Abstract
One significant and cutting-edge analytical tool employed in the pharmaceutical business over the past three decades is ultraviolet spectroscopy. The analytical technique measures the amount of monochromatic light absorbed by colourless substances in the near ultraviolet (200–400 nm) range. The processes required to ascertain the "identity, strength, quality and purity" of such chemicals are included in the pharmaceutical analysis. It also covers the examination of raw materials and intermediates used in the pharmaceutical production process. A spectrophotometer covering the ultraviolet range operates on the basic principle of light passing through a solvent-filled cell and onto a photoelectric cell, which converts radiant energy into electrical energy that can be detected by a galvanometer. To determine the absorbance spectrum of a substance in solution or as a solid, ultraviolet-visible spectroscopy is utilised. The purpose of this review is to present details about the following topics: Q-absorbance quantitative relation methodology, twin wavelength methodology, absorptiontivity methodology, multivariate chemometrics, distinction spectrophotometry, byproduct spectrophotometry, absorbance quantitative relation spectra, by-product quantitative relation spectra, successive quantitative relation by-product spectra, and absorption factor, physical property factor methodology.
Keywords
Ultraviolet Spectroscopic Analysis, UV Spectroscopy, Spectrophotometric Methods,
Introduction
The absorption or reflectance spectroscopy of the ultraviolet and nearby visible parts of the electromagnetic spectrum is known as ultraviolet spectroscopy. UV-visible spectrophotometry, or UV-Vis or UV/Vis, is another name for it. This approach is frequently employed in a wide range of practical and basic applications due to its inexpensiveness and simplicity of implementation [1,2]. All that is necessary for the sample to be a chromophore is for it to absorb in the UV is range. Fluorescence spectroscopy is supplemented by absorption spectroscopy. The metrics of interest, besides wavelength, are absorbance (A), transmittance (%T), and reflectance (%R), together with their temporal fluctuations [3]. White light is going to be scattered into a spectrum when it is directed into a prism or slit. On one extreme of the spectrum is higher frequency purple light with richer energy, while on the other is lower frequency red light with less energy. Additionally, this side is where the invisible electromagnetic radiation is located. There are several types of high intensity invisible lights than ultraviolet (UV) [4]. Standing in direct sunlight can cause damage to your skin due to UV rays. The segments and their wavelengths are described in the table below in accordance with ISO 21348:2007:
Table 1: The segments and their wavelengths are described in the table below in accordance with ISO 21348:2007 [5]

Principle:
The Principle of UV Visible The foundation of spectroscopy is the unique spectra that are produced when chemical substances absorb ultraviolet or visible light. Spectroscopy is based on the interaction of light and matter. A spectrum is created when matter absorbs light and goes through phases of excitation and de-excitation. Transmission, absorption, reflection, and scattering are examples of events that can happen when an electromagnetic wave hits a material [6]. The observed spectrum shows how different wavelengths interact with discrete-dimensional things like atoms, molecules, and macromolecules. When a molecule's excited and ground states have different energies, absorption takes place because the frequency of the incoming light matches that difference [7]. This is how molecular spectroscopy works at its core.

Fig 1: This Figure explains how an electron is energized to go from its ground state to an excited one. This is the basic idea of molecular spectroscopy [8]
Beer-Lambert law:
According to this rule, the route length (b) and the concentration of the absorbing species (c) in the solution are directly correlated with the absorbance of a solution (A).
Absorbance A = molar absorptivity constant x cell length x concentration
A = abc
C = A /a b
Where,
A = absorbance
A = molar absorptivity
b = path length
c = Concentration
Electronic Transition:
When light induces an electronic transition inside the structure of a molecule or ion, absorption occurs in the visible or ultraviolet spectrum. As a result, when a sample absorbs light in the visible or ultraviolet spectrum, its molecules' electrical states change as well. Electrons will be promoted from their ground state orbitals to higher energy excited state orbitals or antibonding orbitals by the energy from the light [9,10].
UV and visible light absorption can result in the following kinds of electronic transitions:
- ? to ?* Transitions:
An electron in the ? orbital of bonding is stimulated to the antibonding orbital that corresponds to it. It takes a lot of energy. For instance, the absorbance maximum of methane, which only contains C-H bonds and can only go through ? to ?* transitions, is observed at 125 nm. Typical UV-Vis spectra (200 - 700 nm) do not show absorption peaks owing to ? to ?* transitions [11].
- n to ?* Transitions:
Transitions from n to ?* are possible in saturated compounds that contain atoms with lone pairs, or non-bonding electrons. Typically, these shifts need a smaller amount of energy than ? to ?*. Changes in Attitude Light with a wavelength between 150 and 250 nm can start them [12,13].
- n to ?* and ? to ?* Transitions:
The majority of organic compound absorption spectroscopy relies on electron transitions from n or ? to the ? * excited state. This is due to the fact that the absorption maxima for these transitions lie in a spectral range (between 200 and 700 nm) that is favourable for experimentation. The ? electrons required for these transitions must come from an unsaturated group in the molecule. Molar absorptivity ranges from 10 to 100 L mol-1 cm-1 during the ? to ?* transitions, which is a comparatively low value. Normal molar absorbtivities for ? to ?* transitions range from 1000 to 10,000 L mol-1 cm-1 [14,15].
Limitation in Laws:
- The reported absorption may change as a result of scattering and reflection.
- The way the solvent react when a concentration is high, the average distance between ions decreases, affecting the charge distribution and bringing particles closer to one another [16].
- The existence of errant light.
Types of UV-visible spectra:
To gather UV-visible spectra, two different kinds of absorbance equipment are utilised:
- Single beam spectrometer:
All of these devices have a sample holding, a detector, and a light source (often a tungsten or deuterium lamp). However, some of them also feature filters that allow the user to choose one wavelength at a time. To analyse one wavelength at a time, the single beam instrument (Figure 2) places a filter or monochromator between the source and the sample [17].
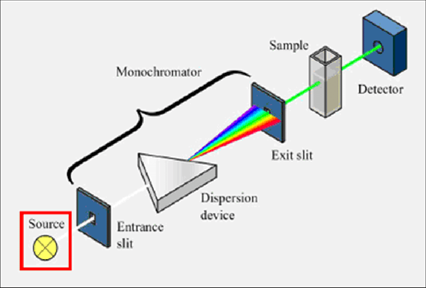
Fig 2: UV-Spectroscopy Single Beam [20]

Fig 3: UV-Spectroscopy Double Beam [21]
INSTRUMENTATION:
The following list of parts make up instruments used to measure the absorption of UV or visible light [22].
- Source
- Monochromator
- Sample cell
- Detector
- Readout system
- Amplifier
- Display
1. Sources:
a. Sources of UV radiation:
It is crucial that the radiation source's power does not fluctuate significantly over its wavelength range. A continuous UV spectrum is produced when deuterium or hydrogen are electrically excited at low pressure. The production of an excited molecular species, which disintegrates into two atomic species and an ultraviolet photon, is the process behind this [23].
D2 + electrical energy • D2* • D' + D'' + hv is one way to represent this.
The wavelength range for radiation emitted by deuterium and hydrogen lamps is 160–375 nm. These lamps require quartz windows and quartz cuvettes since glass absorbs light with wavelengths below 350 nm [24].
b. Sources of visible radiation:
Visible light is frequently provided by tungsten filament lamps. The wavelength range for this kind of light is 350–2500 nm. A tungsten filament lamp's energy output is proportional to the voltage at which it operates, raised to the fourth power. This implies that the voltage to the lamp needs to be extremely consistent in order for the amount of energy produced to be stable. This stability is provided by constant-voltage transformers or electronic voltage regulators [25].
- Monochromator (Wavelength selector):
The following components are found in every monochromator:
- An entrance slit
- A collimating lens
- A dispersing device (usually a prism or a grating)
- A focusing lens
- An exit slit
Via the entry slit, polychromatic radiation—radiation with many wavelengths—enters the monochromator. The beam is collimated before making an angle contact with the dispersion element. The prism or grating divides the beam's wavelengths into their individual components. Radiation of a specific wavelength may only depart the Monochromator through the exit slit by changing the dispersing element or the exit slit [26,27].

Fig 4: Diagrammatic Representation of Monochromator [28]
- Sample cell:
The radiation that will pass between the sample and reference solution's containers has to be transparent to the radiation. For UV spectroscopy, quartz or fused silica cuvettes are necessary. In the visible spectrum, these cells are likewise transparent. Cuvettes for usage in the 350–2000 nm range can be made using silicate glasses [29].
- Detectors:
An electrical signal is created from a lighting signal by a detector. Over a broad range of low noise and high sensitivity, it should respond linearly.
- Photomultiplier tube detector
In UV-Vis spectroscopy, the photomultiplier tube is a frequently employed detector. It is made up of an anode, which releases several electrons for every electron that strikes it, and a photoemissive cathode, which releases electrons when exposed to photons of radiation. When a photon of radiation enters the tube, it strikes the cathode, which releases several electrons. The initial anode, which is 90 volts more positive than the cathode, is where these electrons are propelled. For every incident electron that strikes the first electrode, multiple electrons are emitted [30]. To create extra electrons that are propelled towards the anode, these electrons are then accelerated in the direction of the second anode. At the anode, electrons are gathered. By now, 106–107 electrons have been created for every initial photon. After then, the current has been determined and amplified. Photomultipliers are extremely vulnerable to visible and ultraviolet light. Their reaction times are quick. Photomultipliers are only capable of monitoring low power radiation due to damage from intense light [31].
- Photodiode detector
One type of multichannel photon detector is the photodiode detector. These detectors can measure every component of a scattered radiation beam at the same time. Numerous tiny silicon photodiodes arranged on a single silicon chip make up a linear photodiode array. A single chip can include 64–4096 sensor components; 1024 photodiodes are the most frequent configuration. A switch and a storage capacitor are also present for every diode [32]. One can progressively scan each diode-capacitor circuit in turn. When in utilise, the photodiode array is arranged so that the spectrum hits it at the monochromator's focal plane, which comes after the dispersing element. They can particularly handy when capturing the UV-Vis absorption spectra of materials that are moving quickly through a sample flow cell, such in an HPLC detector [33].
APPLICATION
- Bacterial Culture:
When growing bacteria, UV-Vis spectroscopy is frequently utilised. A wavelength of 600 nm is often and rapidly used for OD measurements, which are used to assess cell concentration and monitor growth. Because of the optical characteristics of the bacterial growth conditions under which they are cultivated and to prevent cell damage in situations where the cells are necessary for further testing, 600 nm is frequently utilised and recommended [34].
- Beverage Analysis:
The identification of certain substances in drinks is another typical use for UV-Vis spectroscopy. UV light can assist in determining the legal limits for caffeine levels. By matching their known peak absorption wavelengths, specific classes of coloured substances—such as anthocyanin, which is present in cherries, blueberries, raspberries, and blackberries—can be easily detected in wine using UV-Vis absorbance for quality control purposes [35].
- DNA and RNA Analysis:
One frequent use is for speedy RNA and DNA concentration and purity measurements. The wavelengths employed in their study and their indications are compiled in Table 2. It is commonly required to make sure that DNA or RNA samples are free of contaminants, such as proteins or chemicals left over from the isolation process, before preparing them for downstream uses like sequencing [36].
Table 2: An overview of the UV absorbance that is important for calculating the 260/280 and 260/230 absorbance ratios [37]

- Pharmaceutical Analysis:
The pharmaceutical business is among the industries that utilise UV-Vis spectroscopy the most frequently. In example, overlapping absorbance peaks in the original spectra can be resolved to identify specific medicinal drugs by processing UV-Vis spectra utilising mathematical derivatives. For example, by using the first mathematical derivative on the absorbance spectra, it is possible to identify the antibiotic chlortetracycline and the local anaesthetic benzocaine concurrently in commercial veterinary powder formulations. Through the creation of calibration functions for each chemical, simultaneous measurement of both compounds was achievable on a microgram per millilitre concentration range [38].
- Other Applications:
Numerous other sectors might potentially make use of this technology. For example, evaluating a colour index can be helpful in keeping an eye on transformer oil as a preventative precaution to guarantee the safe delivery of electricity [39]. Research on cancer may benefit from monitoring haemoglobin absorbance to ascertain haemoglobin concentrations. By comparing their spectra over time, UV-Vis spectroscopy in the treatment of wastewater may be utilised in kinetic and monitoring studies to make sure that certain dyes or dye byproducts have been eliminated correctly. It is also helpful for keeping an eye on air quality and figuring out whether food is legitimate. Additionally, in certain highly specialised studies, UV-Vis spectroscopy is qualitatively beneficial. Determining the composition of batteries and investigating particular structural protein changes can both benefit from tracking fluctuations in the wavelength that corresponds to the peak absorbance. Variations in the wavelengths of peak absorbance can also be helpful in more contemporary applications, such the characterisation of very tiny nanoparticles. There are a wide range of nearly limitless possibilities for this method.
DERIVATIVE UV-SPECTROPHOTOMETRY
For the purpose of obtaining complementary qualitative and quantitative information from spectra that contain unresolved bands, derivative UV spectrophotometry is a widely used analytical technique. First or higher derivatives of absorbance according to wavelength are used for both qualitative and quantitative analysis. When derivative spectroscopy was first introduced in the 1950s, it had several applications. However, due to the difficulty of obtaining derivative spectra using UV-visible spectroscopy, the approach was not widely used. The vulnerability was overcome in the 1970s by microcomputers, which provided derivative spectra in a more precise, straightforward, quick, and repeatable manner [40].
The objective with which derivative methods used in analytical chemistry are:
- Spectral differentiation
As a qualitative technique that discerns minute differences in spectra that are almost identical.
- Spectral resolution enhancement
Resolving overlapping spectral bands just requires estimating the quantity and wavelengths of the bands.
- Quantitative analysis
In addition to correcting the extraneous background absorption, it makes multicomponent analysis easier. The process of differentiating or resolving overlapping bands starts with the derivative spectroscopy approach. One of the key features of this procedure is the suppression of wide bands in comparison to sharp bands.
Measurement Techniques of the Derivative Spectroscopy
The path to a derivative spectrum of any order may be found by differentiating a zero order spectrum of a set of components. Many techniques are employed to discriminate a spectrum, such as the use of an analogue or numerical approach. Spectral differentiation can be thought out either graphically on paper or digitally stored in a computer's memory. Three approaches are used to measure the derivative spectra value: the zero crossing technique, numerical measurement, and graphic measurement.
- Visual measurement:
The theoretical approach of graphic measurement is used to compute the derivative spectra on paper. The manual method has the drawback of producing erroneous findings since the value that can be obtained numerically can be eliminated or diminished indefinitely.
- Quantitative assessment:
The technique makes use of a series of sites where estimating the derivative value at a certain wavelength is done. It uses an appropriate numerical approach to provide derivatives by spectral differentiation.
- Zero crossing methodology:
At a specific wavelength, when the derivative crosses the point at the zero line, the procedure measures the derivative spectra. The zero crossing approach can be used to remove interference from one component's assessment of another component.
Derivative Spectra
Derivative spectra are used in quantitative analysis to resolve overlapping bands by enlarging the difference between spectra. For producing derivative spectra, the Savitzky-Golay digital algorithm approach is highly recommended. Plotting the absorbance spectrum's rate of change against wavelength is part of the universal technique. Numerous experimental methods may be used to acquire derivative spectra, and even if the spectrum was originally digitally captured or is in computer-readable form, differentiation can be performed numerically. Real-time derivative spectra can be obtained via wavelength modulation or by obtaining the time derivative of the spectrum while the spectrum is scanned at a constant rate.
A computerised approach is extensively used to create derivative curves. A wavelength modulation device is employed to record the derivative spectra, where a beam of light alters in wavelength by a minor change (1-2 nm) and the difference between the two readings is recorded [41].

Fig 5: Derivative Spectra
Peak heights of long-wave peak satellites or short-wave peak satellites are measured quantitatively for second or fourth order derivative curves. The inclusion of satellite peaks raises the complexity level of derivative spectra. Two distinct peaks and troughs are observed in second derivative spectra. Over peaks, the solvents have a remarkable impact. Based on the polarity of the solvent, the peaks and troughs move to longer or shorter wavelengths.
The way of obtaining the derivative orders
Converting a normal or zero order spectrum to a first, second, or higher derivative spectrum is achieved by derivative spectroscopy. It results in significant modifications to the derivative's form. Selecting the derivative order appropriately allows overlapping signals to be usefully separated. It is predicted that higher orders will be used for narrow spectral bands and lower orders for large spectral bands in order to achieve criteria such as signals height, breadth, and distance between maxima in the fundamental spectrum. An ideal absorption band that provides a clear understanding of the transition taking place in the derivative spectra is represented by a Gaussian band. When absorbance is plotted against wavelength, a graph is produced that displays a peak with maxima and minima, or points of inflection that should pass through zero on the ordinate.
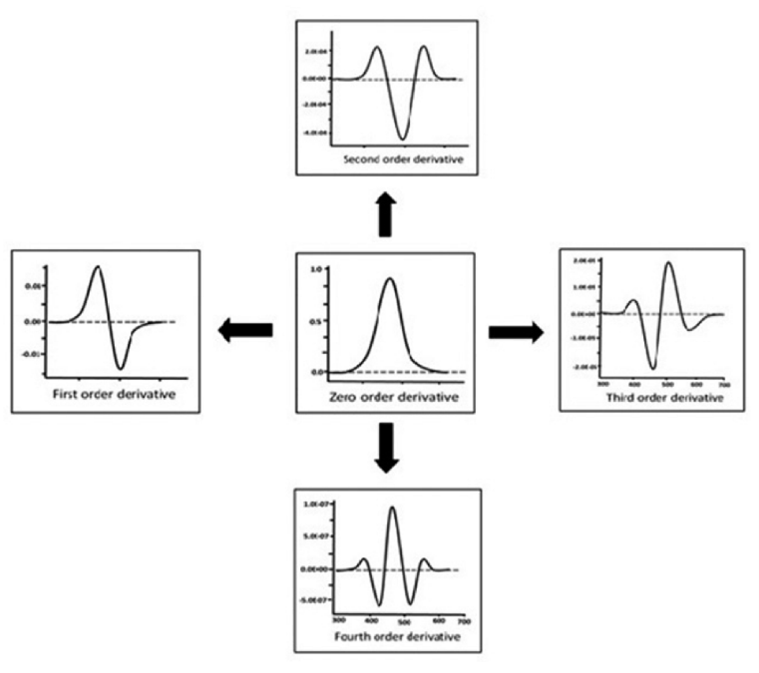
Fig 6 Oder of Derivative Spectra
Zero order derivative spectrum
The first step in providing additional derivatives is the zero order derivative. i.e., the derivative of the nth order may be obtained from infinity. The D0 spectrum, or zeroth order, in derivative spectroscopy is a characteristic of the normal absorption spectrum. The zeroth order spectrum may be used directly to derive the derivative spectra of the first, second, third, and fourth orders. The sensitivity of determination rises with an increase in the order of derivatives. The derivative spectrum is provided as follows if a spectrum is represented as absorbance (A) as a function of wavelength (?).
A = f (?)
First order derivative spectrum
Spectra that result from once derivatizing the zero order spectrum. This is a plot showing the absorbance change with wavelength against wavelength, or the absorbance change rate with wavelength. Even in its derivatized form, the complexity surpasses that of the zero order spectrum. The absorbance band's ? max is passed through by zero in first order spectra. A positive and negative band with peaks and minima may be seen in the absorbance band of the first order derivative. A dual-wavelength spectrophotometer measures the difference between two wavelengths and uses that difference to scan the spectrum and get first-derivative spectra.
dA/d? = f’(?)
Second order derivative spectrum
This kind of spectrum is produced by twice derivatizing the absorbance spectrum. This is a plot of the absorption spectrum's curvature vs wavelength. The second derivative is exactly proportional to the concentration, meaning it is directly related. d2A/d?2 needs to be high; the higher the ratio, the higher the sensitivity. The technique is helpful for acquiring gas and atomic molecular spectra.
d2A/d?2 = f’(?)
Third order derivative spectrum
The third derivative spectrum displays a dispersal function to the original curve, in contrast to the second order spectrum.
d3A/d?3 = f’''(?)
Fourth-derivative spectrum
Fourth order is an inverted spectrum of second order with a centre peak that is sharper than the original band. Fourth derivative (UV-high pressure) selectively determines narrow bands.
d4A/d?4 = f''(?)
Polynomial degree
Instead of determining the form of the derivative, the number of polynomial points is greatly influenced by polynomial degree. The range of a polynomial is narrow; low degree polynomials employ differentiation of half-width spectra, but higher degree polynomials use it for tiny half-width spectra. Inappropriate polynomial degree results in distorted derivative spectrum. Using various polynomial degrees in multicomponent analysis might enhance the spectrum differences of the chemicals being tested and their selective selection.
Signal-to-noise ratio
Using derivative approach with higher orders that result in lower signal-to-noise becomes challenging. S/N decreases with higher orders as a result. The spectrum's sharpest characteristics are caused by noise. Due to derivatization's detrimental influence on S/N, the spectrophotometer's low-noise capabilities are under more pressure. Before derivatization, S/N can be increased if a spectrophotometer were to scan spectra and average many of them. The difference between the highest maximum and the lowest minimum can be used to calculate the best signal-to-noise ratio, although doing so increases the susceptibility to interference from other components. A signal's noise is represented by its standard deviation, or ?. The standard deviation while standard deviation ?n indicates the nth order derivative that may be derived by ?0, ?0 expresses the noise of the normal spectrum of the blank absorbance.
Smoothing of spectra
A technique known as low-pass filtering or smoothing is used to reduce the high-frequency noise or to alleviate the circumstances caused by an increase in the signal-to-noise ratio. Smoothing is an operation that works on neighbouring variables and is carried out on individual spectrum for every row of the data. Closely spaced variables in the data matrix with equivalent information can have much less noise without sacrificing the signal of interest. Care must be exercised since a high degree of smoothing may change the derivative spectrum. The smoothing effect is primarily determined by two variables: (a) the smoothing frequency and (b) the smoothing ratio, or the breadth of the smoothed peak divided by the number M of data points.
Advantages and Disadvantages of Derivative UV-Spectrophotometry
Advantages:
The sensitivity and selectivity of UV Derivative Spectroscopy have been enhanced. Its many benefits include the ability to identify both organic and inorganic chemicals, determine traces in a matrix, analyse proteins and amino acids, identify single components and multiple components in a mixture simultaneously, and determine traces in the environment.
The following are some particular advantages of derivative spectral analysis:
- Absorbance bands can be distinguished even in a narrow wavelength range when there are two or more overlapping peaks present.
- Weak and tiny absorbance peaks can be distinguished when strong and sharp absorbance peaks are present.
- A wide absorbance spectrum provides a precise understanding of the wavelength at that maximum spectrum.
- Because the derivative values and concentration levels have a linear relationship, the quantitative analysis may be conducted even when background absorption is present.
Disadvantages:
Despite being a sensitive approach, it is nevertheless quite vulnerable to several factors. Because of its low repeatability, the procedure is restricted to a specific system and has limited uses. When the current instrumental technique—which measures signal—is unavailable, the approach comes in second. When measuring zero-crossing spectra, it is less precise. Since the derivative and zero order spectra are similar in form, slight changes to the basic spectrum can significantly alter the derivative spectrum. When multiple spectrophotometers used for zero order spectra provide comparable findings but their derivatization displays different results, poor repeatability might affect the results in a way.
APPLICATIONS:
- Single component analysis:
In pharmaceutical formulation, derivative spectrophotometry studies a single component in addition to the Area under the Curve.
- Multicomponent analysis:
In pharmaceutical analysis, derivative spectrophotometry studies several components in the presence of other components, i.e., determining two or more substances at the same time. The predominance brought on by the spectra of bothersome chemicals can be eliminated by spectral derivatization.
- Bioanalytical application:
Derivative spectrophotometry has other uses outside of pharmaceutical analysis. Identification of the chemicals present in different biological samples, including brain tissue, plasma, serum, and urine. The sequence of derivatives of amphotericine and diazepam has been identified in human plasma.
- Forensic toxicology:
Derivative spectroscopy may be applied in mixtures and is particularly useful in the study of illegal substances such as amphetamine, ephedrine, meperidine, and diazepam.
- Trace analysis:
The derivative signal processing method is frequently employed in real-world analytical tasks when measuring trace levels of chemicals in the presence of significant concentrations of compounds that might interfere. Analytical signals become faint, noisy, and overlaid on massive background signals as a result of this interference. Degraded measurement accuracy is caused by sample-to-sample baseline variations and factors such as non-specific broadband interfering absorption, non-reproducible cuvette placement, dirt or fingerprints on the cuvette walls, improper cuvette transmission matching, and solution turbidity. Practical errors can be the cause of baseline shifts; these mistakes can be weakly wavelength dependent (small particle turbidity) or wavelength independent (large suspended particles or bubbles obstructing light). Therefore, it is necessary to distinguish pertinent absorption from the aforementioned baseline shift causes. It is anticipated that differentiation will attenuate the wide background in order to lessen sample-to-sample variability in background amplitude. In many cases, this leads to increased measurement accuracy and precision, particularly when the analyte signal is weak in relation to the background and there exists an abundance of uncontrolled variability in the background.
CONCLUSION:
A crucial technique for researching the optical characteristics of PMCs is UV-Vis spectroscopy. It looks at how nanofillers can improve the characteristics of nanocomposites and advances our understanding of how the matrix and nanofiller interact. UV-Vis spectroscopy is one of the most significant characterisation methods for researching optical characteristics.In the direction of the creation of functional materials with important technological applications; it highlights the value of the UV-Vis spectroscopy technique in characterizing polymer nanocomposites with optically responsive nanofillers like metals, semiconductor nanocrystals, and nano oxides. A derivative these days, spectrophotometers that are controlled by software may do spectrophotometry. This facilitates analysts' ability to extract meaningful information from the spectra of the corresponding chemicals. The UV spectrum derivatives provide useful information for understanding the chemicals used in pharmaceutical formulation.
ACKNOWLEDGEMENT:
The authors are thankful to Department of Pharmacy, LLYOD College, Greater Noida for providing kind guidance and excellent opportunity as well as necessary facilities for the research.
CONFLICTS OF INTEREST:
The authors confirm that the content of the article has no conflict of interest.
AUTHOR’S CONTRIBUTION:
Both the authors Sheikh Wajiha Shabbir and Shilpi Chauhan have contributed equally in the paper.
DATA AVAILABILITY:
The original data that support the findings of this study are included in the Article.
FUNDING:
This research paper received no external funding.
REFERENCES:
- Picollo M, Aceto M, Vitorino T. UV-Vis spectroscopy. Physical sciences reviews. 2019 Mar 26;4(4):20180008.
- Verma G, Mishra M. Development and optimization of UV-Vis spectroscopy-a review. World J. Pharm. Res. 2018 Apr 19;7(11):1170-80.
- Brown JQ, Vishwanath K, Palmer GM, Ramanujam N. Advances in quantitative UV–visible spectroscopy for clinical and pre-clinical application in cancer. Current opinion in biotechnology. 2009 Feb 1;20(1):119-31.
- Förster H. UV/vis spectroscopy. Characterization I: -/-. 2004 Feb 11:337-426.
- Shinde G, Godage RK, Jadhav RS, Manoj B, Aniket B. A review on advances in UV spectroscopy. Research Journal of Science and Technology. 2020;12(1):47-51
- Rocha FS, Gomes AJ, Lunardi CN, Kaliaguine S, Patience GS. Experimental methods in chemical engineering: Ultraviolet visible spectroscopy—UV?Vis. The Canadian Journal of Chemical Engineering. 2018 Dec;96(12):2512-7.
- Van Den Broeke J, Langergraber G, Weingartner A. On-line and in-situ UV/vis spectroscopy for multi-parameter measurements: a brief review. Spectroscopy europe. 2006;18(4):15-8.
- Power AC, Chapman J, Chandra S, Cozzolino D. Ultraviolet-visible spectroscopy for food quality analysis. Evaluation technologies for food quality. 2019 Jan 1:91-104.
- Guo Y, Liu C, Ye R, Duan Q. Advances on water quality detection by uv-vis spectroscopy. Applied Sciences. 2020 Sep 30;10(19):6874.
- Begum R, Farooqi ZH, Naseem K, Ali F, Batool M, Xiao J, Irfan A. Applications of UV/Vis spectroscopy in characterization and catalytic activity of noble metal nanoparticles fabricated in responsive polymer microgels: a review. Critical reviews in analytical chemistry. 2018 Nov 2;48(6):503-16.
- Liauw MA, Baylor LC, O'Rourke PE. UV?Visible Spectroscopy for On?Line Analysis. Process analytical technology: Spectroscopic tools and implementation strategies for the chemical and pharmaceutical industries. 2010 Apr 16:81-106.
- Hou DB, Zhang J, Chen L, Huang PJ, Zhang GX. Water quality analysis by UV-Vis spectroscopy: A review of methodology and application. Spectroscopy and Spectral Analysis. 2013 Jul 15;33(7):1839-44.
- Schoonheydt RA. UV-VIS-NIR spectroscopy and microscopy of heterogeneous catalysts. Chemical Society Reviews. 2010;39(12):5051-66.
- Perkampus HH. UV-VIS Spectroscopy and its Applications. Springer Science & Business Media; 2013 Mar 8.
- Jentoft FC. Ultraviolet–visible–near infrared spectroscopy in catalysis: theory, experiment, analysis, and application under reaction conditions. Advances in catalysis. 2009 Jan 1;52:129-211.
- Urí?ková V, Sádecká J. Determination of geographical origin of alcoholic beverages using ultraviolet, visible and infrared spectroscopy: A review. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2015 Sep 5;148:131-7.
- Venkatachalam S. Ultraviolet and visible spectroscopy studies of nanofillers and their polymer nanocomposites. Spectroscopy of polymer nanocomposites. 2016 Jan 1:130-57.
- Ríos-Reina R, Azcarate SM. How chemometrics revives the UV-Vis spectroscopy applications as an analytical sensor for spectralprint (nontargeted) analysis. Chemosensors. 2022 Dec 22;11(1):8.
- Rojas FS, Ojeda CB. Recent development in derivative ultraviolet/visible absorption spectrophotometry: 2004–2008: A review. Analytica chimica acta. 2009 Mar 2;635(1):22-44.
- Lange R, Balny C. UV-visible derivative spectroscopy under high pressure. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology. 2002 Mar 25;1595(1-2):80-93.
- Sirajuddin M, Ali S, Badshah A. Drug–DNA interactions and their study by UV–Visible, fluorescence spectroscopies and cyclic voltametry. Journal of Photochemistry and Photobiology B: Biology. 2013 Jul 5;124:1-9.
- Yang Z, Zhu M. Case Studies: Ultraviolet-Visible (UV-Vis) Spectroscopy. InSpringer Handbook of Advanced Catalyst Characterization 2023 May 18 (pp. 265-283). Cham: Springer International Publishing.
- Chen J, Li JQ, Li T, Liu HG, Wang YZ. Application of UV-Vis and infrared spectroscopy on wild edible bolete mushrooms discrimination and evaluation: a review. Critical Reviews in Analytical Chemistry. 2023 May 19;53(4):852-68.
- Fedenko VS, Shemet SA, Landi M. UV–vis spectroscopy and colorimetric models for detecting anthocyanin-metal complexes in plants: An overview of in vitro and in vivo techniques. Journal of plant physiology. 2017 May 1;212:13-28.
- Lai Q, Zhu S, Luo X, Zou M, Huang S. Ultraviolet-visible spectroscopy of graphene oxides. AIP advances. 2012 Sep 1;2(3).
- Ojeda CB, Rojas FS. Recent developments in derivative ultraviolet/visible absorption spectrophotometry. Analytica Chimica Acta. 2004 Aug 2;518(1-2):1-24.
- Plane JM, Saiz?Lopez A. UV?Visible differential optical absorption spectroscopy (DOAS). Analytical techniques for atmospheric measurement. 2006 Apr 25:147-88.
- Atole DM, Rajput HH. Ultraviolet spectroscopy and its pharmaceutical applications-a brief review. Asian J pharm clin res. 2018;11(2):59-66.
- Cox H, Stace AJ. Recent advances in the visible and UV spectroscopy of metal dication complexes. International Reviews in Physical Chemistry. 2010 Oct 1;29(4):555-88.
- Imam SS. The future of non-invasive ways to treat cancer. Int J Pharm Sci & Res 2021; 12(8): 4684-96.
- Imam SS, Agarwal S. A Pragmatic Approach To Treat Lung Cancer Through Loading Theaflavin -3,3’-Digallate And Epigallocatechin Gallate In Spanlastic. Asian J Pharm Clin Res. 2021 Nov 7; 14(11): 1-8.
- Imam SS, Imam ST, Mdwasifathar, Kumar R, Ammar MY. Interaction Between Ace 2 And Sars-Cov2, And Use Of EGCG And Theaflavin To Treat Covid 19 In Initial Phases. International Journal of Current Pharmaceutical Research. 2022 Mar; 14(2):5- 10.
- Imam SS, Sharma R. Natural compounds promising way to treat Lung Cancer. International Journal of Pharmaceutical Research and Applications. 2023; 8(2): 552- 558.
- Imam SS, Sharma S, Kumari D, Khan S, Pathak P, Katiyar D. An Expedient Approach to Treat Asthma through Non-Steroidal, Natural Transferosomes Aerosol System. Innovare journal of medical sciences. 2022; 10(6): 7-11.
- Imam SS, Imam ST, Agarwal S, Kumar R, Ammar MY, Athar MW, Akthar A. Lung Cancer Therapy Using Naturally Occurring Products and Nanotechnology. Innovare journal of medical sciences. 2022; 10(4): 1-5.
- Imam ST, Imam SS. The Cream which relieves the pain of Menstrual cramps without interfering with the Hormones or Period Cycle. Research Journal of Pharmacy and Technology. 2023; 16(3):1239-6.
- Imam SS. Topical Formulation Constituted with Transferosomes for the Treatment Of Non-Melanoma Skin Cancer. Asian J Pharm Clin Res. 2023 May 7;16(5):27-32.
- IMAM SS. NANOPARTICLES: THE FUTURE OF DRUG DELIVERY. Int J Curr Pharm Sci. 2023 Nov. 15;15(6):8-15.
- Imam SS, Mehdi S, Mansuri A. A COMPREHENSIVE REVIEW ON THE PHARMACOLOGICAL POTENTIAL OF RED GINSENG OIL, TEA TREE OIL AND HEMP SEED OIL. European Journal of Biomedical. 2024;11(4):167-77.
- Imam SS. Sublingual Tablets Amalgamated with Nano-particles and natural products to treat Oral Cancer. 2024; 17(5): 2056-62.
- Roberts J, Power A, Chapman J, Chandra S, Cozzolino D. The use of UV-Vis spectroscopy in bioprocess and fermentation monitoring. Fermentation. 2018 Mar 13;4(1):18.


 Sheikh Wajiha Shabbir*
Sheikh Wajiha Shabbir*
 Shilpi Chauhan
Shilpi Chauhan







 10.5281/zenodo.12570882
10.5281/zenodo.12570882