Abstract
Periodontitis is a chronic inflammatory disease that affects the supporting structures of the teeth, leading to tissue destruction and tooth loss if left untreated. Conventional treatments for periodontitis, such as scaling and root planing , may be limited in effectiveness, particularly in cases of advanced disease or in the management of infections within deep periodontal pockets. In recent years, in situ gel systems have emerged as a promising novel approach for the local delivery of therapeutic agents directly to the site of infection, offering numerous advantages over traditional methods. These gels are unique in their ability to transition from a liquid to a gel upon contact with the oral cavity, providing a sustained release of antibiotics or anti-inflammatory drugs, thereby enhancing treatment outcomes and minimizing systemic side effects. This review explores the potential of in situ gels in the management of periodontitis, focusing on their composition, mechanism of action, and efficacy in delivering active pharmaceutical ingredients. The review also highlights various biocompatible polymers used in the formulation of in situ gels, and their role in ensuring controlled drug release and therapeutic efficacy. Additionally, challenges related to patient compliance, gel stability, and scalability for clinical application are discussed. Overall, in situ gels represent a promising therapeutic strategy in the localized treatment of periodontitis, offering a targeted, effective, and minimally invasive alternative to conventional periodontal therapies. The characterisation of oral in situ gels, encompassing several evaluations including drug content study, pH & viscosity determination, texture analysis, spreadibility, gel strength, gelling time, sterility testing, in-vitro drug release study, stability study, etc., is finally summarized in this review. Experimental data indicates that in situ gel forming systems may be helpful in treating a number of common oral cavity illnesses, according to the reviewed literature. Clinical studies should be the main focus of future research if the in situ gel is to significantly reduce periodontitis.
Keywords
In situ gel, Periodontitis, Oral drug delivery, Polymers, Evaluation.
Introduction
The transitional condition between the solid and liquid phases is called gel. The liquid phase is immobilized by the solid component's three-dimensional network of interconnected molecules.[1] After the formulation is administered to the site, a process known as "in situ gelation" occurs, where a gel forms at the site of action. Based on a liquid medication formulation solution, the in situ gel phenomenon transforms into a semi-solid mucoadhesive important depot.[2] Drugs can now be administered to patients in a liquid dose form using in situ gel forming formulations, which provide a sustained release of the medication for the intended amount of time. Various polymer-based delivery systems have been developed that can extend the formulation's residence time at the drug's absorption site.[3] In the buccal cavity, its mucoadhesive quality also keeps saliva from washing it away. Water-soluble polymers with gel-forming properties that can be applied to a delivery location have garnered more attention recently. Water-soluble polymers with gel-forming properties that can be applied to a delivery location have garnered more attention recently. Unlike very strong gels, these so-called in situ gelling polymers are simply delivered in liquid form to the site of drug absorption, which makes them a particularly favourable alternative to other polymers. They swell at the drag absorption site to create a robust gel that can extend the active ingredient's residence duration. Because of their excellent biocompatibility, solute permeability, and adjustable release properties, gels three-dimensional networks of polymeric materials have drawn a lot of interest in the biomedical domain as drug, protein, and cell transporters.[4] The feature that compromises the surrounding tissues and promotes good biocompatibility is their capacity to hold a significant quantity of water within their structures, which results in a high water content and soft-surface qualities.[5-7] A group of issues known as periodontal disease occur in the sulcus, the space between the gums and teeth. Actinobacillus and actinomy cetemcomitans are common pathogens that cause juvenile periodontitis, while staphylococci subspecies epidermidis and aureus are responsible for adult periodontal disease.[8] Three potential benefits of site-specific therapy for periodontitis include lower medication dosages, higher drug concentration at the infection site, and fewer systemic side effects such gastrointestinal upset.[9] The goal of treating periodontal illnesses with a localized drug delivery system is to minimize the negative effects of systemic medication administration while simultaneously providing the therapeutic agent at a level that is adequate inside the periodontal pocket. Consequently, it improves patient compliance.[10]
Periodontitis
Etiology of periodontitis[11-13]
Periodontal disease is caused by the proliferation of bacteria and spirochetes, among other pathogens. Tooth loss occurs in periodontal disease. The clinical indicators used to gauge the effectiveness of the illness include the plaque index (PI), gingival index (GI), probing pocket depth (PPD), and clinical attachment level (CAL). The primary source of microbial feeding is the probing pocket depth (PPD).

Fig No: 1 Normal tooth and periodontitis
Teeth with periodontitis eventually lose their ability to function or suffer damage as a result of the disease's on going progression. The etiology of periodontal disease is known to be influenced by the bacterial flora found in the gingival crevice. When gingival and teeth are in good health, there is a 2 mm gap between them. The sulcus depth is typically greater than 5 mm when periodontitis and other bacterial collagenase enzymes are present. The condition of infectious disease at the location of nuclear cell polymorphisms caused by the anti-inflammatory reaction. and may aid in halting tooth loss. There is intriguing data from a recent study regarding a number of significant systemic disorders and periodontal infections. Theoretically, systemic health impacts from periodontal disease may be predicted using one or more of numerous approaches. Infection spreads directly to the periodontium from deep and nearby tissue (brain, sinus infection, facial, or aircraft). The work is impacted by circulating inflammatory mediators in the periodontium passage from distant places (atherosclerosis). Oral bacteria can penetrate infections through distant areas in the systemic circulation, such as endocarditis and thrombosis/atherosclerosis. Conditions like gastrointestinal or pulmonary infections encourage or contribute to oral bacteria's dissemination of their products or host products to distant mucosal locations. The teeth are surrounded by a 1 mm broad gingival border and a tight collar of healthy gingival tissue. The gingival margin creates the sulcus, or external wall crevice. A little crevice emerges between the tooth and the gingiva. The gingival sulcus is never totally devoid of microorganisms, and there is a small amount of microflora that is primarily composed of aerobic bacteria that are both analogous to tissue and work in harmony with the host's defense system. Periodontal pathogens grow only and only when environmental and nourishes and their requirements, 3.5 mL/day or more in diseased condition. Firstly a rise in the number of gingival aerobic bacteria, then a shift in the composition of the micro flora is observed with the development of periodontal disease.
Mechanism Of Periodontitis-Induced Tooth Loss
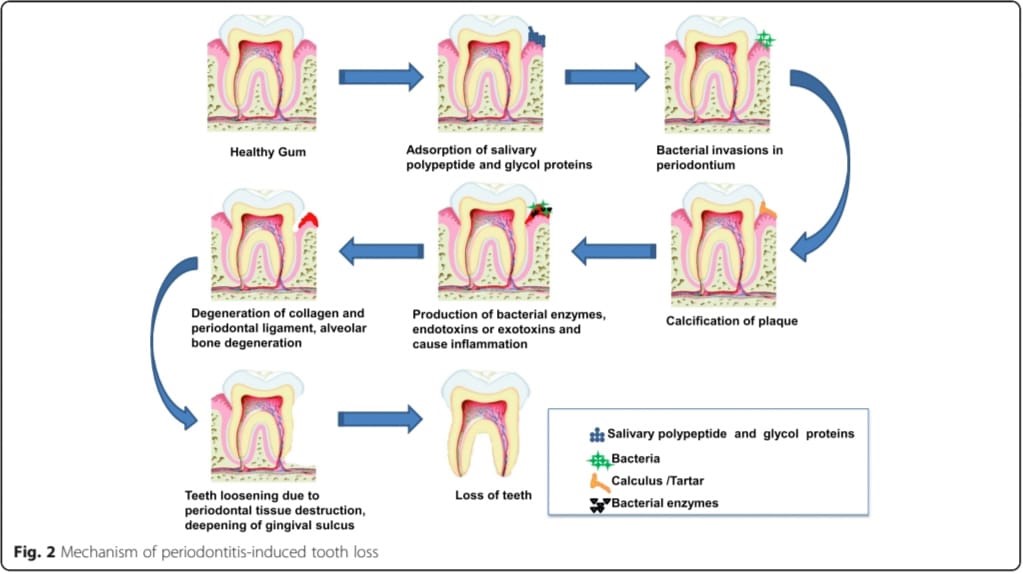
Fig No: 2 Mechanism of periodontitis-induced tooth loss
Periodontal Infection [14,15]
When bacteria and other pathogens infiltrate the pocket, proliferate there under ideal conditions, and reduce the clinical attachment level (CAL), probing pocket depth (PPD), and other parameters, periodontal diseases result.
- Causes
Numerous factors, such as the introduction of pathogens or an imbalance in the native flora, can result in dental infections. The elevated levels of pro-inflammatory substances and cytokines demonstrate that the systems controlling these genes are broken in periodontitis.
- Organisms causing the infection
Infections are caused by organisms that are antibacterial and antiprotozoal. Numerous causes, including the invasion of pathogens, the alteration of the balance of native flora, and the invasion of germs by various ways, are accountable for periodontal infections.
- Systemic factors and periodontitis
- One of the biggest risk factors is smoking, which is followed by diabetes. Smoking tobacco was linked to a higher incidence of dental caries. Smokers are more likely to develop a periodontal pocket and experience greater bone loss because they often have a higher plague index and fewer bleeding sites than non-smokers. However, stopping smoking can reverse the disease.
- Patients with diabetes experience more bleeding episodes, bone loss, and gingival recession than people without the disease. Diabetes patients' monocytic hyperresponsiveness to bacterial antigens is the mechanism that explains the potential link between diabetes and periodontitis.
- Stress reduces salivary flow, which creates an environment that is conducive to bacterial growth. Saliva's pH and content are altered by emotional stress. Periodontitis is exacerbated by hormonal changes that occur during puberty or pregnancy.
- A more dangerous illness called Sjogren's syndrome, an autoimmune systemic condition that causes xerostomia, can frequently develop from periodontitis.
- Pneumonia: Pathogens found in periodontal fluid are thought to be a risk factor for various illnesses.
- Genetic factors and periodontitis
People with Down syndrome, Chediak-Higashi syndrome, and Papillon-Lefevre syndrome are more susceptible to severe periodontitis. Periodontal disease, which also affects humoral immunity and cell-mediated immunity, is caused by a change in humoral immunity. In children with Chediak-Higiashi syndrome, the periodontal disease presents as early-onset periodontitis. The hallmark of Down syndrome is widespread, severe periodontitis. Early tooth loss is a Papillon-Lefevre symptom of periodontitis
Types Of Periodontitis [16-17]
i. Mild periodontitis
Teeth with gingivitis have a spongy, glossy, mushy, red, and enlarged surface. The bacteria in plaque produce toxins that irritate the gums. A patient with gingivitis will have a 3 mm pocket depth.
ii. Moderate periodontitis
More bone and tissue are lost, and deeper pockets occur as a result. probing pocket depth (PPD) of this kind is 5 mm, and bacteria or other pathogens cause tissue injury.
iii. Advances periodontitis
Up to 6 mm is the probing pocket depth (PPD) for this primary form of periodontitis. Additional tooth damage could cause them to be destroyed.
iv. Refractory periodontitis
Permanent tooth loss and damage occur in cases of periodontitis.
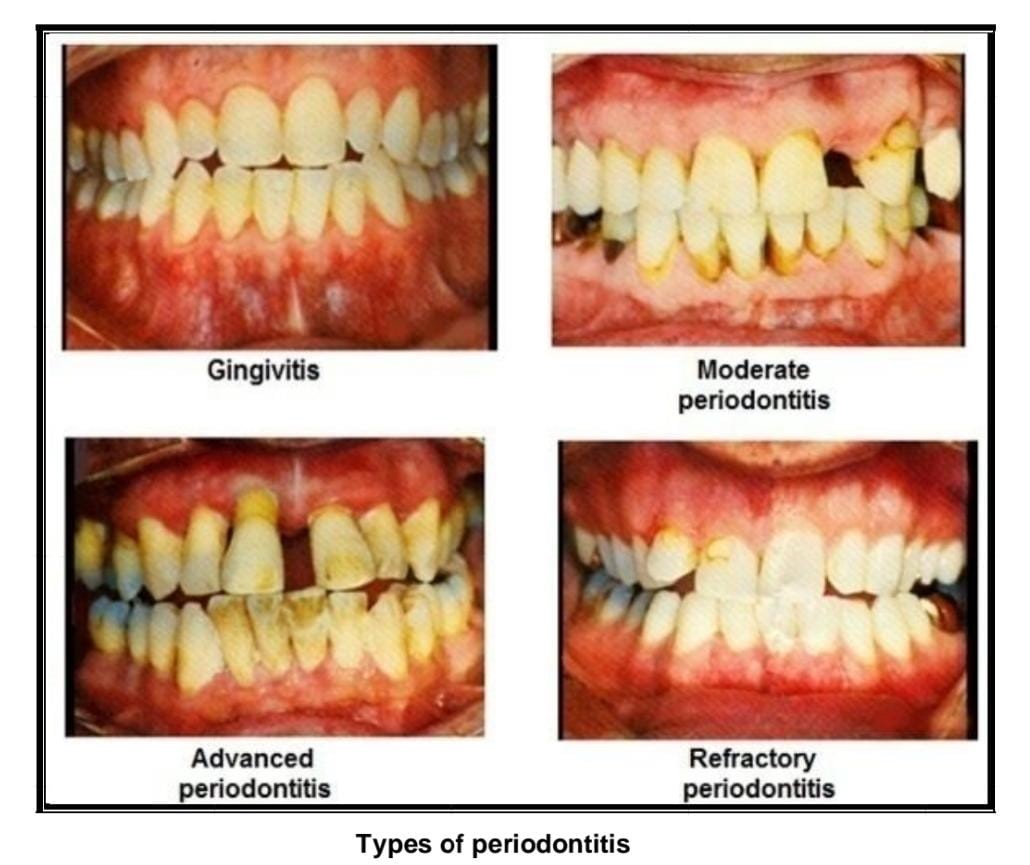
Fig No: 3 Types of periodontitis
Treatment
It's also crucial to remember that systemic antibiotic therapy has been shown to be clinically effective in treating superimposed infections, patient non-compliance, etc. Because periodontitis is the source of local pharmacological treatments, elevated levels of the toxic side effects of systemic administration of high dosages to the condition are considered a localized disease. [18,19] Because the drug's distribution in the periodontal pocket is typically not particularly obvious and the gingival sulcus flow swiftly removes the drug from the site of action, treating periodontitis is extremely difficult. For example, 40 times an hour, material may be replenished in a periodontal pocket that is 5 mm deep.[20] Currently, scaling and root planning the mechanical removal of bacteria are the mainstays of conventional nonsurgical periodontal therapy. Even though the patient's pocket geometry may not be conducive to total excision, it nevertheless causes discomfort for the patient, and in some situations, infections return to cavities following treatment. It has been suggested that using various antimicrobial treatments in conjunction with root planning can reduce the likelihood of pathogenic germs reoccurring.[21] The correct delivery of this medication is a significant difficulty in its therapy. Multiple dosages cause bacterial resistance, and systemic delivery exposes medications to the entire organ, resulting in unwanted side effects. Mouthwash prevents periodontal pockets from receiving adequate medication concentrations. The most effective strategy at the moment is thought to be local drug delivery systems, which release the active ingredient at the site of action at a regulated pace over a predefined amount of time. Easy delivery, adequate retention at the administration site, and ideally controlled drug release are all characteristics of the perfect drug delivery system.[22]
Symptoms [23]
In comparison to early gum disease, periodontitis is a more advanced stage of periodontal disease and might present with more severe symptoms. These are a few common signs:
i. Deepening Gum Pockets
A dentist can identify this during an examination: a deeper gap between your teeth and gums.
ii. Gum Recession
More gum recession, revealing more of the tooth's surface or perhaps the root.
iii. Loose Teeth
Teeth that seem unsteady or loose because the gums and supporting bone have been lost.
iv. Persistent Bad Breath
Increased severity and persistence of foul breath that is unabated by good mouth hygiene.
v. Pain While Chewing
Gum disease can make chewing painful or uncomfortable.
vi. Gum Pockets
Infection may develop in the pockets or gaps that develop between the teeth and gums.
vii. Change in Bite
Noticeable alterations in your teeth's bite fit, frequently brought on by teeth shifting.
viii. Gum Pain or Sensitivity
Increased pain or sensitivity in the gums, which can become more intense as the condition progresses.
ix. Pus Between Teeth and Gums
An infection is indicated by the presence of pus or an unpleasant taste emanating from the pockets surrounding the teeth.
In-Situ Gelling System
The ability to provide precise and repeatable amounts in contrast to gel that has already formed is of utmost importance. Easy administration and lower administration frequency are two benefits of the in-situ forming polymeric delivery method that enhance patient comfort and compliance. The usage of gel systems that are injected locally can help overcome the poor bioavailability and therapeutic response that conventional dosage forms demonstrate because of the drug's quick metabolism and excretion.[24] In situ medication delivery devices present an intriguing opportunity to get over this significant barrier. Easily injected into the periodontal pocket, these liquid preparations solidify to create a gel with a specific geometry after the solvent is replaced.[25] Under non physiological circumstances, the gel in situ stays in the form of a solution; under physiological conditions, it transforms into a gel in response to stimuli like pH, temperature, ions, and the solvent that is present in the mouth cavity.[26,27] In situ gel lowers side effects and increases patient compliance by delivering the drug release at a regulated pace straight to the target spot.[28] It seems that in situ forming gel is a promising option for satisfying the needs of both high adhesiveness and low viscosity. Injectable One especially efficient medication delivery method is in situ gel. The in situ forming system is a sol before administration, and it turns into a gel or solid depot as it is gradually supplied. Because it can sustain high drug concentrations in the gingival crevicular fluid for extended periods of time in order to achieve the intended clinical advantages, in situ gel is one of the most promising local drug delivery technologies. The success of using in situ gels to treat periodontitis depends on the phase transition from the fluid to the gel. The in-situ gel gelling technology has a number of benefits, such as simple dosage form applications, less frequent administration, and even drug protection against environmental changes. In-situ gel formation is a process that can be used to ocular, transdermal, buccal, intraperitoneal, parenteral, injectable, rectal, and vaginal routes for a variety of natural and synthetic polymers.[29,30] There are three approaches: ion-activated systems, pH-triggered systems, and thermally-triggered systems. To improve product retention in the periodontal pocket, use polymers such as mucoadhesive polymers to make the solution more viscous. The ability of the mucoadhesive polymer to interact with the gingival crevicular fluid found on the tooth's surface is essential.[31]
Advantages Of In Situ Gel [32,33]
- Ease of administration
- Increased residence time.
- Low dose is required for treatment.
- Improved bioavailability.
- Improved patient compliance and comfort.
- Minimum local and systemic side effects.
- Reduced frequency of drug administration.
- It can also be administered to unconscious patient.
Disadvantages Of In Situ Gel
- It requires high level of fluids.
- It leads to degradation due to storage problems.
- It is more susceptible to stability problems due to chemical degradation.
Limitation Of In Situ Gel [34]
- For local irritants is not useful.
- It should little area the dose is to small.
- The patient having erythema or other diseases they can't administered by this route.
- It should be avoided of irritancy or a sensitization.
Ideal Characteristics of a Suitable Drug Candidate [35]
- The drug should have good stability and solubility in water.
- It should have ability to permeate the oral mucosal tissue.
- The drug should have smaller and moderate molecular weight.
- The drug should have pleasant taste.
- It should be partially unionized at the pH of oral cavity.
Ideal Properties Of Polymers For In-Situ Gels [35]
- It should have good shelf life.
- It should have a good mouth feel property.
- The polymer should exhibit sufficient peel, shear and tensile strengths.
- The polymer should be readily available and should not be very expensive.
- It should have good wetting and spreadability property.
- It would be ideal to have a polymer that would have local enzyme inhibition action along with penetration enhancing property.
Various Mechanisms For In Situ Gel
i. Stimuli responsive in situ gel [36,37]
In this mechanisms solution covert to gel like structure after physiological condition is change like temperature, pH and ion. This type of gel is called stimuli responsive in situ gel.
- Temperature triggered in situ gel systems
These systems are injectable liquids that can be administered minimally invasively to the body and then solidify in the organ, tissue, or bodily cavity of choice . The idea pertains to the creation of mucoadhesive formulations that include a temperature-activated gel transition polymer solution at temperatures between 25 and 37 °C. At body temperature, the polymers that have a low critical solution temperature of about 32 °C go through a phase change.[38]
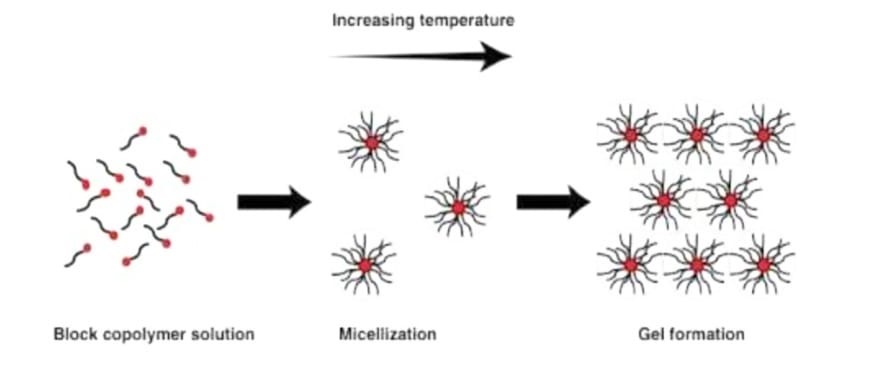
Fig. 4: Mechanism of Temperature induced in situ gel systems.
pH triggered in situ gelling systems
The class of pH-sensitive polymers includes any polymer with an acid or basic group. These polymers undergo polymerization as a result of proton acceptance or release when exposed to varying physiological and ambient pH levels. A shift in physiological pH causes the solution-gel transition, which results in in situ gel.[39]

Fig 4 : pH triggered system.
- In situ gel formation due to ion-activated system
Ionic stimulation causes these in-situ gelling systems to convert from solution to gel. It comprises an ion-sensitive polymer that gels in the body's natural setting. The most often utilized ion-sensitive gelling agents for in-situ gelling systems are gellan gum, alginate, and pectin. They cause cationic complexation and the creation of a three-dimensional network structure by interacting with cations found in the physiological fluid, such as Na+, K+, Ca++, Mg++, etc. Because the periodontal pocket is rich in cations like Ca++, cationic complexation and polymeric interaction occur when formulations containing these kinds of polymers are present, leading to a solution to gel transition.[40-42]
ii. In situ gel formation due to solvent exchange [43]
Because of the solvent exchange with the surrounding aqueous environment, the gel solidifies.
- Swelling
One of the key processes for creating in situ gel is the substance's absorption of water from the surrounding environment, which causes the material to expand to fill the required space.
- Diffusion:
In situ gel formation, which is based on the diffusion method, precipitates the polymer matrix because solvent diffuses from the polymer to surrounding tissue. Diffusion is a more accurate and dependable mechanism of drug release from the system than erosion. Diffusion is the movement of atoms, ions, and molecules from a region of higher concentration to a region of lower concentration and is driven by a concentration gradient. The porosity of the polymer matrix, which ultimately depends on the pore formation process during the diffusion process, reverse phase. The diffusion of the medication takes place during the coagulation of the polymeric solution.
iii. Chemically induced in situ gel [44]
- Ionic crosslinking
In this type of method ionic strength is responsible for converting in to gel like structure.
- Enzymatic crosslinking
Also in situ gel structure depend up on enzymatic activity like glucoseoxidase. In this method enzymes are catalysed in to the body and convert into gel like structure.
- Photo polymerization
In this method radiation, UV rays, X rays, are also responsible for convert in to solution in to gel like structure.
Polymers Commonly Used For Periodontal In-Situ Gelling Systems
Carbopol
Carbopol is a polymer comprising acrylic acid and cross-linked poly alkyl esters of sugars or polyalcohols, according to the European Pharmacopoeia. The carboxyl (-COOH) groups in this polymer range from 56.0% to 68.0%, depending on the dried material. During hydration and neutralization, carbomers swell and produce colloidal dispersions that appear viscous despite not being soluble in deionized water. [45] It is well-known pH-dependent polymer, carbopol remains in solution at acidic pH values but gels with low viscosity at alkaline pH values. HPMC and carbopol are combined to give the carbopol solution viscosity while lowering the solution's acidity. The group of water-soluble polymers known as pH-induced in-situ precipitating polymeric systems includes the carbopol system-hydroxy propylmethyl cellulose system and the poly (ethylene glycol)-poly (methacrylic acid).[46]
Hydroxypropyl methylcellulose (HPMC)
HPMC is a member of the class of cellulose ethers that have had one or more hydroxyl groups in the cellulose ring swapped out for hydroxyl groups. Deionized water dissolves HPMC, a hydrophilic polymer. It has numerous uses in medication delivery and is also biodegradable and biocompatible. HPMC is a white, yellowish-white, or greyish-white powder or granule. After drying, it might take in moisture from the surrounding air. Acetone, anhydrous ethanol, toluene, and hot deionized water do not dissolve HPMC. Nevertheless, it can form a colloidal solution when dissolved in cold deionized water.[47]
Methylcellulose (MC)
MC is a methyl ester of cellulose that has between 27.5 and 31.5% methoxy groups. The aqueous solution of MC has thermal gelation capabilities and is soluble in deionized water.[48]
Pectin
Pectins are a family of polysaccharides that mostly consist of ?-(1-4)-Dgalacturonic acid residues in their polymer backbone. In aqueous solution, low methoxypectins easily gel when free calcium ions are present. This crosslinking of the galacturonic acid chains is explained by the egg-box concept. In order to create gels that are appropriate for drug delivery, calcium ions are typically needed, even though pectin will gel when H + ions, a source of divalent ions, are present. Pectin's water solubility eliminates the need for organic solvents in these formulations, which is its primary benefit. When taken orally, divalent cations in the stomach facilitate the conversion of pectin to a gel form.[49,50]
Chitosan
Chitosan is a polycationic, biodegradable, and thermosensitive polymer that is produced by alkaline deacetylation of chitin, a naturally occurring substance found in crab and shrimp shells. As a pH-dependent cationic polymer that is biocompatible, chitosan dissolves in aqueous solutions up to 6.236. When the chitosan aqueous solution is neutralized to a pH higher than 6.2, a hydrated gel-like precipitate forms. The addition of polyol salts with a single anionic head, such as glycerol, sorbitol, fructose, or glucose phosphate salts, to chitosan aqueous solution converts the pH gelling cationic polysaccharides solution into thermally sensitive pH dependent gel forming aqueous solutions without any chemical modification or cross linking.[51,52]
Poloxamer
Poloxamers, which are nonionic triblock copolymers made up of two hydrophilic chains of polyepoxyethane on either side of a core hydrophobic chain of polyepoxypropane, are frequently employed in research on drug delivery systems.[53,54] There are numerous uses for the non-ionic surfactant polxamer 407. BASF Laboratories has registered it under the Pluronic® F127 brand, whereas ICP Laboratories has registered it under the Synperonic PE/F127® trademark. Poloxamer 407 is recognized by the U.S. Food and Drug Administration (FDA) as a "inactive ingredient" in a number of pharmaceutical preparations, including topical, ophthalmic, intravenous (IV), inhalation, suspension, and oral solutions.It is a nonionic surfactant with good solubility, low toxicity, and good drug release properties that is compatible with cells, bodily fluids, and a wide range of substances. '[55] Poloxamer 188, also known as Pluronic F-68 or Flocork, is a nonionic block copolymer that contains segments of polypropylene oxide and polyethelyene oxide organized in an ABA structure. Despite having the same chemical structure, poloxamers differ in the quantity of poly (propylene oxide) and poly (ethylene oxide) units they contain, which causes a variance in their molecular weight. Hypromellose, methyl hydroxypropyl cellulose, propylene glycol ether of methylcellulose, and methylcellulose propylene glycol ether are other names for hydroxypropyl methylcellulose (HPMC).
The formula for this substance is C8H15O6-(C10H18O6)-C8H15O5.[56,57]
Gellangum
An anionic deacetylated exocellular polymer, gellan gum (marketed as Gelrite TM or Kelcogel TM) is secreted by Pseudomonas elodea and contains a tetrasaccharide repeating unit consisting of one ?-L-rhamnose, one ?-D-glucuronic acid, and two ?-D-glucuronic acid residues. It has a propensity to gel, which can be caused by cations or temperature. Following the creation of double helical junction zones, the double helical segments aggregate to create a three-dimensional network through complexation with cations and hydrogen bonding with water. A gellan solution with a calcium chloride and sodium citrate complex made up the composition.[58,59]
Xanthum gum
Xanthomonas campestris is a gram-negative bacterium that ferments to create xanthan gum, a high molecular weight extracellular polymer. A cellulosic backbone (?D-glucose residues) and a trisaccharide side chain of ?-D-mannose-?-D-glucuronic acid-?-D-mannose are connected to the main chain's alternating glucose residues in the fundamental structure of this naturally occurring cellulose derivative. This polymer's anionic nature results from the side chain's existence of both pyruvic and glucuronic acid groups.[60]
Xyloglucan
Tamarind seeds are the source of xyloglucan, a polysaccharide made up of a (1-4)-?-D-glucan backbone chain with (1-6)-?-D xylose branches that are partially replaced by (1-2)-?-D-galactoxylose. The product of ?-galactosidase's partial degradation of xyloglucan shows thermally reversible gelation through the lateral stacking of the rod-like chains. The degree of galactose removal affects the sol-gel transition temperature. When it warms up to body temperature, it creates thermally reversible gels.[61,62]
Alginic acid
The linear block copolymer polysaccharide alginic acid is made up of residues of ?-D-mannuronic acid and ?-L-glucuronic acid connected by 1,4-glycosidic bonds. The algae source affects the percentage of each block and how the blocks are arranged along the molecule. When divalent and trivalent metal ions are added to diluted water solutions of alginates, the alginate chain's ?-L-glucuronic acid blocks' successive glucuronic residues work together to generate solid gels.[63]
Evaluation Of In-Situ Gelling System
Clarity
The in situ gel's clarity is usually evaluated visually. To look for particles or cloudiness, the gel is examined under black and white background. In situ gel formulations require clearness testing to ensure quality, efficacy, transparency, purity, and physical stability as well as to confirm the gel's applicability for a range of applications.[64]
pH
Measurement of pH was done by using a digital type pH meter by dipping the electrode completely into the gel so as to cover the electrode.[65]
Viscosity
Various viscometers, such as the Brookfield viscometer, cone, and plate viscometer, can be used to measure the viscosity and rheological characteristics of the polymeric formulations, whether they are in solution or in gel formed with artificial tissue fluid. These formulations' viscosity should be such that patients comply with them.[65]
Gel strength
A rheometer can be used to assess this parameter. A certain amount of gel is made in a beaker from the sol form, depending on the gelling agent's process. A probe is pushed slowly through the gel because the beaker containing the gel rises at a specific rate. The probe's depth of immersion below the gel surface can be used to measure changes in the load on the probe.[66]
Spreadability
One glass plate (10 x 10 cm) was carefully covered with another glass plate after 1 gram of gel had been weighed and placed in the middle. The plate was centered with a 2 kg weight, and caution should be used to prevent the plate from moving. Following 30 minutes, the gel's diameter was measured in centimeters.[66]
Drug content
1 ml of each formulation taken in 10 ml volumetric flask, diluted with distilled water and make up the volume to 10 ml. 1ml solution was taken and diluted to 10 ml with distilled water. Measured the absorbance of final solution using double beam UV visible spectrophotometer. [66]
Sol-gel transition temperature and gelling time
For In-Situ gel forming systems, the sol-gel transition temperature and pH should be determined. Gelling time is the time required for the first detection of gelation of in-situ gelling system. Thermo sensitive in-situ gel should be checked for in-situ gelling at body temperature.[66]
In vitro drug release studies
The in-vitro drug release study of from in-situ gel was determined by using a Franz-diffusion cell. The cellophane membrane was set on the receptor cell. The donor cell was packed with 1 g of in-situ gel formulation. The receptor compartment was filled with simulated salivary fluid pH 6.8 and constantly stirred using magnetic stirrer at a speed of 200-250 rpm throughout the experiments to prove homogeneity. The temperature was maintained at 37± 1°C by circulating hot water through the jacket of Franz-diffusion cell. 1 ml of sample was withdrawn at scheduled time intervals of 1hr and was replaced with same volume of pH 6.8 simulated salivary fluids to maintain the sink condition. Samples were analysed UV-visible spectrophotometer.[66]
Texture analysis
The firmness, consistency and cohesiveness of formulation may be determined using texture analyzer which mainly indicates the syringe ability of sol so the formulation can easily administered in-vivo.[67]
Accelerated stability studies
Formulation is replaced in amber colored vials and sealed with aluminum foil for the short term accelerated stability at 40?±20?C and 75±5% RH as per ICH state guidelines.[67]
CONCLUSION
In conclusion, in situ gel systems represent a highly promising and novel strategy for the management of periodontitis. By combining the benefits of sustained drug release, localized treatment, and improved patient compliance, these gels offer significant advantages over traditional therapeutic approaches. Their ability to deliver drugs directly to the affected periodontal tissues ensures enhanced therapeutic efficacy and minimal systemic side effects. Moreover, the customization of gel formulations for different drugs and periodontal conditions opens new avenues for personalized treatment options. While further clinical trials and research are needed to fine-tune their application, in situ gels stand as a transformative approach in periodontitis therapy, offering potential for better long-term outcomes in periodontal health management.
REFERENCES
- Chavan P et al. Nasal drug delivery system: a review. World J Pharm and Pharma Sci., 2014; 3(12): 598-617.
- Ramya DD et al. In-Situ Gelling System – Potential Tool For Improving Therapeutic Effects Of Drugs. Int J Pharm And Pharma Sci., 2013; 5(3): 27-30.
- Bajpai V. In situ Gel Nasal Drug Delivery System – A Review. Int J Pharma Sci., 2014; 4: 577-580.
- Aikawa K et al. Drug release from pH-response polyvinylacetal diethyl aminoacetate hydrogel and application to nasal delivery. Int J Pharm, 1998; 168: 181-8.
- Almeida H et al. In situ gelling systems: a strategy to improve the bioavailability of ophthalmic pharmaceutical formulations. Drug Discov Today, 2014; 19: 400–412.
- 6. Al-Shamklani A et al. Evaluation of the biological properties of alginates and gellan and xanthan gum. Proc Int Symp Control Release Bioact Mater, 1991; 18: 2134.
- 7. Bhardwaj TR et al. Natural gums and modified natural gums as sustained release carriers. Drug Devel Ind Pharm, 2000; 26: 1025-38.
- Burkoth AK, Anseth KS. A review of photocrosslinked polyanhydrides: In situ forming degradable networks. Biomaterials, 2000; 21: 2395404.
- Chand P et al. In situ gel: A Review. Indian J. Pharm. Biol. Res., 2016; 4(2): 11-19.
- Chenite A et al. Novel injectable solution of chitosan form biodegradable gels in situ. Biomaterials, 2000; 21: 2155-61.
- Novak John, Classification of Disease and condition affecting the periodontium, Chapter-4 in Clinical Periodontalogy, Newman, Takri, ninth edition, Harcount Pvt. Ltd., New Delhi, 2003, 64- 72.
- Carranza FA, The Periodontal pocket, in; Fermin, A. Carranza, and Michael, G. Newman, Clinical Periodontology, 8 Edition, Prism Books Pvt. Ltd., Bangalore, India, 1996, 2814.
- Neha bisht, Laxmi goswami, Preeti kothiyal. In situ gel: A study of dental disease. Indian Journel of Novel Drug Delivery, 2014; 6(3): 208-214.
- Novak John, Classification of Disease and condition affecting the periodontium, Chapter-4 in Clinical Periodontalogy, Newman, Takri, ninth edition, Harcount Pvt. Ltd., New Delhi, 2003, 64-72
- Dr.Pandit JK. Targeted Devices for Periodontal disease, Chapter-6 in. controlled and novel drug delivery, Jain MK. First edition, C.B.S. Publishers and Distribution, New Delhi, 1997, 130-146.
- Loe H. Theilade E, and Jensen SB. Experimental gingivitis in man. J.Periodontal, Res., 1965, 36,177.
- Socransky SS, Microbiology of periodontal disease present status and Future considerations. J. Periodontal, 1977, 48, 497.
- WHO, Epidemiology, Etiology, Prevention of periodontal disease, 1978. Technical Report Series No. 621.
- 37. Jain N, Jain GK, Javed S, Iqbal Z, Talegaonkar S, Ahmad FJ. Recent approaches for the treatment of periodontitis. Drug Discov Today. 2008;13:932-943.
- Phaechamud T, Thurein SM, Chantadee T (2018) Role of clove oil in solvent exchange-induced doxycycline hyclate-loaded Eudragit RS in situ forming gel. Asian J Pharma Sci 13(2):131-142.
- AlAhmari F, Ahmed HB, Al-Kheraif AA, Javed F, Akram Z (2019) Effectiveness of scaling and root planning with and without adjunct antimicrobial photodynamic therapy in the treatment of chronic periodontitis among cigarette-smokers and never-smokers: a randomized controlled clinical trial. Photodiagn Photodyn Ther 25:247–252.
- Sah AK, Dewangan M, Suresh PK (2019) Potential of chitosan-based carrier for periodontal drug delivery. Colloids Surf B Biointerfaces 178:185–198.
- Borderwala K, Patel S, Gandhi J. Naik H. Pillai L, Shah P. Advanced local drug de- livery approaches for periodontitis: a strategic intervention. Res Rev J Pharmaceut Sci. 2008;9:4-11.
- Fakhari A, Subramony JA. Engineered in-situ depot-forming hydrogels for intratumoral drug delivery. J Cont Release 2015; 220: 465–475.
- Do M, Neut C, Metz H, Delcourt E, Mäder K, Siepmann J et al (2015) In-situ forming composite implants for periodontitis treatment: how the formulation determines system performance. Int J Pharm 486(1-2):38–51.
- Agossa K, Lizambard M, Rongthong T, Delcourt-Debruyne E, Siepmann J, Siepmann F (2017) Physical key properties of antibiotic-free, PLGA/HPMCbased in-situ forming implants for local periodontitis treatment. Int J Pharm 521(1-2):282–293.
- Ranjan R, Patil SR, Veena H R (2017) Effect of in-situ application of simvastatin gel in surgical management of osseous defects in chronic periodontitis-a randomized clinical trial. J Oral Biol Craniofac Res 7(2):113-118.
- Kassem AA, Ismail FA, Naggar VF, Aboulmagd E (2014) Comparative study to investigate the effect of meloxicam or minocycline HCl in situ gel system on local treatment of periodontal pockets. AAPS PharmSciTech 15(4):1021–1028.
- Dr.Prakash Supra, Dr. Dhaval Patel, Dr Moinuddin Soniwala, Dr.Jayant Chavda. "Development And optimization of In Situ Periodontal Gel For the treatment of Periodontitis. Journal of Scientific and Innovative Research 2013; 2(3) 608-627.
- Kevin Garala, Parth Joshi Malay shah, Jaydeep Patel "Formulation and Evaluation Of Periodontal In situ gel. Published by International Journal of Pharmaceutical Investigation January 2013 Volume 3 Issue 1.
- Khule MR Vyavahare SB. A review: In situ drug delivery system. Int. J Res Education and scientific methods. 2021; 9(3):899-909.
- Guo JH et al. Pharmaceutical applications of naturally occurring water-soluble polymers. Pharm Sci & Technol Today, 1998; 1: 254-61.
- Haffajee AD, Socransky SS. Attachment level changes in destructive periodontal diseases. J Clin Periodontol, 1986; 13: 46-175.
- Bansal M, Mittal N, Yadav SK, Khan G. Gupta P. Mishra B. Periodontal thermo- responsive, mucoadhesive dual antimicrobial loaded in-situ gel for the treatment of periodontal disease: preparation, in-vitro characterization and antimicrobial study. J Oral Biol Craniofac Res. 2017:5:2- 12 2017.
- Sanheeta Chakrabarty, Bipul Nath. Oral In Situ Gel For Periodontitis: A Review. World Journal And Pharmaceutical Research. 2018; 7(11):262-275.
- Padmasri BU, Nagaraju RA, Prasanth DA. A comprehensive review on in situ gels. Int. J.Appl pharm 2020;129(6):24-33.
- Parvathy S, Arun Unnikrishnan, Oshin P. George and Sreeja C. Nair. Thermoreversible- pH sensitive cephalexin insitu gel for treating periodontal disease. J. Chem. Pharm. Res., 2015; 7(5): 555-567.
- Ruel-Gariepy E, Leroux J-C (2004) In situ-forming hydrogels—review of temperature-sensitive systems. Eur J Pharm Biopharm 58(2):409–426.
- Devasani SR, Dev A, Rathod S, Deshmukh G (2016) An overview of in situ gelling systems. Pharmaceut Biolog Evaluat 3(1):60–69.
- Pandey M, Choudhury H, Aziz ABA, et al. Potential of stimuli-responsive in situ gel system for sustained ocular drug delivery: Recent progress and contemporary research. Polymers. 2021;13(8).
- Agrawal M, Saraf S, Saraf S, et al. Stimuli-responsive In situ gelling system for nose-to-brain drug delivery. Journal of Controlled Release. 2020;327(July):235-265.
- Patel KS, Vadalia KR, Patel JK. Development and evaluation of in situ gelling system for treatment of periodontitis. International Journal of PharmTech Research. 2014;6(7):2102-2112.
- Devasani SR, Dev A, Rathod S, Deshmukh G (2016) An overview of in situ gelling systems. Pharmaceut Biolog Evaluat 3(1):60–69.
- Snehal Nikode, Gauri Dixit, Kanchan Upadhya. Insitu gel: Application and uses of polymers. WJPPS, 2016; 5(7): 1638-16.
- Benet LZ, Broccatelli F, Oprea TI. BDDCS applied to over 900 drugs. AAPS Journal. 2011;13(4):519-547.
- Vyas SP et al. Controlled and targeted drug delivery strategies towards intra-periodontal pocket diseases. J Clin Pharm Ther, 2000; 25: 21-42.
- Deshmukh K, Basheer Ahamed M, Deshmukh RR, Khadheer Pasha SK, Bhagat PR, Chidambaram K. Biopolymer Composites with High Dielectric Performance: Interface Engineering. Elsevier Inc.; 2017.
- Brady J, Drig T, Lee PI, Li JX. Polymer Properties and Characterization.; 2017.
- Pagar SA et al. A Review on Intranasal Drug Delivery System. J Adv Pharm Educ & Res., 2013; 3(4): 333-346.
- Podual K et al. Dynamic behavior of glucose oxidase-containing microparticles of poly(ethylene)- grafted cationic hydrogels in an environment of changing pH. Biomaterials, 2000; 21: 1439-50.
- Suisha F et al. Xyloglucan gels as sustained release vehicles for intraperitoneal administration of mitomycin C. Int J Pharm, 1998; 172: 27-32.
- Swamy NGN, Zaheer A. Mucoadhesive In situ Gels as Nasal Drug Delivery Systems: An Overview. Asian J Pharma Sci., 2012; 7(3): 168-180.
- Chen Y, Lee JH, Meng M, et al. An overview on thermosensitive oral gel based on poloxamer 407. Materials. 2021;14(16).
- Braun S. Encapsulation of Cells (Cellular Delivery) Using Sol-Gel Systems. Vol 4. Elsevier Ltd.; 2011.
- Giuliano E, Paolino D, Fresta M, Cosco D. Mucosal applications of poloxamer 407-based hydrogels: An overview. Pharmaceutics. 2018;10(3):1-26.
- Moghimi SM, Hunter AC, Dadswell CM, Savay S, Alving CR, Szebeni J. Causative factors behind poloxamer 188 (Pluronic F68, FlocorTM)- induced complement activation in human sera. A protective role against poloxamer-mediated complement activation by elevated serum lipoprotein levels. Biochimica et Biophysica Acta - Molecular Basis of Disease. 2004;1689(2):103-113.
- Carvalho GC, Araujo VHS, Fonseca-Santos B, et al. Highlights in poloxamer-based drug delivery systems as strategy at local application for vaginal infections. International Journal of Pharmaceutics. 2021;602(April).
- Riva R et al. Chitosan and chitosan derivatives in drug delivery and tissue engineering. In: Jayakumar R, Prabaharan M, Muzzarelli RAA. Chitosan for biomaterials II. Berlin: Springer-Verlag Berlin, 2011; 19–44.
- Sawhney AS et al. Photopolymerizable biodegradable hydrogels as tissue contacting materials and controlled release carriers. US Patent 5410016. 1995.
- Soppimath KS et al. Stimulus-responsive “smart” hydrogels as novel drug delivery systems. Drug Dev Ind Pharm, 2002; 28: 957-74.
- Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev., 2001; 53: 321-39.
- Rehaman S et al. Site Specific Delivery System for the treatment of Periodontitis. Ind J Pharma Sci March-April, 2003; 106 – 112.
- Sechoy O et al. A new long acting ophthalmic formulation of carteolol containing Alginic acid. Int J Pharm, 2000; 207: 109-16.
- Parekh H, Jivani R, Jivani N, Patel L, Makwana A, Sameja K. Novel in situ polymeric drug delivery system: A review. J Drug Deliv Ther 2012;2:136?45.
- Miyazaki S, Kawasaki N. Comparison of in situ gelling formulations for the oral delivery of cimetidine. Int J Pharm, 2001; 220: 161-8.
- Rahana P. V, Dr. Sujith S. Nair.''Formulation and Evaluation of in situ gel for the Treatement of Perodontal Desease. World Journal Of Pharmacy And Pharmaceutical Science.2018 7(11) 1306-1319.
- Wang XQ et al. In situ gel-forming system: an attractive alternative for nasal drug delivery. Crit Rev Ther Drug Carrier Syst, 2013; 30: 411-434.
- Abdulla R. Abdulla, Mohanad A. Alfahad, Zeyad A. Hameed. In-situ Gelling System: A Promising Delivery Method for Treating Periodontitis. Iraqi Journal of Pharmacy. 2024 21(3) 89-101.


 Darshad Rane*
Darshad Rane*
 Rashmi Mahabal
Rashmi Mahabal
 Namita Bhosale
Namita Bhosale
 Vijay Jagtap
Vijay Jagtap





 10.5281/zenodo.14551009
10.5281/zenodo.14551009