The eye is an important organ as our vision totally depends on it. Ocular diseases are a major public health challenge as it potentially impacts the quality of life and leads to vision impairment, if not properly treated causing blindness. As per the WHO Report 2019, approximately 2.2 billion people have visual impairment; this number can be reduced if treated properly. According to a survey conducted in 39 nations, 285 million people are visually impaired. Among them, 65% are older than 50, and 82% of the blind patients are older than 50 [1]. The complexity of the eye’s anatomical structure and physiological barriers makes the treatment of various ocular disorders difficult. The delivery of therapeutic products into the anterior segment of the eye is difficult, due to a number of obstacles such as anatomy and physiology. In short, the anatomical barriers are divided into two types: static and dynamic. There are two types of static barriers: corneal epithelial and blood stromal barrier; whereas dynamic barriers consist of tears drainage, conjunctival blood and lymphoid flow. On the other hand, physiological barriers include blinking activities, tear film turnover and nasolacrimal drainage [2]. More than 70% of cases of blindness are due to anterior segment eye diseases (ASED), which include uncorrected refractive errors, cataracts, glaucoma, corneal opacity, and trachoma. ASED are among the seven most common conditions that impair vision [3]. Topical or periocular administration is used to treat anterior segment diseases such as blepharitis, conjunctivitis, scleritis, keratitis and dry eye syndrome. Delivering drugs to both the posterior and anterior segments of the eye, whether for conditions like glaucoma, endophthalmitis, or uveitis, presents a common challenge: achieving adequate bioavailability due to barriers in drug delivery. Nonetheless, despite the risk for complications, i.e. administration of an lntraocular solution could be preferred [4]. With the advancement in the field of nanoscience, a range of nanomaterials have been developed for ocular drug delivery systems. These nanomaterials possess promising characteristics and novel properties which makes them apt for the formulation of ocular drug delivery systems. Biopharmaceuticals are being exploited more and more by new nanotechnology and nanoscience techniques. Nanoscience is an interdisciplinary field that combines material science, physics, chemistry, and biology, whereas nanotechnology involves the design and fabrication of different materials in nanometer scale at least in one dimension [1]. Many innovative drug delivery systems have been designed with a view to improving bioavailability of the eye. These devices may also be able to penetrate the ocular barrier. Examples include mic, nanospheres, liposomes, dendrimers, water-soluble gels, nanometer emulsions and fluid suspensions,in situ gel etc [5].This comprehensive scientific review provides an extensive analysis of various ocular diseases, nanotechnology-driven drug delivery methodologies, and an array of associated patents, existing hurdles, and prospective future applications. Furthermore, the review delves into the examination of different polymers utilized in the development of ocular drug delivery systems.
- Anatomy Of Eye:
The eye, from an anatomical point of view, can broadly be regarded as a series of overlapping layers of tissue. Eye's external structures consist of eyelashes, eyelids, muscles, attachment glands and conjunctiva. Three layers of tissue are laid out in concentric circles within the internal structure of the eye: The outer layers are made up of the sclera and cornea. The uvea is composed of a vascular layers in the middle, separated by irises,ciliary bones and choroids. The inner layer is the retina, which consists of nerve tissue. When scrutinizing an individual's ocular structures, one can readily discern several prominent components. Firstly, there is the visually conspicuous, darkened aperture known as the pupil. The pupil serves the vital function of facilitating the entry of light into the eye, its apparent darkness stemming from the absorption properties of retinal pigments. Adjacent to this pivotal element is the iris, a circular muscular structure adorned with intricate pigmentation patterns, thereby defining an individual's eye color. Notably, the central opening within the iris corresponds to the pupil and plays a pivotal role in modulating the amount of incoming light, adapting to prevailing environmental conditions. The captivating diversity of eye colors, more accurately referred to as iris colors, emerges from the varying levels of eumelanin (responsible for brown and black pigments) and pheomelanin (responsible for red and yellow pigments) synthesized by melanocytes. [6-8].
- Ocular Barriers:
In the light of this, a brief review is given of the introduction into the Ophthalmological literature of the concept of blood ocular barriers. Two fundamental blood-ocular barriers are posited: the blood-aqueous barrier and the blood-retinal barrier.
-
- Blood Aqueous Barrier:
Blood-aqueous barriers are made up of the non-porous epithelium on the ciliary side of the body, the epithelium behind the iris (the posterior iris), the leaky endothelium on the iris (iris vessels with leaky junctions), and the schlemm's canal endothelium [9].
-
- Blood Retinal Barrier:
Basically, the BRB is super tight and constricted, and it's a physical barrier that controls how ions, proteins, and water get in and out of your retina. It's made up of both the inner and outer BRB, with the inner one made up of tiny junctions between the cells that make up your retina, called retinal capillaries, and the outer one being made up of tiny connections between the cells made up of your retina's pigment epithelium. It's really important for keeping your eye like a special place, and it's also really important for your vision [10,11].
- Ocular Disorders
- Glaucoma:
The term "glaucoma" originates from the Greek word for "green" or "light gray". This group of disorders is characterized by their distinct pathophysiological and risk factors, as well as their various manifestations, treatment options, and prognosis. All of these disorders share one common characteristic: progressive degeneration in the optic nerve. This degeneration is characterized by the loss of visual neurons, the thinning of retinal nerve fibers, and the progressive erasure of the optic discs [12]. Glaucoma is a progressive disease of the optic neuropathies, in which retinal ganglionic cells are degenerated and the optic nerve head undergoes alterations. The loss of ganglionic cells is associated with an increase in intraocular pressure, although other factors may also contribute to the disease. The only effective treatment for glaucoma is to reduce intraocular pressure. Treatment is typically initiated with ocular hypoporosis drops, but other methods may be used to slow the progression of the disease, such as laser therapy and surgery [13].
-
- Dry eye disease (DED):
Dry Eye Disease is a multifaceted condition of the tear system and the eye's surface that is characterized by symptoms of pain, visual disturbances, and instability of the tear system, with the potential for damage to the eye's surface. Additionally, it is characterized by an increase in the amount of osmolality in the tear system, as well as a decrease in ocular surface inflammation [14].Tear dysfunction happens when the LFU (Lacrimal Functional Unit) made up of the tear secreting glands (lacrimal gedgets, conjunctive goblet cockets, meibomian gedgets) and their nervous and immunological systems, is no longer capable of maintaining a stable preneuronal tear layer [15]. There are a number of risk factors associated with dry eye disease, especially in the elderly, women who have gone through the menopause and those who suffer from autoimmune diseases. According to the NEI classification, dry eye disease is divided into two categories: aqueous-depleting and evaporative. Other risk factors associated with DED include: High altitude, Pterygium, Smoking, and Excessive consumption of multi-vitamins and caffeine [16].
-
- Keratitis:
Keratitis consists of inflammation in the cornea, which is characterised by corneal edema, inflammatory cell infiltration and ciliary congestion. This is accompanied by infectious and noninfectious diseases that may be systemic or localised to the ocular surface. The majority of keratitis is caused by "microbial keratitis", which has been the primary cause of concern in developing countries [17].
-
-
- Infectious Keratitis:
Infectious Keratitis is a type of corneal infection that is also referred to as Infectious Cornea Ulcer or Infectious Cornea Opacity. It can be divided into microbial and viral categories. Microbial keratitis refers to infections caused by bacteria, fungi, or parasites. Viral keratitis, on the other hand, is caused by herpes viruses [18].
-
-
- Non infectious Keratitis:
Trichiasis, giant papillae, and a foreign body in the sulcus subtarsalis are examples of local causes ulcerative keratitis of the periphery, rheumatoid arthritis, granulomatosis with polyangiitis, polyarteritis nodosa, relapsing polychondritis, systemic lupus erythematosus, and others are collagen vascular diseases. Trigeminal nerve damage as a result of surgery or a tumor may cause neurotrophic corneal ulcers (post-herpetic zoster ophthalmicus).
-
- Conjunctvitis:
Inflammation and swelling of the conjunctival tissue, engorgement of the blood vessels, ocular discharge, and pain are all symptoms of conjunctivitis. Conjunctivitis affects a large number of people globally and is one of the most common causes of office visits to general medical and ophthalmology clinics. Acute conjunctivitis is reported to be diagnosed by non-ophthalmologists such as internists, family practitioners, pediatricians, and nurse practitioners in more than 80% of cases [19]. Infectious and noninfectious causes of conjunctivitis can be distinguished. Bacteria and viruses are the most typical infectious causes. Noninfectious conjunctivitis includes inflammation brought on by immune-mediated illnesses and neoplastic processes, as well as allergic, toxic, and cicatricial conjunctivitis [20].
-
- Cataract:
A cataract is an eye condition where the normally clear lens has become opaque, obstructing the passage of light. It is a slowly progressing illness that accounts for a sizable portion of global blindness. Infants, adults, and seniors can all develop this blinding disease, but older people are disproportionately affected. The severity can vary and it can be bilateral. If the cataract has advanced to the point where it is interfering with daily activities, surgery may be recommended, which is very effective. Treatment options include correction with refractive glasses only at earlier stages [21]. Finding the risk factors that cause cataract development could lead to the development of preventative measures. Only a small number of risk factors meet the requirements for a causal relationship, including smoking, which increases the risk of nuclear cataract, excessive UV-B exposure and diabetes, which raises the risk of cortical cataract, and steroidal therapy, diabetes, and ionizing radiation, which causes posterior subcapsular opacity [22].
- Nanotechnology-Based Ocular Drug Delivery Systems
- Nanoparticles:
Nanoparticles (NPs) are a diverse class of materials that include substances that are particulate and have at least one dimension that is less than 100 nm. These materials can be 0D, 1D, 2D, or 3D depending on the overall shape. Based on their characteristics, shapes, or sizes, they can be divided into various classes. Fullerenes, metal NPs, ceramic NPs, and polymeric NPs are some of the various groups. Nanoparticles (NPs) exhibit unique physical and chemical properties owing to their tiny size and extensive surface area. In contrast to conventional eye drops, nanoparticles (NPs) have been developed to overcome obstacles, boost drug penetration at the target region, and prolong drug levels by a few internals of medication administrations in lower doses.Through intravitreal injection and surface applications, NPs could target the cornea, retina, and choroid. The ocular system's obstacles were more easily overcome by the use of nanoparticles (NPs) with sizes ranging from 10 nm to 1000 nm [23]. Direct administration via either of these two routes has a number of issues with drug bioavailability, such as adverse effects and the need for numerous unpleasant treatments to reach therapeutic drug levels. Improved topical transit of big, inefficiently water-soluble compounds, like glucocorticoids or cyclosporine for immune-related, vision-threatening disorders, is one benefit of utilizing nanoparticles in this context [24]. The two main types of NPs used for drug delivery are organic and inorganic NPs. Polymer NPs, nanomicells, liposomes, quantum dots, nanoemulsions, and hybridized NPs are examples of organic NPs, while silica NPs, gold NPs, and carbon nanotubes are examples of inorganic NPsAdditionally, optical coherence tomography (OCT) can use NPs' strong stability and high light-scattering ability to enhance the early detection and diagnosis of eye diseases [25].
-
- Niosomes:
Niosomes are amphiphilic, nonionic, bilayered, biodegradable, and non-immunogenic vesicles that are nanoscale in size [26].Drugs that are both hydrophilic and lipophilic can be contained by niosomes, which are bilayered, non-ionic surfactant vesicles. Chemically, niosomes are stable, and because they are non-ionic, their toxicity is low. They are chosen for ocular use over other vesicular formulations because of their many benefits [27]. Because of their high stability and permeability, hydrophobic and hydrophilic drugs have been delivered using liposomes, spherical vesicles made from biocompatible lipids that resemble cell membranes [25].
-
- Nanowafers:
A tiny, transparent disc called a nanowafer can be applied to the surface of the eye with the tip of a finger and can withstand repeated blinking without moving. It has a variety of drug-filled nanoreservoir arrays from which the drug will be released under strict control for a few hours to days. Due to the nanowafer's slow drug release, the drug spends more time on the ocular surface before being absorbed into the surrounding ocular tissue. The nanowafer will dissolve and disappear at the conclusion of the predetermined time for drug release [28]. Dexamethasone-loaded nanowafers (Dex-NW) were created to increase convenience and effectiveness for dry eye patients. The Dex-NW nanowafers, which feature 500 nm square reservoirs filled with dexamethasone, were made using carboxymethyl cellulose [26].
-
- Nanosuspension:
Nanosuspensions are colloidal dispersions of drug particles that are nanoscale in size and are stabilized by surfactants. Poorly water-soluble drugs without any matrix material are suspended in dispersion as nanosuspensions [29].
Recently, a high pressure homogenization process has made it possible to mill drug micro-particle suspensions. The increase in saturation solubility and subsequent increase in the compound's rate of dissolution are two outstanding characteristics of the nanosuspension [30]. By keeping the active pharmaceutical ingredients (API) in a crystalline state and enabling them with increased drug loading during formulation development, nanosuspensions can resolve such specific drug delivery problems related to them. Due to the reduced use of harmful non-aqueous solvents and extreme pH, accommodating large drug amounts with minimal dose volume has additional benefits in parenteral and ophthalmic drug delivery systems. Additional benefits include improved stability, prolonged drug release, increased effectiveness via tissue targeting, reduced first pass metabolism, and deep lung deposition [31].
-
- Nanoemulsion:
Nanoemulsions are transparent, kinetically stable formulations with inner-phase droplets that are typically between 20 and 200 nm in size (some authors raise this upper limit to 500 nm). Ophthalmic o/w nanoemulsions are made up of two immiscible phases of the nanoemulsion—an immiscible phase of oil and an immiscible phase of water—as well as a carefully chosen mixture of surfactants and cosurfactants that allows for the reduction of surface tension at the interphase [32]. Due to their ability to reduce interfacial tension and produce small particle sizes as a result of their role in the formation of stable preparations as a result of the repellent electrostatic interaction and steric hindrance, the surfactant and cosurfactant molecules play an effective role in the formation of nanoemulsions [33]. Due to their capacity to increase drug bioavailability, NEs are extensively researched as a cost-effective formulation and non-invasive method. Ophthalmic NEs also have the following benefits: Compared to gels or ointments, the drug has (i) a longer pre-corneal retention time, (ii) high penetration ability, (iii) improved ocular bioavailability, (iv) improved drop drainage through the cornea, and reproducible amounts in the eye,(v) ocular formulations are retained in the conjunctival sac for a longer period of time due to the interface of lipid present in NEs to the lipid layer of tear film, (vi) By electrostatically interacting with the anionic surface of the corneal mucin when using cationic NEs, it is possible to extend the drug's residence time and, as a result, increase the bioavailability of the drug in the eye. The interaction of the mucin's surface with the cationic NEs lengthens their time in the pre-corneal site [34].
-
- Nanomicelles:
Nanomicelles are self-assembling colloidal dispersions with a hydrophobic core and a hydrophilic shell, typically with particle sizes between 10 and 100 nm. Nanomicelles exhibit some distinctive or novel characteristics due to their size, solubility, customized surface, or exposure to the environment. This multifunctionality makes nanomicelles essential for use in biomedical applications as well as numerous other fields. The process of targeted drug delivery uses nanomicelles, allowing for deeper tissue penetration and greater drug bioavailability [35]. Reverse micelles are amphiphilic copolymer self-assemblies in a non-aqueous medium, whereas regular micelles are amphiphilic copolymer self-assemblies in an aqueous medium. Block copolymers, such as core (laur)-polyethylene glycol (core [laur]PEG), are used to create monomolecular micelles. One molecule of these polymers contains a number of hydrophilic and hydrophobic regions, allowing it to self-assemble into a micelle [36]. In contrast to reverse nanomicelles, which are used to encapsulate and deliver hydrophilic drugs, positive micelles are used to encapsulate, solubilize, and deliver hydrophobic drugs. Nanomicelles are thought to be safe substitutes for intraocular drug delivery because of their distinctive chemical structure, which can solubilize drugs internally, reduce side effects, improve drug stability, and have a sustained release effect [37].
-
- Dendrimers:
Dendrimers are nanostructured polymers with a "tree-like" structure that have potential for ocular drug delivery. Due to their range of nanosizes, capacity to display multiple surface groups that allow for targeting, ease of preparation, and functionalization, they are desirable systems for drug delivery. Ongoing research into creating better ocular dendrimeric systems may not only improve drug delivery to the ocular surface but may also enable noninvasive delivery of therapeutic agents to intraocular tissues like the retina or choroid [38]. Dendrimers are promising new scaffolds for drug delivery because of their special qualities, which include their high degree of branching, multivalency, globular architecture, and well-defined molecular weight. The design and synthesis of biocompatible dendrimers, as well as their use in the development of vaccines, antimicrobials, and antivirals, as well as drug delivery, have all been the subject of increased research over the past ten years [39]. Both divergent and convergent methods can be used to prepare dendrimers. These two construction ideas are fundamentally different from one another. Dendrimer expands from a multipurpose core molecule using the divergent techniques. The core molecule interacts with monomer molecules that have one reactive group and two dormant groups to produce the first generation dendrimer. The divergent synthesis's flaws led to the development of the convergent methods. The dendrimer is built in stages using the convergent approach, working inwardly from the end groups. When the expanding dendrons, or branched polymeric arms, reach a sufficient size, they are joined to the multipurpose core molecule [40].
-
- Cubosomes:
Cubosomes are special structures made of self-assembled amphiphilic lipid molecules dispersed in aqueous media as a liquid crystalline phase with cubic crystallographic symmetry. Due to the presence of two continuous water channels separated by a twisted lipid bilayer, they are distinguished by having a large surface area. They range in size from 100 to 500 nm and have a structure resembling honeycomb (cavernous) structures [41]. Cubosomes are reversibly polarized bicontinuous cubic phases with distinctive physicochemical properties. Because they can deliver a wide variety of hydrophobic, hydrophilic, and amphiphilic medications with improved bioavailability and loading potential, these special systems are a study area of interest. They are frequently used in chemotherapy, oral, transdermal, ocular, and other drug delivery methods [42].
-
-
- Types of Cubosomes:
Depending on the formulation technique, cubosome precursors can be divided into liquid and powdered forms. By combining monoolein with a hydrotropic solvent, such as ethanol, cubosomes can form on their own. Particles can form through the nucleation process and grow through the crystallization and precipitation processes. Powdered cubosomes can also be created using dehydrated surface-active agents combined with a suitable polymer, in addition to the liquid cubosome precursors. Powdered cubosomes can be created by spray-drying after liquid droplet particles have been encapsulated in emulsion and dispersion [43].Figure 1 show different Nano based systems for Ocular drug Delivery System.
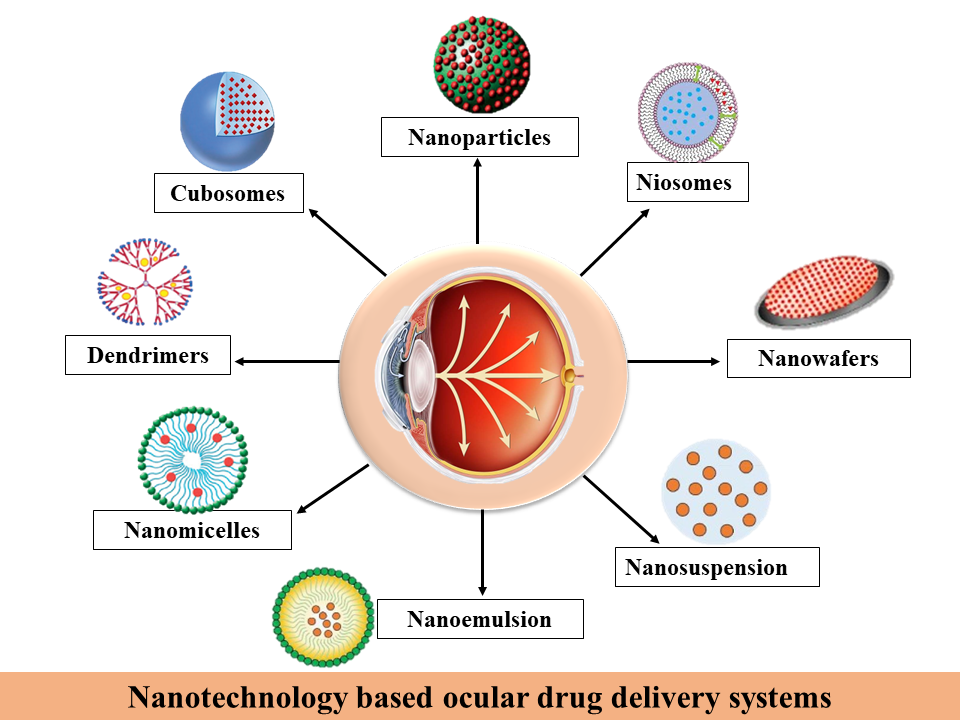
Figure: 1 Different Nano based systems for Ocular drug Delivery System


 Anuradha Verma*
Anuradha Verma*
 Anshika Garg
Anshika Garg
 10.5281/zenodo.14786780
10.5281/zenodo.14786780