skin permeability of Drugs and biomolecules, as compared to the delivery of Drugs by topical application on the skin [73]. TDDS supplemented by appropriate equipment is termed as active Transdermal delivery, which is known to deliver drugs Quickly and reliably into the skin. In addition, this mode Of enhanced TDDS can accelerate the therapeutic of delivered drugs.
Iontophoresis:

Iontophoresis promotes the movement of ions across The membrane under the influence of a small externally Applied potential difference (less than 0.5 mA/cm2),Which has been proven to enhance skin penetration and Increase release rate of several drugs with poor absorption/permeation profiles. This technique has been utilized in the in vivo transport of ionic or nonionic drugs By the application of an electrochemical potential gradient [25]. The efficacy of iontophoresis depends on the Polarity, valence, and mobility of the drug molecule, the Nature of the applied electrical cycle, and the formulation containing the drug. In particular, the dependence On current makes drug absorption through iontophoresis Less dependent on biological parameters, unlike most Other drug delivery systems (Fig. 2A, B) [26]. This modality could additionally include electronic means of Reminding patients to change dosages, if desired, to increase patient compliance [27, 28].
Sonophoresis:

The desired range of ultrasound frequencies generated By an ultrasound device can improve transdermal drug Delivery [30, 31]. Low-frequency ultrasound is more effective, because it facilitates drug movement by creating an aqueous path in the perturbed bilayer through cavitation (Fig. 2C) [32]. The drug under consideration is Mixed with a specific coupler, such as a gel or a cream, Which transmits ultrasonic waves to the skin and disturbs the skin layers, thereby creating an aqueous path Through which the drug can be injected. Drugs typically Pass through passages created by the application of Ultrasonic waves with energy values between 20 kHz and 16 MHz. Ultrasound also increases the local temperature Of the skin area and creates a thermal effect, which further promotes drug penetration. Several drugs of different classes have been delivered by this method Regardless of their solubility, dissociation and ionization Constants, and electrical properties (including hydrophilicity), such as mannitol and high molecular weight (MW) drugs such as insulin. However, the exactly mechanism of drug penetration through this method is Not yet completely understood, and problems with device availability, optimization of duration of exposure And treatment cycles for delivery, and undesirable side Effects including burns persist.
Electroporation :
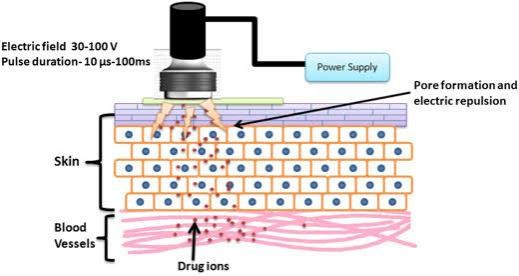
This method uses the application of high voltage electric Pulses ranging from 5 to 500 V for short exposure times (~ms) to the skin, which leads to the formation of small Pores in the SC that improve permeability and aid drug Diffusion [34, 35]. For safe and painless drug administration, electric pulses are introduced using close positioned electrodes. This is a very safe and painless Procedure involving permeabilization of the skin and has Been used to demonstrate the successful delivery of not only low MW drugs, such as doxorubicin, mannitol, Calcein, but also high MW ones such as antiangiogenic Peptides, oligonucleotides, and the negatively charged Anticoagulant heparin. However, this method has the Disadvantages of small delivery loads, massive cellular Perturbation sometimes including cell death, heating Induced drug damage, and denaturation of protein and Other biomacromolecular therapeutics.
Photomechanical waves :
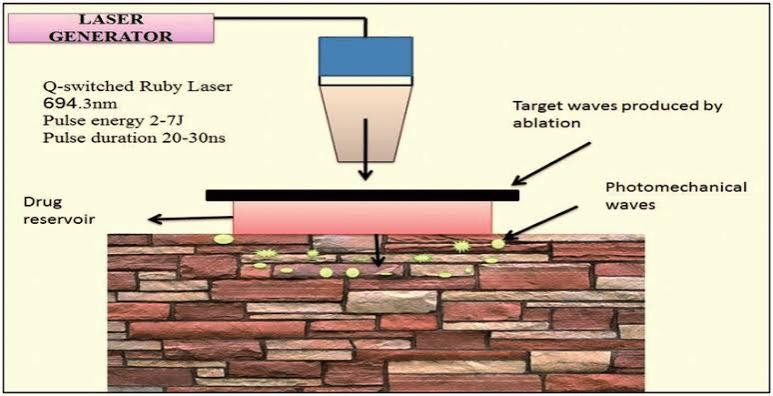
Photodynamic waves transmitted to the skin can penetrate the SC, allowing the drug to pass through the transiently created channel [37, 39]. The incident wave Produces limited ablation, which is achieved by low radiation exposure of approximately 5–7 J/cm2 to increase The depth to 50–400 ?m for successful transmission. This limited ablation showed a longer increase and duration as compared to that in other direct ablation techniques, which made it necessary to control properties of The photodynamic waves to ensure delivery of the product to the intended depth in the skin. The wave generated by a single laser pulse also showed increased skin Permeability within minutes, allowing macromolecules To diffuse into the skin. Dextran macromolecules of 40 kDa weight and 20 nm latex particles could be delivered by a single photodynamic laser pulse of a 23-ns duration.
Microneedle:
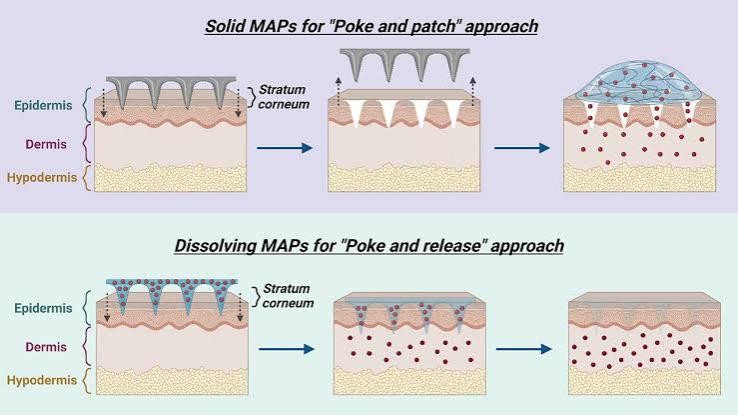
The microneedle drug delivery system is a novel drug Delivery system, in which drugs are delivered to the circulatory system through a needle [41]. This represents One of the most popular methods for transdermal drug Delivery and is an active area of current research. This Involves a system in which micron-sized needles pierce The superficial layer of the skin, resulting in drug diffusion across the epidermal layer. Because these microneedle are short and thin, these deliver drugs directly to The blood capillary area for active absorption, which Helps in avoiding pain [42]. Scientists have attempted to Use multiple techniques for appropriate optimization And geometric measurements required for effective insertion of microneedles into human skin, which also Represents the broad objective of research on Microneedle. The fabrication of microneedle system has been widely Investigated with considering the objective, drug type And dose, and targets for use [43]. Up to now, the microneedles can be fabricated with laser-mediated techniques And photolithography. The laser-mediated fabrication Techniques are used for manufacturing metal or polymer Microneedle. The 3D structure of a microneedle is generated through cutting or ablating on a flat metal/polymer surface using a laser [44, 45]. Photolithography is Known as the method of elaborately fabricating microneedle and has the advantage of being able to manufacture needles of various shapes using various materials. This method is mainly used to manufacture dissolving/Hydrogel microneedles or silicon microneedles via making an inverse mold based on the microneedle structure Through etching of photoresist [46]. In addition, 3D Printing [47], Microstereolithography [48], and Two-Photon polymerization [49] are also investigated for preparing various microneedle system.
The prepared microneedles could be of several types, Such as solid microneedles that simply make a physical Path through which drugs can be absorbed, drug-coated Microneedles which facilitate delivery of drugs coated on The surfaces of the needles as the latter enter the skin, Dissolving microneedles made of drug formulations that Dissolve in the body, naturally delivered melting needles Which involve drug storage in hollow needles followed By administration (such as a specific injection type), and Microneedle patches combined with diverse patch types.
Thermal ablation :
Thermal ablation, also known as thermophoresis, is a Promising technique for selectively disrupting the Stratum corneum structure by localized heat which provides enhanced drug delivery through microchannels Created in the skin [55]. To ablate the stratum corneum By thermal ablation, a high temperature above 100 °C is Required and this leads to heating and vaporization of keratin. Additionally, the degree of alteration of the Stratum corneum structure is proportional to the locally Elevated temperature, indicating that it is an ideal technique for precise control of drug delivery. The thermal Exposure should be short within microseconds to create A high enough temperature gradient across the skin for Selective ablation of the stratum corneum without damaging the viable epidermis. Micron-scale defects created From thermal ablation are small enough (50–100 ?m in Diameter) to avoid the potential to cause pain, bleeding, Irritation, and infection. Therefore, the patient is well Tolerated if there is no damage to the cells of the deeper Tissues. In addition, thermal ablation has better control And reproducibility than other approaches such as mechanical abrasion, chemical treatment, or tape-stripping. And it offers effective delivery of small molecules as well As high molecular weight compounds. However, the Structural changes in the skin must be evaluated, especially when using higher energy for enhancing the diffusion rate of drug molecules.
Thermal ablation can usually be induced by laser and Radiofrequency methods depending on the different Sources of thermal energy [56, 57]. Laser thermal ablation methodologies utilize a laser to induce micropore Structure of skin as well as the increase of the skin. Temperature which increases skin diffusivity. Laser light Energy is absorbed by water and pigments of the skin And transforms to thermal energy leading to water excitation and explosive evaporation from the epidermis. The degree of the ablated skin depth can be precisely Controlled upon tuning many parameters such as wavelength, pulse length, energy, number and repetition rate, Tissue thickness, absorption coefficient, and duration Time of laser exposure. Laser thermal ablation, especially When using Er:YAG laser, makes it possible to increase The penetration of drugs by more than 100 times and enhance the delivery of both lipophilic and hydrophilic Drugs including biomacromolecules such as peptides, Proteins, vaccines, and DNAs [56–58].
TDDS using chemical enhancers (passive delivery) :
To achieve enhanced transdermal delivery and therapeutic efficacy, drugs should have low MW (less than 1 kDa), an affinity for lipophilic and hydrophilic phases, short half-life, and a lack of skin irritability [64]. Many factors affect drug penetration through the skin, such as species differences, skin age and site, skin temperature, state of the skin, area of application, duration of exposure, moisture content of the skin, pretreatment methods, and physical characteristics of the penetrant. Recent studies that have focused on aspects of transdermal drug delivery technologies ranging from the development of chemical enhancers that increase the Spread of drugs across the skin or increase the solubility Of drugs in the skin to novel innovative approaches that Extend this concept to the design of super-strong formulations, microemulsions, and vesicles [65, 66] (Fig. 3).Penetration enhancers can be used alone or in combination with chemical penetration enhancers with proven Superior skin penetration as compared to that of individual chemicals. These synergistic systems include eutectic Mixtures and nanoparticle composite self-assembled vesicles. Therefore, research in recent years have focused On the application of suitable molecular simulation Methodologies in understanding the skin lipid barrier, Mechanisms regulating penetration of molecules across The skin and transport of penetration enhancers, and Perturbations in the skin barrier function.
Vesicles :
Vesicles are colloidal particles filled with water and consist of Amphiphilic molecules in a bilayer arrangement. Under conditions of excess water, these Amphiphilic Molecules form concentric bilayers with one or more Shells (multilayer vesicles). Vesicles can carry water Soluble and fat-soluble drugs to achieve transdermal absorption. When utilized for topical applications, vesicles Can be used to achieve sustained release of stored drugs. It is also possible to employ vesicles in TDDS to control The absorption rate through a multilayered structure. Owing to the presence of different components, vesicle Systems can be divided into several types, such as liposomes, transfersomes, and ethosomes, depending on the Properties of the constituent substances [60].
Liposomes are circular soft vesicles formed by one or More bilayer membranes that separate an aqueous Medium from another. Their main components are usually phospholipids, with or without cholesterol. Phospholipid molecules are mainly composed of different polar head groups and two hydrophobic hydrocarbon chains. Polar groups can be either positively or Negatively charged. Hydrocarbon chain molecules have Different lengths and different degrees of unsaturation. The formation of liposomes occurs spontaneously upon Reconstitution of a dry lipid film in an aqueous solution. This unique structure allows liposomes to be both Hydrophilic and hydrophobic and affords encapsulation Of both water-soluble and fat-soluble substances. However, some studies have shown that liposomes can only Remain on the surface of the skin and cannot pass Through the granular layer of the epidermis, thereby Minimizing the amount of drug absorbed into the blood circulation. This property increases the retention of Drugs that stay on the skin, prolong their activity at the Site of the lesion, and allow long-term sustained release. Therefore, liposomes are the preferred system of choice For the topical treatment of skin diseases [61].
Polymeric nanoparticles :
Nanoparticles (NPs) are nanocarriers with sizes ranging Between 1 and 1000 nm and can be classified into several Types according to their composition. Drug administration in the form of NPs leads to targeted and controlled Release behavior, changes in in vivo dynamics of the Drug, and extends the drug residence time in the blood, Which further lead to improved drug bioavailability and Reduced toxicity and side effects. NPs are conventionally Generated by polymerization and crosslinking, and biodegradable polymeric materials such as gelatin and polylactic acid (PLA) are often used [67, 69, 70]. In the field Of TDDS, polymeric NPs are gaining increased attention Because they can overcome the limitations of other Lipid-based systems, such as by conferring protection to Unstable drugs against degradation and denaturation and Achieving continuous drug release to reduce side effects. Increase in the concentration gradient improves transdermal penetration of the drug. Depending on the Manufacturing method and structure, polymeric NPs Can be classified as nanospheres, nanocapsules, and polymer micelles. Widely used polymers include polylactic acid , poly(D,L-lactide-co-glycolide) (PLGA), polycaproLactone, polyacrylic acid, and natural poly esters (including chitosan, gelatin, and alginate). These polymer Chains can be synthesized by covalent linkage of two or more single polymeric units under specific conditions, Such as the presence of a synthetic membrane that Mimics the cellular lipid bilayer membrane.
Nanoemulsion :
Nanoemulsions are a mixture characterized by low viscosity and isotropic, thermodynamic, and dynamic stability [71]. The mixture consists of transparent or Translucent oil globules dispersed in an aqueous phase Stabilized by an interfacial membrane formed by surfactant or co-surfactant molecules of extremely small droplet size. The particle size of commonly used Nanoemulsions ranges from 100 to 1000 nm, although An upper limit to the particle size has been proposed on Account of its nanoscale dimensions. Nanoemulsions are Different from microemulsions; although nanoemulsions Have almost the same droplet size range, composition, And appearance as microemulsions, they differ greatly in Terms of structural aspects and long-term thermo-Dynamic stability. The small particle size, large specific Surface area, and low surface tension of nanoemulsions Provide excellent wettability that ensures close contact With the skin. In addition, nanoemulsions offer many Other benefits such as high solubilization capacity and Physical stability, improved bioavailability, ease of preparation, production with less energy input, and long shelf Life. Nanoemulsions exhibit a shorter transdermal time And better transdermal absorption than commonly used Topical skin preparations. Depending on the composition, nanoemulsions can include oil-in-water (O/W: oil Phase dispersed in a continuous aqueous phase), water-In-oil (W/O: aqueous phase dispersed in a continuous Oil phase), and bicontinuous/multiphasic emulsion. SevEral studies have reported the increased use of O/W Nanoemulsions as a delivery system for encapsulating Lipophilic components in pharmaceuticals, highlighting The immense potential of nanoemulsions in contributing To novel TDDS-based advances in pharmaceutical applications [58, 68].
Diffusion cell method :
Tests employing diffusion cells represent the gold standard in the evaluation of TDDS, with Franz diffusion cells Being the most common used setup (Fig. 4) [84, 85]. This technique determines important relationships Among the skin, active pharmaceutical ingredients, and The nature of the formulation. The diffusion cell consist Of a chamber for drug application, a membrane within Which the drug may diffuse, and an acceptor media Chamber from which samples may be investigated. DiffuSion cells are categorized into two main classes, namely, Static and flow-through cells. In static cells, as in the Popular Franz diffusion cell, the donor, the membrane, And the acceptor modules could be placed either vertically or horizontally. There are Franz cells that open From above; therefore, the measurement runs under conditions of atmospheric pressure. However, most of these Cells are closed from the top, leading to increased pressure, which translates to an overestimation of penetration values. Nowadays, “hand-sampler” Franz diffusion Cells have been replaced by systems connected to an automated sampler. These automated sampling systems facilitate the work of researchers and reduce errors from Manually conducted experiment.
Tape stripping :
Tape stripping is a commonly used minimally invasive Method to test the penetration of topically applied formulations through the SC, where a layer of the SC is removed with an

adhesive tape followed by examination of The skin layer on the adhesive tape (Fig. 5) [74, 83, 86,87]. The tape stripping process is performed after an appropriate incubation time post topical application of the Test composition. The composition may be removed or Left on the skin to provide the original amount of components to be used during the measurement. The adhesive tape is placed on the skin surface and is always Removed from the same selection. It is important that The adhesive tape is always flattened with the same force As the roller to eliminate the effect of creases and recesses on tape stripping. In addition, the removal rate is An important factor.
CONCLUSION
The development of TDDS technology is widely recognized as the development of a mass delivery methodology, which makes it the preferred drug injection Modality for transdermal delivery across skin types, While preventing first-pass metabolism and other sensitivities associated with various alternative drug administration routes. In various devices and TDDSs, drugs can Be delivered through the skin to the systemic circulation. Drugs are generally reliably and safely delivered through TDDS and are safe and stable from biochemical modifications until they reach the target tissue. TDDS is noninvasive, nonallergenic, and has a set duration and dose Delivery method, which allows for uniform distribution Of drugs at prescribed and controlled rates. Many new And old formulations are in the process of improving the Bioavailability of low-absorption drugs via easy routes of Administration that allow large doses to be administered Over a long period of time. Therefore, the TDDS technology is growing rapidly in the pharmaceutical field.
REFERENCES
- Chein YW. Transdermal Controlled-Release Drug Administration, Novel Drug Delivery System: Fundamental Development concepts and Biochemical Applications. New York: Marcel Dekker; 1982.
- Puttipipatkhachorn S. Journal of Controlled Release, 2001; 75:143-153
- Wilkosz MF. Transdermal Drug Delivery: Part I. U.S.Pharmacist. Jobson publication; 28:04; 2003.
- Jain NK. (Ed. First). Controlled and Novel Drug Delivery. CBS Publishers and distributors; 1997.
- Barry BW. Dermatological Formulations. New York: Marcel Dekker; 1983.
- Penna LE. Topical Drug Delivery Formulations, New York: Marcel Dekker; 1990.
- Chien, YW, Novel drug delivery systems, Drugs and the Pharmaceutical Sciences, Vol.50, New York: Marcel Dekker;1992.
- Panner Selvam R, Anoop Kumar Singh, Sivakumar T, Transdermal drug delivery systems for antihypertensive drugs A review, IJPBR, 2010, 1(1), 1-8.
- Kakkar A, P and Ajay Gupta, Gelatin Based Transdermal Therapeutic System, Indian Drugs, 1991, 29 (7), 308-315
- Vega-Vásquez P, Mosier NS, Irudayaraj J. Nanoscale drug delivery systems: From medicine to agriculture. Front Bioeng Biotechnol. 2020;8:79.
- Vargason AM, Anselmo AC, Mitragotri S. The evolution of commercial drug Delivery technologies. Nat Biomed Eng. 2021.
- Mali AD, Bathe R, Patil M. An updated review on transdermal drug delivery Systems. Int J Adv Sci Res. 2015;1(6):244–54.
- Li C, Wang J, Wang Y, Gao H, Wei G, Huang Y, et al. Recent progress in drug Delivery. Acta Pharm Sin B. 2019;9(6):1145–62.
- Kumar JA, Pullakandam N, Prabu SL, Gopal V. Transdermal drug delivery System: An overview. Int J Pharm Sci Rev Res. 2010;3(2):49–54.
- Roohnikan M, Laszlo E, Babity S, Brambilla DA. Snapshot of transdermal and Tropical drug delivery research in Canada. Pharmaceutics. 2019;11(6):256
- Peña-Juárez MC, Guadarrama-Escobar OR, Escobar-Chávez JJ. Transdermal Delivery Systems for Biomolecules. J Pharm Innov. 2021;6:1–14.
- Ali H. Transdermal drug delivery system & patient compliance. MOJ Bioequiv Availab. 2017;3(2):47–8.
- Leppert W, Malec–Milewska M, Zajaczkowska R, Wordliczek J. Transdermal And Topical Drug Administration in the Treatment of Pain. Molecules. 2018;23(3):681.
- Akhter N, Singh V, Yusuf M, Khan RA. Non-invasive drug delivery Technology: development and current status of transdermal drug delivery Devices, techniques and biomedical applications. Biomed Tech. 2020;65(3):243–72
- Pires LR, Vinayakumar KB, Turos M, Miguel V, Gaspar J. A perspective on Microneedle-based drug delivery and diagnostics in Paediatrics. J Pers Med.2019;9(4):49
- Ruby PK, Pathak SM, Aggarwal D. Critical attributes of transdermal drug Delivery system (TDDS) – a generic product development review. Drug Delivery system.
- Ali S, Shabbir M, Shahid N. The structure of skin and transdermal drug Delivery system – a review. Res J Pharm Tech. 2015;8(2):103–9.
- Wang M, Luo Y, Wang T, Wan C, Pan L, Pan S, et al. Artificial skin perception. Adv Mater. 2020;33:e2003014.
- Hutton AR, McCrudden MT, Larrañeta E, Donnelly RF. Influence of molecular Weight on transdermal delivery of model macromolecules using hydrogel- Forming microneedles: potential to enhance the administration of novel low Molecular weight biotherapeutics. J Mater Chem B. 2020;8(19):4202–9.
- Andrews SM, Jeong EH, Prausnitz MR. Transdermal delivery of molecules is Limited by full epidermis, Not Just Stratum Corneum. Pharm Res. 2013;30(4) 1099–109.
- Chaulagain B, Jain A, Tiwari A, Verma A, Jain SK. Passive delivery of protein Drugs through transdermal route. Artificial Cells Nanomed Biotechnol. 2018;46(1):472–87.
- Uchechi O, Ogbonna J, Attama AA. Nanoparticles for dermal and Transdermal drug delivery. In: Application of nanotechnology in drug Delivery. Sezer AD: InTech C; 2014. P. 193–235.
- . Zhou X, Hao Y, Yuan L, Pradhan S, Shrestha K, Pradhan O. NanoFormulations for transdermal drug delivery: a review. Chin Chem Lett. 2018 29(12):1713–24.
- Ková?ik A, Kope?ná M, Vávrová K. Permeation enhancers in transdermal Drug delivery: benefits and limitations. Expert Opin Drug Deliv. 2020;17(2):145–55.
- Pawar PM, Solanki KP, Mandali VA. Recent advancements in transdermal Drug delivery system. Int J Pharm Clin Res. 2018;10(3):65–73.
- Mujoriya R, Dhamande KA. Review on transdermal drug delivery system. ResJ Sci Tech. 2011;3(4):227–31.
- Patel R, Patel A, Prajapati B, Shinde G, Dharamsi A. Transdermal drug Delivery systems: A mini review. Int J Adv Res. 2018;6(5):891–900.
- Kakar S, Singh R, Rani P. A review on transdermal drug delivery. Innoriginal Int J Sci. 2016;3(4):1–5.
- Wang Y, Zeng L, Song W, Liu J. Influencing factors and drug Application of iontophoresis in transdermal drug delivery: an overview Of recent progress. Drug Deliv Transl Res. 2021.
- Dhal S, Pal K, Giri S. Transdermal delivery of gold nanoparticles by a Soybean oil-based oleogel under iontophoresis. ACS Appl Bio Mater. 2020; 3(10):7029–39.
- Escobar-Chávez JJ, Díaz-Torres R, Domínguez-Delgado CL, Rodríguez-CruzIM, López-Arellano R, Hipólito EAM. Therapeutic applications of Sonophoresis and sonophoretic devices. In: Percutaneous Penetration Enhancers Physical Methods in Penetration Enhancement. Springer-Verlag Berlin Heidelberg; 2017. P. 31–58.
- Charoo NA, Rahman Z, Repka MA, Murthy SN. Electroporation: An avenue For transdermal drug delivery. Curr Drug Deliv. 2010;7(2):125–36
- Agrawal S, Gandhi SN, Gurgar P, Saraswathy N. Microneedles: An Advancement to transdermal drug delivery system approach. J Appl Pharm Sci. 2020;10(3):149–59.
- Zhao Z, Chen Y, Shi Y. Microneedles: a potential strategy in transdermal Delivery and application in the management of psoriasis. RSC Adv. 2020 10(24):14040–9
- Lim J, Tahk D, Yu J, Min DH, Jeon NL. Design rules for a tunable merged-tip Microneedle. Microsyst Nanoeng. 2018;4(1):1–10. Dardano P, Caliò A, Palma VD, Bevilacqua MF, Matteo AD, Stefano LD. A
- Photolithographic approach to polymeric microneedles array fabrication. Materials. 2015;8(12):8661–73.


 Rutik Kachare*
Rutik Kachare*
 Ulka Mote
Ulka Mote








 10.5281/zenodo.14220788
10.5281/zenodo.14220788