Abstract
Transdermal drug delivery systems offer numerous advantages over conventional routes, including improved patient compliance and reduced systemic side effects. Spray-on film forming systems (SFFS) have emerged as a novel and versatile approach for delivering drugs through the skin. This abstract presents an overview of SFFS technology, highlighting its potential in transdermal drug delivery. SFFS formulations consist of a solution or suspension of drug molecules and polymers, which upon spraying onto the skin, form a thin, uniform film that adheres to the application site. This film acts as a reservoir for drug release, providing controlled and sustained delivery over time. The versatility of SFFS allows for the incorporation of various drugs, including small molecules, peptides, and proteins, catering to a wide range of therapeutic needs. Moreover, SFFS offers advantages such as ease of application, improved bioavailability, and enhanced skin permeation compared to traditional dosage forms. This abstract summarizes recent advancements in SFFS technology, including formulation strategies, characterization methods, and in vitro/in vivo evaluations. Additionally, challenges such as skin irritation, film integrity, and scalability are discussed, along with potential solutions. Overall, SFFS holds great promise as a transdermal drug delivery system, offering opportunities for enhanced therapeutic outcomes and patient convenience.
Keywords
film forming solution, film forming polymer, TDDS.
Introduction
Transdermal drug delivery systems (TDDS) are self-reliant, distinct dosage forms that, when pragmatic to intact skin, distribute drugs through the skin into the body at a controlled rate [1]. This is a dosage form that involves delivering the drug into the epidermis of the skin and possibly into the dermal tissue of the skin, thus creating a local treatment [2]. Transdermal drug delivery is an beautiful alternative to oral drug delivery and is expected to subcutaneous drugs [3][4][5]. However, it still cannot reached its full potential due to the skin barrier. Skin is an external organ with many layers and its role is to protect our body from environmental hazards such as chemicals, heat and toxins[6][7]. Controlled drug delivery, as well as continuous drug delivery, transdermal drug delivery also allows for short-lived continuous drug delivery and avoids pulses ejected from the body and prevents pulses from entering the body, which often causes adverse side effects. For this reason, many new drugs delivery methods have emerged. Many of the key benefits of transdermal delivery are limiting first-pass metabolism in the liver, improving performance, and maintaining stable plasma drug levels. The first Transderm SCOP was approved by the FDA in 1979 for the prevention of nausea and vomiting caused primarily by accidents on cruise ships. Evidence of transdermal drug absorption can be determined by measuring blood levels of the drug, urinary excretion of the drug and its metabolites, and determining the patient's response to treatment[8]. Transdermal drug delivery devices can be active or passive designs and are devices that provide alternative route for drug delivery. These devices can deliver drugs across the skin barrier [9]. The transdermal method has become one of the most successful and innovative methods of medicine [10]. While spraying equipment is required for the film process, other chemical applications are not required. Using spraying equipment Injection of solution into film is more accurate than semi-solid process[11]. Transderm-Scop, the first transdermal drug delivery (TDD) system, was developed in 1980 and contained the drug scopolamine for pain management. Transdermal devices are one method of use. The membrane in this system is a microporous polypropylene membrane. Pharmacy is a solution in a mixture of mineral oil and polyisobutylene[12].
Advantages of TDDS[13]
- Drug concentration can be decreased due to progressed bioavailability.
- First pass digestion system by liver can be escaped.
- They can anticipate gastrointestinal pharmaceutical assimilation issues cause by stomach pH, enzymatic action, and medicate interaction with nourishment, drink and other orally managed pharmaceuticals.
- Decreases the plasma concentration levels of drugs, with lower side effects.
- They anticipate the bother of parenteral treatment since they are non-invasive.
- They moved forward compliance compared to past dose shapes that required more visit dosage organization since they advertised longer treatment with a single application.
- Drug treatment may be ended quickly by expulsion of the application from the superficial of the skin.
- Self-administration is conceivable with these systems
- It diminishes systemic sedate interactions.
- It offers longer term of activity.
Disadvantage of TDDS:
- Only strong drugs are required for the treatment of skin changes
- Some patients may experience skin irritation at the application site
- This procedure is not commercial
- Binding of the drugs to the skin causes wastage of the drug chronic pain requires the use of medication
- Hypertension, angina, diabetes etc. long- term treatment such as.
- Medication are effective. May be affected by skin metabolism.
- Ionic drugs are not suitable for transdermal treatment.
- It is suitable for drugs with small molecular weight, that is, drugs below 500 daltons are suitable for transdermal application.
Limitations of TDDS:
- Limited skin permeability.
- Limited to strong chemicals.
- Not suitable for large molecules, eg. Over 500 daltons
- Patch not suitable for adhesion to skin.
- Chemicals can damage the skin
- Due to the low solubility, too much solvent cannot be used with this method.
- Not at all suitable if they cause irritation to skin.
Anatomy and physiology of skin
Human skin consists of three different but interconnected tissues:
- Layered, vascular, cellular epidermis,
- Connective tissue hypodermis,
- Hypodermis
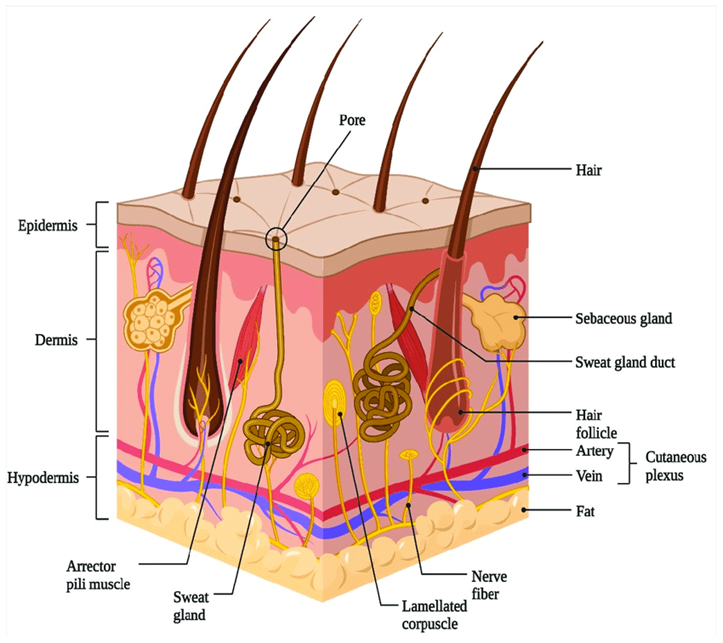
Figure 1: Structure Of The Skin
Epidermis
The thickness of the multilayered epidermal layer on the palms and feet varies between 0.8 mm, depending on the size and number of epidermal cells. Point 0.06 mm to the eyelid. Stratum corneum This is the outer layer of the skin, also called the stratum corneum. It contains 10 to 25 layers of dead keratinocytes called keratinocytes. It is both flexible and impermeable. The stratum corneum is the main barrier to drug penetration.
Dermis
The dermis is 3 to 5 mm thick and contains tissue containing blood vessels, lymphatic vessels, and blood vessels. Blood supply to the skin plays an important role in regulating body temperature. It also provides nutrients and oxygen to the skin while removing toxins and waste products. Capillaries are approximately 0.2 mm away from the skin and provide the necessary conditions for the sinking of most molecules that can cross the skin barrier. Therefore, although the dermal concentration of the permeate provided by the blood is very low, the concentration difference established in the epidermis provides the concentration gradient required for transdermal penetration.
Hypodermis
The hypodermis, or subcutaneous fatty tissue, supports the dermis and epidermis. It works as a fuel storage area. These layers help regulate temperature, provide nutritional support, and provide overall protection. It carries major blood vessels and nerves to the skin and may contain pressure-sensitive cells[14].
Definition and mechanism of film-forming spray
An FFS may be a medicate conveyance framework within the shape of a showered arrangement that will shape a film when it contacts the target restorative location by using the polymer as a network for film arrangement [15][16][17]. After shaping the film, the medicate discharged prepare is comparative to fix, in which the polymer network containing the medicate will discharge it in maintained mold [18].
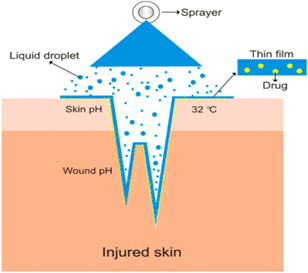
Figure 2: Mechanism Of Film Forming Spray
However, unlike cosmetics and other cosmetic products, film casts cause skin or scar formation because small beads of film material can expose a deep depth of the film (see Figure 2). Of course, ensure the gel reaches the target tissue. In the filmmaking leap, the dose of sedation may also be balanced according to the balance of the challenge to maintain social or local impact. FFS distributed medicine and spread well. Ease of utilize can moreover increment understanding compliance[16][17][19]. Other than the film arrangement, the vanishing of unstable solvents leads to the noteworthy misfortune of volume and subsequently to the fast increment in concentration of dynamic substance within the definition[20]. The concept of supersaturation can be clarified by the altered shape of Fick’s law of dissemination. Fick’s law of diffusion is given by Eq. (1):
J=DKCv/h (1)
Where,
J = rate of sedate penetration/unit range of skin per unit time (flux)
D = dissemination coefficient of drug
Cv = conc. of drug
h = thickness of boundary to diffusion
from this situation, it is obvious that the rate of sedate penetration over the skin is proportionate to the conc. of the sedate. In any case typically genuine when all the sedate is broken down within the vehicle. Equation (2) portrays the altered shape of Fick’s law of diffusion:
J=?D/?h (2)
Where,
? = thermodynamic movement of sedate inside formulation
? = thermodynamic movement of medicate inside film according to this eqn, the flux of the drug is directly proportional to the thermodynamic action of the system, which is related to capacity. However increasing the supersaturation increases thermodynamically unstable[21].
Film-Forming sprayes
- Ordinal spray
Sequential spraying is a type of spray that usages a aluminum or plastic bottle, usually with a 1.2mm hollow tube and a 0.3mm orifice diameter. This type of spraying does not involve any distinct treatment while spraying. The average angle of the spray formed is 78.69 – 87.39°, and generally 0.11-0.35g or ml of film enhancing drug can be sprayed. The average penetration value of spray volume is 0.01-0.03%. antibiotics can be vertical or horizontal. The 3K straight nozzle is designed to keep liquid sterile during use and storage. The category and conc. of polymer used is also related to the spray strength of the compound. 31 preparation of extracts can also be done by spraying[22].
- Metered Dose Spray
Metered spray (MDS) is a spray trick with a variable spray pattern. The device is generally used to deliver medications to the body’s organs a transmucosal or transcutaneous route. As it relates to dosage, volume of spray must be taken into account when evaluating the film production of the spray. The quantity of Metered dose spray that can be sprayed will be affected by the capacity of bottle, how different the product is, and where the container is sited during use. 45 most FFS sprays have a volume between 90 to 102 ml. the average firing angle of the 30, 32 and MDS is 83.51°. the average understanding rate for 20 MDS volumes was 0.01% to 0.02% [23].
C. Electrostatic Spray
A Common method for spread over insecticides in agribusiness is electrostatic splash (ES). Electrostatic spray can progress misfortune from float, bead arrangement rapidity, cover-age consistency and statement productivity. The adequacy of electrostatic spray rest on the thickness, electrical resistivity and surface pressure of the arrangement. The conductivity of the arrangement must be among 108 and 105 s/m in arrange to be showered with electrostatic spray. The beads delivered by electrostatic spray have an normal breadth of 6.3 to 26 m [24].
- Ultrasonic Spray
Great probable exists for film forming courses of action to be passed on by ultrasonic shower. The coming approximately dot has incline properties of film and can method the nano scale. The ultrasonic sprinkle gush can work at both moo and tall weights, making solid globules with > 10 m in separate over. The globule breadth of the ultrasonic shoer is 1-10 m, and the gush is 0.5 mm in separate over. The cathode being utilized incorporates a resonation repeat of 10 mHz layer -by layer (LBL) coating motion pictures can be made utilizing an ultrasonic shower for restorative applications that have more noticeable particle degree consistency. Judgments for each sort of sprayer compare to particular polymers. The FFS system has utilized a grouping of polymers, both common and made [25].
Applications of film forming systems
Originally, the filmmaking frame was widely used in surgery or medicine. Films or gels are used as tissue to cover wounds. The film agents used for this may be original, such as fibrin, or produced, such as cyanoacrylates. These treatment plans can predict wound infection without the use of medications or antibiotics [21] .

Table 1: Film Forming Wound Care Product:[21].
The film shaping wound care items are recorded in table[1]. It can moreover be utilized for non medical employments, such as the conveyance of dynamic fixings contained in excellence items like silicone film shaping innovations utilized to plan corrective creams and treatments[26].
Film forming formulation
Film equipment is the most common product of the film industry. As described so far, the preparation consists mainly of unstable water in which the film-forming polymer is dissolved or broken down, as well as plastic, sedatives and other additives. The dispersion of unstable solutes forms a polymer film on the skin. Schroeder et al. Film preparations were prepared by separating or separating 14 specific film-forming polymers with each plastic (triethyl citrate, enzactin, or dibutyl phthalate) in ethanol, silicone oil, or water. Film products are evaluated for drying time, cure attractiveness, visible adhesion, skin sensitivity and consistency. Some properties of the dry film, such as ductility, stretching at rest, and water vapor permeability, were also tested. The experimental design is sufficient for in-vitro analysis. Although the results cannot be transferred to in-vivo environments, this information may provide the basis for advancements in filmmaking [27] . The topical administration of anti-infective agents is one area where film-forming systems are used. For cleaning agents and antimicrobials to effectively reduce or eliminate microorganisms, their concentration must be sufficiently high and stable. It is especially important in this case to deliver the dynamic substance to the site of activity in the shortest amount of time possible and to keep a safe distance from orderly application in the event of dermal or subordinate tissue disease, as the side impact profile increases with higher concentrations [28]. Conventional formulations need to be linked at regular intervals in order to ensure a constant sedate concentration. Impediment of the dermal framework may be a viable technique for successful treatment and moved forward compliance [29]. The four main parts of sprays and solutions include medicines, dissolvable frame works (i.e., nonvolatile and unstable carriers), polymers, and entry enhancers. When the unstable dissolvable component disappears, the nonvolatile component display in the dissolvable framework prevents the sedate from accelerating in arrangement. The non-volatile component is selected such that it fast distributes across the stratum corneum, helps in the distribution of the sedate there, and increases the diffusivity of the sedate by upsetting the desired intercellular lipids and enhancing saturation. By creating an undetectable station of sedate within the stratum corneum, this type of conveyance system allows the sedate to be progressively absorbed into the systemic circulation. This allows for the improved and supported penetration of medication over the skin following a once-daily application [30][31]. An interesting technique for transdermal measuring shape is the use of film shaping arrangements and splashes. This involves the polymeric structure being applied to the skin as a liquid or misted onto it, where it dissolves to form a nearly clear layer[32]. To ensure complete breakdown of the polymer, the detailed arrangement calls for expanding the polymer to the vehicle and combining the mixture overnight. Upon achieving a distinct polymeric configuration, further optional excipients like plasticizer or cross linker are added. The mixture is combined for twenty-four hours after all excipients have expanded [33] . To ensure the physical stability of the API, the selected polymer must be specialized to prevent nucleation and crystallization inhibitor, which is expected to cause crystallization of sedatives after dissolution of the sedative, free of polyvinylpyrrolidone, polyethylene glycol, hydroxypropyl methylcellulose. Film shaping arrangements can be applied to the skin with an instrument and left to dry. The film-forming shower is designed as a metered-measurement pump container that delivers a settled amount of sedative, which is sprayed onto the affected area to create a film. For transdermal therapy, these frameworks form a consistent, rapidly drying, non-irritating, undetectable film from which the medication can be applied [34].
Components of film forming systems
Drug
Notwithstanding of whether the medication is indicated to be utilized in topical or transdermal application, it must be able to enter well into the stratum corneum. Because the stratum corneum's structure also has a lipophilic quality, medicines with a transcendently lipophilic character typically penetrate the skin more than hydrophilic medications. In an ideal environment, the conveyance coefficient log P is located midway between 1 and 3 [35][36]. Here, the weight of the atoms is important because smaller atoms spread more quickly. Less than 1000 Da should be the atomic weight [35] , Less than 500 Da is optimal [37] . The solvency of the medication is another essential feature that must be considered while making details better. The medicine must dissolve in this if the vehicle of film-forming definitions primarily consists of naturally dissolvable materials. The sedate concentration inside the details increases as a result of the unstable dissolvable dissipating. In order to prevent the sedative from taking shape during the dissipation process, it is equally important that it has sufficient solvency within the non-volatile components of the definition. It appears that a definition containing the broken-down medication should have a pH value between 5 and 10 [35]. Since the skin's pH value is approximately 5, the detailing's pH value should also be in this range to prevent skin irritation during application. The optimal pH value for entry is typically more than 7. A compromise pH range of 5–10 is thought to be appropriate for both[38][39].
Polymers
The optimal of film-forming polymer has the most prominent impact on the substantivity of the definition. Polymers are useful either alone or in combination. They must be able to produce a flexible, streamlined, uncomplicated, and secure movie. Basically, a refinement is made between water soluble and water insoluble film formers [40]. Water-soluble polymers often do not meet descriptions for skin development due to their hydrophilic nature; however, they are ideal for the context in which sedatives penetrate the stratum corneum to produce the drug found there. Some chemicals, such as methylcellulose, can extend the gap between high and low temperature emulsions through thermal gelation. The purpose of film translation is to create a water-filled substance, so hydrophilic polymers themselves are not suitable. Water-insoluble polymers form a water-free film, which has good affinity, but is generally brittle and brittle, making the skin brittle and easy to break, the description picture. To increase the consistency and flexibility of the film, plasticizers are often added to the content or the polymer is mixed with water-soluble polymers [33]. Small polymers with low atomic weight are generally suitable for use in the process of forming thin films. In muscle, resolution is lost and definition intensifies; This is especially true for high atomic weight polymers. In addition, short chains allow small polymers to settle better, making the elimination of cross-chains closer to the ideal for the gel structure. Once the unstable water is eliminated, the film always forms close to the skin, preventing significant changes in film thickness. Dynamic fixation is especially necessary to penetrate the skin due to the concentration gradient of the film and the stratum corneum remaining fixed throughout the skin for a long time. [41].

Table 2: Polymers for use in film-forming formulations[21][42][43]
Plasticizer
A very important part of the film-forming detailing are plasticizers. To give the film the necessary adaptability and to increase the water saturation throughout the picture, they should be very little added to the details [44] . In addition to providing the film with adaptability, several plasticizers also serve as facilitators of sedate distribution. They function by lowering the film's glass transition temperature [45]. Plasticizers can also help maintain the stability of API[46][47].

Table 3: Plasticizers used in the film forming system [45][48]
Solvent
It is possible to achieve the goal of modifying the film's drying rate within the FFS framework by using both volatile and non-volatile solvents. Drugs struggle to escape and find their way into films that harden and dry out quickly. In order to ensure the necessary and sufficient influence on the drying time, film arrangement, and medication release, the solvents are typically used in combination [30] . The dissolvable ought to match the skin tone. In addition, it shouldn't irritate the skin if the skin's edge is injured during the disappearing plan. It is necessary for the film-forming polymer to scatter or disintegrate [21][49]. Less time should be allotted for the film's arrangement. It shouldn't happen too quickly as that could affect the way the film forms[30]. Ethanol and isopropanol are particularly good solvents for subtleties related to film formation. Propylene glycol and isopropyl myristate both have extra penetration-enhancing qualities, but they don't go away[21][49][30]. Two types of solvents are used in the FFS framework: volatile and non-volatile solvents. Equality of the film drying rate is the goal. Drugs have a hard time escaping and entering films that dry out too quickly and create a tough film. In order to promote the film drying process, the dynamic substance is often broken down to immersion
Evaluation of film forming spray
pH
The pH value is measured and adjusted to improve the dynamic fabric's soundness or to make it suitable for the application area. The pH range of diabetic wounds is 6.5–8, while the pH range of skin is 4-6. Burns heal more quickly below pH 7.32. The pH of the preparation is adjusted to account for discomfort and variations in the physiological state of the wound during the healing process. Furthermore, the amount of ionization and the pH value of the dose might alter how well a medication is absorbed through the skin.
Viscosity
Every polymer type and concentration variation will have a different consistency. The spray ability of the film shaping arrangement is determined by its consistency, thus often a vital component to consider. MDS specifically. It is possible to reduce the shower's scope range by increasing the film-forming arrangement's concentration. Harmony The mucosa of the eyes and wounds are examples of body locations where the tonicity of the film-forming arrangement needs to be adjusted. Non-isotonic layouts may cause ocular discomfort and irritate the mucosa. Therefore, in order to determine and adjust the tonicity of the medications, the Kahar method must be applied. The criterion below is used to compute the materials' isotonic concentration.
Cb = ci /1.92[?(cnx?Tfn)] (1)
Where, Cb is the material's isotonic concentration, Ci is the material's beginning concentration, and (Cn x Tfn) is the item of the duplication of the concentration and the esteem of the solidifying point misery of each element [19].
Rheological Properties
Stream testing points to decide whether a chemical is thixotropic or not. If a blend has certain stream qualities, it can successfully flow through the sprayer spout more than once. Because it moves past the spout (focused) and returns to its distinct thickness after being soaked, this streaming property allows the film shaping arrangement to be diminished (stretch is misplaced). A rheometer can be used to determine the kind of flow by showing a logarithmic increment in the shear rate range of 1-900 s?1 and back again from 900–10 s?1. Both ambient temperature and the film-forming arrangement's capacity temperature are used for this test. It is also possible to test the temperature and excipient's influence on the change in gel consistency by testing the stream qualities using the swaying time clear and plentifulness clear technique in a range of excipient concentrations.
Bioadhesive Strength of the Film
A film can be joined to the mouse skin's surface (2 x 5 cm) to estimate the film's bio adhesive quality. At that moment, 0.5 mL of purified water is applied to the skin to hydrate it. For five minutes, the film is allowed to remain attached to the tissue surface. The entire drive (F) to separate the film from the skin's surface is noted. Per film unit zone (A), the bio adhesive quality (Fb) is computed[51].
Fb = F / A (2)
Determination of the water vapor permeability
The amount of water that is conveyed through a unit zone of film in a unit of time is known as the water vapor porousness. The information on water vapor saturation is crucial in determining the film's penetration qualities, as it affects skin parameters such as blood flow, skin temperature, and stratum corneum moisture. On a Teflon plate, movies are applied utilizing a dissolvable dissipation technique and allowed to dry for 72 hours at room temp. The dried film sheets are cut into circular tests. Glass vials with openings are used for the test planning. The vials are filled with refined water, sealed with silicone rings and circular film tests, and tightly fastened with an aluminum vial lid. Next, the vial's weight is determined. put into a desiccator that creates an atmosphere with a relative humidity of either 58% or 0%. They are weighed after predetermined intervals and maintained at a specified temperature for 72 hours. The water vapor penetrability is computed as the total amount of water that saturates through the film in relation to the surface range A (cm2) and the time t (h) based on the weight misfortune of the vials W(g):
WVP ??W A??t [50] (3)
Film formation /drying /evaporation time
To determine how rapidly the film shapes after the arrangement is splattered, the drying time of the film is measured. The arrangement is sprayed on the glass's surface and then left to dry at ambient temperature, according to a few requirements. In alternative theories, the glass plate's surface is coated with a combination of colloidal silica gel and dye to provide retention. Additionally, by applying a film-forming arrangement to the skin, drying time can be precisely monitored. A glass plate is pressed, but not compressed, up against the film to see if the film has dried. The film is considered to have dried if it is not attached to the glass with any water. Since the skin has pores and body heat, this method is more representative of real-world settings than using glass plates in the film drying time test, which could result in a different drying time.
Stickiness
Cotton fleece is used to gently squeeze the dry film. The total number of cotton fleece filaments attached to the film is used to determine the film's thickness. When there is a high degree of joined fiber, the film adhesiveness is seen as tall; when there is little to no joined fiber, it is regarded as medium; and when there is no joined fiber, it is regarded as moo. is small or no connected fiber. Stickiness is tried to discover out whether the film will end up effectively connected to clothing or other objects, so it needs consideration when on the move[51].
Spray angle, pattern and droplet size distribution [51]
It's easy to view the shower designs shape when paper sprayed with pointer reagents is used. The type of dissolvable and the pH of the film-shaping configuration are the determining factors here. Using a material that is sensitive to solvents will make the design and shower bead measure delivery more clear. At that stage, the design's width is measured to determine the splash point and secured range.
Spray angle ? = tan -1 (l/r) (4)
where ? is the remove of the paper surface from the spout and r is the sweep of the circle. The remove of the spout from the paper is for the most part around 15 cm. The higher the shower point, the more troublesome is it for the film-forming arrangement to spread when showered. An outline of the shower design estimation can be seen in Figure 3.

Figure 3: Measurement Of Spray Angle
To degree the secured range, the shower design is checked at 600x600 dpi (Konica Minolta scanner, bizhub c3350). The picture is at that point changed over to a two fold picture utilizing imageJ computer program. At that point, the rate of the secured region is calculated utilizing the taking after equation.
%scope =Zone secured (white pixel) /Region secured (dark pixel) × 100% (5)
The measure of the beads is determined using the ImageJ program's molecular investigation plugin. You can also use Spraytec®, a product of Malvern (Malvern, UK), which operates on the principle of laser diffraction. Units of measurement for bead distance across are mm, from which D10%, D50%, and D90% are determined. Next, using the following equation, the relative span calculate (RSF) is computed to determine the consistency of the bead estimate conveyance.
RSF = D90% - D10 % / D50% (6)
Swab studied
Swab testing can be used to measure the normal duration of a video production frame. Glass was used as the polar hydrophilic substrate for the retention test. Glass was chosen as the test site because both materials have a polar surface structure and a film that makes the glass appear to adhere strongly to the skin. [52].
Dry swab test:
This test demonstrates how FFS behaves when the skin is dry. A glass plate can be used to conduct a dry swab test. Six 1 × 1 cm stamps are placed on the glass plate. In this zone, created definition is linked. After extracting the medication from the swab, the linked film is swabbed at min, 30 min, 2 h, 4 h, 6 h, and 8 h, and the presence of medication is evaluated. Test for damp swabs: This test simulates the movements of the FFS when it comes into contact with perspiration or water. The damp swab test and dry swab test work in the same way; however, the damp swab is used to swab the definitions after it has been freshly soaked in water.
In vitro drug release study
Franz dissemination cells or USP disintegration device sort II (paddle Strategy), are generally utilized in this test as compartment separators, beside cellulose layers (pore estimate 0.44 ?m), nylon layers (molecule measure 0.21 mm), or silicone films. The medium is phosphate buffer with a pH of 6.8. The film-making arrangement is poured into the giver compartment once the compartment framework has been assembled. The hardware being used to measure the arrangement is then properly balanced once a particular amount of the arrangement is taken from the cells at specific intermissions of time. The very similar volume of liquid is substituted for the tests after they are completed. Following that, a UV unmistakable spectrometer was used to examine the drawn tests [23].
Drug content per each spray actuation
Measure the volume or amount of solution with each spray to determine the drug content and use the concentration of the drug film to measure the stability of the drug [53]
V= Wt – Wo / D (7)
Where,
V = volume of spray produced by each threshold,
Wt = represents the weight of the solution after spraying
Wo = weight of the solution before spraying,
D = density of the solution
CONCLUSION AND FUTURE PROSPECTS
Film-forming systems provide a new method for both topical and transdermal delivery of skin medications. The basis and essence of this film process is convenience, oil-free, less skin irritation, tensile resistance, longer retention, the important thing is to predict how it works, revealing the true distinction of best performance and beautiful beauty. Significant work has been done on these systems, but not much information has been published about their transportation. So some high-level stuff is obvious. Further research will be important to demonstrate the importance of the underlying membrane process of image prediction, but their results will almost certainly lead to new meaning for Thrive. According to previous studies, it proved to be suitable for use in sports forming due to its high strength and good control of the film forming system. The drug penetrates from the body into the skin, allowing stable dermal and transdermal application. This content also serves as promotional material. Although the application of film-forming nozzles in therapy is now mature and widely accepted by patients, film technology is still unique in the treatment of diseases. For transdermal application, there is the testosterone inhibitor Axiron, which is now widely available on the market. In transdermal applications, the film process has not yet reached the stage where it can prepare itself to support other transdermal systems such as patches. Since it is an action movie for the treatment of local infections such as Terbinafine Lamisil Once® Spray for dermal application; Although treatment of persistent dermatoses may be considered, there are no recommendations for patients with this condition. One or another routine treatment or a non-steroidal anti-inflammatory drug is not prescribed within the scope of the action of cortisone as a strong treatment. With regularly occurring changes in dermatoses and local dissemination of film-forming substances, the drug can seek internal aggression. Especially when the film process is sprayed on the skin, it creates a small skin feeling, unlike the patch system. Patches, on the other hand - when used without patches, application is accurate and much easier, allowing the skin to be treated for a long time and offer physical benefits. Film-forming lotions also have a lipophilic texture and can be used on dry skin. The advantage of the gel is that it requires less effort and is more accurate to apply due to its partial structure. All machines are suitable for treatment. The composition of semi-solids and liquids can be displayed in separate concentrations and only film can be printed. In addition, little progress has been made in converting film formation into hydrophilic substances. Changes to the finer details of the site will help restore the area. By adjusting the concentration of all ingredients and using unused ingredients, the skin performance follows, and the focus can be close to the perfect state of skin softness. In this case, gels and lotions may be more important because they are less likely to be used than the film-based sprays that have been widely used to date. Another area of future research is the development of lipophilic film-forming systems with oil-soluble film-forming managers. This product works well in treating dark red heat on the skin and can delay the release of energy and produce beneficial effects on the skin by releasing high concentrations. This will allow for daily treatment with medications such as cortisone along with significant treatments or therapies.
REFERENCES
- S. B. Shirsand, G. M. Ladhane, S. J. Prathap, and P. Prakash, “Design and evaluation of matrix transdermal patches of meloxicam,” RGUHS J. Pharm. Sci., vol. 2, 2012, [Online]. Available: https://api.semanticscholar.org/CorpusID:137209801
- Himanshi Tanwar * and Ruchika Sachdeva Guru, “TRANSDERMAL DRUG DELIVERY SYSTEM - A REVIEW HTML Full Text TRANSDERMAL,” Guru Jambheshwar Univ. Sci. Technol. Hisar, Haryana, India, vol. 0, no. August, pp. 1–5, 2020.
- M. R. Prausnitz and R. Langer, “Nihms121685,” Nat Biotechnol., vol. 26, no. 11, pp. 1261–1268, 2009, doi: 10.1038/nbt.1504.Transdermal.
- A. C. Williams, “Book Review,” Pharm. Educ., vol. 4, no. 1, pp. 49–50, 2004, doi: 10.1080/15602210410001682551.
- M. R. Prausnitz, S. Mitragotri, and R. Langer, “Current status and future potential of transdermal drug delivery,” Nat. Rev. Drug Discov., vol. 3, no. 2, pp. 115–124, 2004, doi: 10.1038/nrd1304.
- H. J. Lee and M. Kim, “Skin Barrier Function and the Microbiome,” Int. J. Mol. Sci., vol. 23, no. 21, 2022, doi: 10.3390/ijms232113071.
- M. Wang et al., “Artificial Skin Perception.,” Adv. Mater., vol. 33, no. 19, p. e2003014, May 2021, doi: 10.1002/adma.202003014.
- M. AN., “Controlled and novel drug delivery.?;;,” , CBS Publ. Distrib., vol. 4th ed., pp. 107-109., 1997.
- H. C. Ansel, L. V Allen, and N. G. Popovich, Pharmaceutical dosage forms and drug delivery systems, 7th ed. Philadelphia, Pa. SE - x, 595 pages?: illustrations?; 26 cm: Lippincott-Williams & Wilkins Philadelphia, Pa., 1999. doi: LK - https://worldcat.org/title/40881470.
- Ramteke KH, Dhole SN, and Patil SV, “Journal of Advanced Scientific Research TRANSDERMAL DRUG DELIVERY SYSTEM: A REVIEW,” J. Adv. Sci. Res., vol. 2012, no. 1, pp. 22–35, 2012, [Online]. Available: http://www.sciensage.info/jasr
- B. J. Thomas and B. C. Finnin, “The transdermal revolution.,” Drug Discov. Today, vol. 9, no. 16, pp. 697–703, Aug. 2004, doi: 10.1016/S1359-6446(04)03180-0.
- D. Prabhakar, J. Sreekanth, and K. N. Jayaveera, “Transdermal Drug Delivery Patches: a Review,” J. Drug Deliv. Ther., vol. 3, no. 4, 2013, doi: 10.22270/jddt.v3i4.590.
- R. S. Kumar, D. A. Devi, N. G. Raj, and M. Deepa, “A Review on Transdermal Drug Delivery Patches,” J. Pharm. Res. Int., vol. 22, no. 02, pp. 39–47, 2022, doi: 10.9734/jpri/2022/v34i31a36085.
- S. N. Deshmukh et al., “Novel Film Forming Spray from Tea Tree Leaves with Special Emphasis on Development, Formulation and Evaluation,” J. Posit. Sch. Psychol., vol. 2022, no. 5, pp. 5179–5184, 2022, [Online]. Available: http://journalppw.com
- P. Mohite, H. Patel, M. Patel, C. Shah, and U. Upadhyay, “Film Forming Spray: A Comprehensive Review I. DEFINITION AND MECHANISM OF A FILM-FORMING SPRAY [1-4],” Int. J. Innov. Sci. Res. Technol., vol. 7, no. 12, pp. 1163–1171, 2022, [Online]. Available: www.ijisrt.com
- A. Lisitsyn et al., “Approaches in Animal Proteins and Natural Polysaccharides Application for Food Packaging?: Edible Film Production and Quality Estimation,” pp. 1–59, 2021.
- A. K. Umar, M. Butarbutar, S. Sriwidodo, and N. Wathoni, “Film-forming sprays for topical drug delivery,” Drug Des. Devel. Ther., vol. 14, no. July, pp. 2909–2925, 2020, doi: 10.2147/DDDT.S256666.
- A. Soni, J. S. Dua, and D. N. Prasad, “Article Reviewing Transdermal Drug Delivery System,” J. Drug Deliv. Ther., vol. 12, no. 1, pp. 176–180, 2022, doi: 10.22270/jddt.v12i1.5159
- S. K. Tilekar, S. F. Sayyad, and B. K. Phopse, “a Comprehensive Review on Film Forming Topical Spray Drug Delivery,” World J. Pharm. Pharm. Sci., vol. 11, no. 10, pp. 496–527, 2022, doi: 10.20959/wjpps202210-23311.
- K. Frederiksen, R. H. Guy, and K. Petersson, “The potential of polymeric film-forming systems as sustained delivery platforms for topical drugs,” Expert Opin. Drug Deliv., vol. 13, no. 3, pp. 349–360, 2016, doi: 10.1517/17425247.2016.1124412.
- K. Kathe and H. Kathpalia, “Film forming systems for topical and transdermal drug delivery,” Asian J. Pharm. Sci., vol. 12, no. 6, pp. 487–497, 2017, doi: 10.1016/j.ajps.2017.07.004.
- M. C. Gohel and S. A. Nagori, “Fabrication of modified transport fluconazole transdermal spray containing ethyl cellulose and Eudragit RS100 as film formers.,” AAPS PharmSciTech, vol. 10, no. 2, pp. 684–691, 2009, doi: 10.1208/s12249-009-9256-8
- T. Garg, G. Rath, and A. K. Goyal, “Comprehensive review on additives of topical dosage forms for drug delivery.,” Drug Deliv., vol. 22, no. 8, pp. 969–987, Dec. 2015, doi: 10.3109/10717544.2013.879355.
- R.-K. Chang, A. Raw, R. Lionberger, and L. Yu, “Generic development of topical dermatologic products: formulation development, process development, and testing of topical dermatologic products.,” AAPS J., vol. 15, no. 1, pp. 41–52, Jan. 2013, doi: 10.1208/s12248-012-9411-0.
- S. A. Ibrahim, “Spray-on transdermal drug delivery systems.,” Expert Opin. Drug Deliv., vol. 12, no. 2, pp. 195–205, Feb. 2015, doi: 10.1517/17425247.2015.961419.
- M. Freccero, “Me ,, ~ ,,,’: Me \ i Me ’ M . e,” vol. 54, pp. 12323–12336, 1998.
- T. T. D. Tran and P. H. L. Tran, “Controlled release film forming systems in drug delivery,” Pharmaceutics, vol. 11, no. 6, pp. 1–16, 2019.
- Z. Mohammadi and P. V. Abbott, “On the local applications of antibiotics and antibiotic-based agents in endodontics and dental traumatology,” Int. Endod. J., vol. 42, no. 7, pp. 555–567, 2009, doi: 10.1111/j.1365-2591.2009.01564.x.
- P. Gao, X. Nie, M. Zou, Y. Shi, and G. Cheng, “Recent advances in materials for extended-release antibiotic delivery system,” J. Antibiot. (Tokyo)., vol. 64, no. 9, pp. 625–634, 2011, doi: 10.1038/ja.2011.58.
- C. G. M. Gennari, F. Selmin, P. Minghetti, and F. Cilurzo, “Medicated Foams and Film Forming Dosage Forms as Tools to Improve the Thermodynamic Activity of Drugs to be Administered Through the Skin,” Curr. Drug Deliv., vol. 16, no. 5, pp. 461–471, 2019, doi: 10.2174/1567201816666190118124439
- U. Kumar Mandal, B. Chatterjee, F. Husna, and B. Pauzi, “A Review on Transdermal Spray: Formulation Aspect,” A Rev. Transdermal Spray Formul. Asp. M J Pharma, vol. 1, no. 1, p. 6, 2016, [Online]. Available: www.mathewsopenaccess.com
- I. Zurdo Schroeder, P. Franke, U. F. Schaefer, and C.-M. Lehr, “Development and characterization of film forming polymeric solutions for skin drug delivery.,” Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft fur Pharm. Verfahrenstechnik e.V, vol. 65, no. 1, pp. 111–121, Jan. 2007, doi: 10.1016/j.ejpb.2006.07.015.
- F. F. D. de Oliveira, L. R. de Menezes, and M. I. B. Tavares, “Film-Forming Systems in Topically Administered Pharmaceutical Formulations,” Mater. Sci. Appl., vol. 11, no. 08, pp. 576–590, 2020, doi: 10.4236/msa.2020.118038.
- D. Rashmi, “Journal of drug delivery and therapeutics (jddt),” J. Drug Deliv. Ther., vol. 8, no. 6, pp. 124–8, 2018, [Online]. Available: http://dx.doi.org/10.22270/jddt.v9i3.2678
- I. Alberti, A. Grenier, H. Kraus, and D. N. Carrara, “Pharmaceutical development and clinical effectiveness of a novel gel technology for transdermal drug delivery.,” Expert Opin. Drug Deliv., vol. 2, no. 5, pp. 935–950, Sep. 2005, doi: 10.1517/17425247.2.5.935.
- Y. N. Kalia, V. Merino, and R. H. Guy, “Transdermal drug delivery. Clinical aspects.,” Dermatol. Clin., vol. 16, no. 2, pp. 289–299, Apr. 1998, doi: 10.1016/s0733-8635(05)70011-5.
- J. Hadgraft, G. Cordes, and M. Wolff, “Prediction of the Transdermal Delivery of ß-blockers,” Die Haut als Transp. für Arzneistoffe, pp. 133–143, 1990, doi: 10.1007/978-3-642-72452-7_15.
- J. L. Matousek, K. L. Campbell, I. Kakoma, P. F. Solter, and D. J. Schaeffer, “Evaluation of the effect of pH on in vitro growth of Malassezia pachydermatis.,” Can. J. Vet. Res. = Rev. Can. Rech. Vet., vol. 67, no. 1, pp. 56–59, Jan. 2003.
- K. Bucher, K. E. Bucher, and D. Walz, “Irritant actions of unphysiological pH values. A controlled procedure to test for topical irritancy.,” Agents Actions, vol. 9, no. 1, pp. 124–132, Apr. 1979, doi: 10.1007/BF02024143.
- S. Karki, H. Kim, S. J. Na, D. Shin, K. Jo, and J. Lee, “Thin films as an emerging platform for drug delivery,” Asian J. Pharm. Sci., vol. 11, no. 5, pp. 559–574, 2016, doi: 10.1016/j.ajps.2016.05.004.
- L. A. Felton, “Mechanisms of polymeric film formation.,” Int. J. Pharm., vol. 457, no. 2, pp. 423–427, Dec. 2013, doi: 10.1016/j.ijpharm.2012.12.027.
- S. Nandi and S. Mondal, “Fabrication and Evaluation of Matrix Type Novel Transdermal Patch Loaded with Tramadol Hydrochloride,” Turkish J. Pharm. Sci., vol. 19, no. 5, pp. 572–582, 2022, doi: 10.4274/tjps.galenos.2021.43678.
- V. M. Platon, B. Dragoi, and L. Marin, “Erythromycin Formulations—A Journey to Advanced Drug Delivery,” Pharmaceutics, vol. 14, no. 10, 2022, doi: 10.3390/pharmaceutics14102180
- S. Gngr, M. Sedef, and Y. zsoy, “Plasticizers in Transdermal Drug Delivery Systems,” Recent Adv. Plast., 2012, doi: 10.5772/38156.
- J. Grip, R. Einar, I. Skjæveland, N. Škalko-basnet, A. Mari, and A. M. Holsæter, “Ingredient for Wound Healing – Development and in-Vivo Evaluation,” Eur. J. Pharm. Sci., vol. 107, pp. 34–31, 2017.
- Y. Zhong, C. Zhuang, W. Gu, and Y. Zhao, “Effect of molecular weight on the properties of chitosan films prepared using electrostatic spraying technique.,” Carbohydr. Polym., vol. 212, pp. 197–205, May 2019, doi: 10.1016/j.carbpol.2019.02.048.
- R. N. Mirajkar, A. R. Madgulkar, A. G. Harne, and S. L. Dhage, “In Situ Film Forming Systems,” Int. J. Pharm. Pharm. Res. , vol. 24, no. 3, pp. 389–405, 2022, [Online]. Available: www.ijppr.humanjournals.com
- S. S. Bornare, S. S. Aher, and R. B. Saudagar, “A REVIEW?: FILM FORMING GEL NOVEL DRUG DELIVERY SYSTEM,” vol. 10, no. 2, 2018.
- S. Ranade, A. Bajaj, V. Londhe, N. Babul, and D. Kao, “Fabrication of topical metered dose film forming sprays for pain management.,” Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci., vol. 100, pp. 132–141, Mar. 2017, doi: 10.1016/j.ejps.2017.01.004.
- N. A. Rajab, “Preparation and Evaluation of ketoprofen as Dermal Spray Film,” Kerbala J. Pharm. Sci., vol. 6, no. 6, pp. 1–8, 2013.
- A. K. Umar, M. Butarbutar, S. Sriwidodo, and N. Wathoni, “Film-Forming Sprays for Topical Drug Delivery.,” Drug Des. Devel. Ther., vol. 14, pp. 2909–2925, 2020, doi: 10.2147/DDDT.S256666.
- A. L. Morais Ruela, A. Gravinez Perissinato, M. De Sousa Lino, P. Silva Mudrik, and G. Ribeiro Pereiro, “Evaluation of skin absorption of drugs from topical and transdermal formulations,” Brazilian J. Pharm. Sci., vol. 52, no. 3, pp. 527–544, 2016.
- Reem Wael Shahadha and Nidhal Khazaal Maraie, “Mucoadhesive Film Forming Spray for Buccal Drug Delivery: A Review,” Al Mustansiriyah J. Pharm. Sci., vol. 23, no. 1, pp. 105–116, 2023, doi: 10.32947/ajps.v23i1.994.


 Maya Y. Gaikwad 2
Maya Y. Gaikwad 2






 10.5281/zenodo.10931631
10.5281/zenodo.10931631