Abstract
The aim of this article is to focus on buccal patches. Buccal route found to be more suitable for the delivery of pharmaceutical agents using mucoadhesive polymers due to presence of relative lystatic and smooth surface on which various mucoadhesive dosage forms can be placed. The mucosa of buccal cavity is the most easily accessible transmucosal site delivery help to bypass first-pass metabolism by allowing direct access to systemic circulation. Buccal patch has been become an interesting area of novel drug delivery system as the dosage forms designed for buccal administration should not cause irritation and should be small and flexible enough to be accepted by the patient. Buccal delivery medication gives a convenient route of administration for both local and systemic effects. This presents a brief description the study of buccal patches and include its introduction, types of buccal patches, advantages, limitation, anatomical structure of oral mucosa, potential uses of buccal patches, methods of preparation, evaluation.
Keywords
buccal patches Mucoadhesion, Buccal mucosa and Polymers NDDS
Introduction
The buccal patch is the non-dissolving modifying released dosage form that is placed between the cheek and upper gums to deliver drug into the oral cavity or submucosal layers. Buccal Patches are used to treat local and systemic condition. they can be useful when a medicine needs to take effect quickly or when the patient is unconscious.
Mucoadhesion has gained popularity over the years due to its potential to improve localized drug delivery by keeping a dosage form at the site of action (e.g., By keeping the formulation in close contact with the absorption site (e.g., within the gastrointestinal tract), the formulation can be administered systemically. G. Cavity in the mouth. A well-defined definition of bio adhesion is a substance's propensity to stick for an extended period of time to biological tissues, whether it be synthetic or biological. Mucoadhesion is the term used to describe a phenomenon where adhesion is to a mucous coat. There are more applications for mucoadhesive polymers in buccal drug delivery. There are many mucoadhesive products that have recently been developed, including tablets films, patches, disks, strips, ointments and gels. But compared to ho the devices, buccal patches offer more comfort and flexibility [1,2]. buccal drug
The Structure Of The Oral Mucosa:
Structure
The oral mucosa is composed of an outermost layer of stratified squamous epithelium (Figure 1). Below
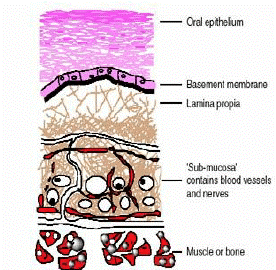
Figure 1: Structure of the Oral Mucosa
this lies a basement membrane, a lamina propria followed by the submucosa as the innermost layer. The epithelium is similar to stratified squamous epithelia found in the rest of the body in that it has a mitotically active basal cell layer, advancing through a number of differentiating intermediate layers to the superficial layers, where cells are shed from the surface of the epithelium. [2],[3]
Advantages of Buccal Patches:
1. The oral mucosa has a rich blood supply.Drugs are absorbed from the oral cavity through the oral mucosa, and transported through the deep lingual or facial vein, internal jugular vein and braciocephalic vein into the systemic circulation.
2. Buccal administration, the drug gains direct entry into the systemic circulation thereby bypassing the first pass effect. Contact with the digestive fluids of gastrointestinal tract is avoided which might be unsuitable for stability of many drugs like insulin or other proteins, peptides and steroids. In addition, the rate of drug absorption is not influenced by food or gastric emptying rate.
3. The area of buccal membrane is sufficiently large to allow a delivery system to be placed at different occasions, additionally; there are two areas of buccal membranes per mouth, which would allow buccal drug delivery systems to be placed, alternatively on the left and right buccal membranes.
4. Buccal patch has been well known for its good accessibility to the membranes that line the oral cavity, which makes application painless and with comfort.
5. Patients can control the period of administration or terminate delivery in case of emergencies.
6. The buccal drug delivery systems easily administered into the buccal cavity.
7. The novel buccal dosage forms exhibit better patient compliance.
Limitations:
i. The buccal route cannot be used to administer mucosa-bothering or strongly-flavored medications.
ii. Small dose medications can only be given.
iii. Saliva production is constant, causing drugs to be quickly eliminated.
iv. Little area for absorption.
v. Involuntary salivation gulping causes a sizable portion of the delivered drug to disintegrate or suspend and be removed from the site of retention. The delivery system itself could also be swallowed, which is another risk. [4-6]
vi. Small absorption area: the absorptive membrane is relatively small,and delivery system dimention can make the area smaller .
vii. Law drug concentration: The concentration of drugs at the surface of the absorbing membrane is law. [24]
Method Of Preparation:
Solvent casting
It involves dispersing all of the patch excipients, including the medication, in an organic solvent before coating the mixture on a sheet of release liner. The coated release liner sheet is laminated with a thin layer of protective backing material after the solvent has evaporated. This laminate is then die-cut into patches with the desired size and geometry. A boiling tube was filled with weighed-out HPMC E15. This was then mixed with 20 ml of the solvent solution (1:1 dichloromethane: methanol). An adequate amount of care was taken to avoid lump formation. To give the polymer time to swell, the boiling tube was left idle for six hours. Propylene glycol was added in a precise amount after swelling, and the mixture was vortexed. The final CPH amount was weighed out, and 5 ml of the solvent mixture was used to dissolve it. It was then added to the polymer solution and thoroughly mixed. It was transferred into an anumbra petriplate that had already been cleaned after being set aside for a while to let any trapped air escape. These patches were dried in an oven that was positioned over a flat surface for 8 hours. With no plasticizer added, the process is repeated for HP.
Direct milling
In this process, patches are made without the use of solvents. Direct milling or kneading, typically without the use of any liquids, is used to mechanically combine the drug and excipients. The final product of the mixing process is rolled on a release liner until the desired thickness is reached. Following that, the backing material is laminated as before. [7-8]
Potential benefits of buccal patches:
1. Buccal films provide large surface area that leads to rapid disintegration and dissolution in the oral cavity due to which it promotes the systemic absorption of Active pharmaceutical ingredient.
2. No need of chewing and swallowing.
3. No risk of choking.
4. The film increases the systemic bioavailability of the drugs, as it bypasses the hepatic first pass metabolism.
5. Drug can be protected from degradation by GI enzymes and the acidic environment.
6. Rapid onset of action and minimum side effects.
7. Self-administration is possible.
8. Accurate dosing compared to liquid dosage forms.
9. Taste masking is possible.
10. Prolongs the residence time of the dosage form at the site of absorption, hence increases the
bioavailability.
11. Ease of administration to paediatric, geriatric patients, and also to the patients who are mentally
retarded, disabled or non-cooperative.
13.As a result of the large surface area that buccal films offer, the active pharmaceutical ingredient quickly disintegrates and dissolves in the oral cavity, promoting systemic absorption.
14.There is no need to swallow or chew.
15. No chance of choking.
16. Because the hepatic first pass metabolism is not affected, the film increases the drugs' systemic
bioavailability.
17. By GI enzymes and the acidic environment, drugs can be shielded from degradation.
18.Efficacy with a quick onset and few side effects.[9]
Bio adhesive Delivery of Drug system in Oral Cavity:
1)Sublingual Delivery: which is systemic delivery of drugs through the mucosal membranes lining the floor of the mouth.
2)Buccal Delivery: This is drug administration through the mucosal membranes lining the cheeks (buccal mucosa).
3) Local Delivery: for the treatment of conditions of the oral cavity, principally ulcers, fungal conditions and periodontal disease. These oral mucosal sites differ greatly from one another in terms of anatomy, permeability to an applied drug and their ability to retain a delivery system for a desired length of time. [6],[10],[11]
Physiological factors affecting buccal bioavailability inherent permeability of the -
Epithelium
The permeability of the oral mucosal epithelium is intermediate between that of the skin epithelium, which is highly specialized for barrier function and the gut, which is highly specialized for an adsorptive function.
Thickness of epithelium
The thickness of the oral epithelium varies considerably between sites in the oral cavity. The buccal mucosa measures approximately 500-800?m in thickness.
Blood Supply
A rich blood supply and lymphatic network in the lamina propria serve the oral cavity, thus drug moieties which traverse the oral epithelium are readily absorbed into the systemic circulation. The blood flow in the buccal mucosa is 2.4mL min-1.
Metabolic activity
Drug moieties absorbed via the oral epithelium are delivered directly into the blood, avoiding first-pass metabolism effect of the liver and gut wall. Thus, oral mucosal delivery may be particularly attractive for the delivery of enzymatically labile drugs such as therapeutic peptides and proteins. [12-14]
Saliva and mucous
The activity of the salivary gland means that the oral mucosal surfaces are constantly washed by a stream of saliva, approximately 0.5-2L per day. The sublingual area in particular, is exposed to a lot of saliva which can enhance drug dissolution and therefore increase bioavailability.
Saliva and mucus are both important substance in the body that serve different functions:
Mucus
Produced in the nose, mucus is a slimy material that coats mucosal surface. in following function:
- Trapping
- Humidifying
- Protecting
Saliva:
Produced in the salivary glands in the mouth, saliva helps with digestion and oral hygiene in following function:
- Protecting the mouth
- Moistening food
- Starting digestion [23]
Patches and film
Buccal patches consist of two laminates, with an aqueous solution of the adhesive polymer being cast onto an impermeable backing sheet, which is then cut into the required oval shape. A novel mucosal adhesive film called “Zilactin” consisting of an alcoholic solution of hydroxyl propyl cellulose and three organic acids. The film which is applied to the oral mucosal can be retained in place for at least 12 hours even when it is challenged with fluids. [15]
Evluation Of Buccal Films:
The buccal films are evaluated by the following parameters:
1) Surface PH of the film
For determination of surface pH, three films of each formulation are allowed to swell for 2 h on the surface of an agar plate. The surface pH is to be measured by using a pH paper placed on the surface of the swollen patch. A mean of three readings is to be recorded.
2) Swelling index
After determination of the original film weight and diameter, the samples are allowed to swell on the surface of agar plate kept in an incubator maintained at 37 ± 0.2ºC. The weight of the films (n=3) is determined at different time intervals (1-5 h). The percent swelling is calculated using the following equation:
Percent swelling [% S] = [Xt–Xo/Xo] ×100, Where, Xt=The weight of the swollen film
after time t, Xo= The initial film weight at zero time.
3) Moisture content
The prepared films are to be weighed individually and kept in a desiccator containing calcium chloride at room temperature for 24 h. The films are to be weighed again after a specified interval, until they show a constant weight. The percent moisture content is to be calculated by using following formula-
%Moisture content =[initial wieght -final weight / final weight ]
4) Moisture uptake
Weighed films are kept in desiccators at room temperature for 24 h. These are then taken out and exposed to 84% relative humidity using saturated solution of potassium chloride in desiccators until a constant weight is achieved. % moisture uptake is calculated as given below.
% Moisture uptake= [Final weight–Initial weight/Initial weight]×100
5) Drug-excipients interaction studies
The development of solid dosage forms requires studies of the interactions between drugs and excipients. Differential scanning calorimeters (DSCs), X-ray diffraction (XRDs), Fourier Transform Infrared Spectrum (FTIRs) and thin layer chromatography are all possible methods to assess potential drug excipient interaction studies. Because they display changes in melting endotherms and exotherms, changes in appearance, and variations in the corresponding reaction enthalpies, differential scanning calorimeters are used for quick evaluations of potential incompatibilities.
6) Physical evaluation
It is made up of three parts: content uniformity, weight uniformity, and thickness uniformity. By contrasting the average weight of ten patches from each batch that were chosen at random with the weight of a single patch, weight variation was evaluated. The film's thickness should be measured five times (at the centre and in each of the four corners), and the mean thickness should be computed. Samples that have air bubbles, nicks, tears, or a mean thickness variation of more than 5% are disqualified from analysis. Each formulation's three 20 mm-diameter patches were placed separately in 100 ml volumetric flasks with 100 ml of pH 6-point 8 phosphate buffer solution, which was then continuously stirred for 24 hours. Filtered, properly diluted, and then examined with a UV spectrophotometer, the solutions were. As a final reading, the average of three patches was used.
7) In vitro drug release
To examine the drug release rate from the bilayer and multi-layered tablets, the United States Pharmacopoeia (USP) XXIII rotating paddle method was used. Phosphate buffer with a pH of 6–8 is the dissolution medium. As 37°0°5°C and 50 rpm, the study was conducted. Instant adhesive (cyanoacrylate adhesive) was used to attach the buccal tablet's backing layer membrane to the glass disk. The dissolution vessel's bottom was given to the disc. 5 ml samples were taken out and fresh medium was added at regular intervals. After the appropriate dilution, the samples were filtered through Whatman filter paper and subjected to UV spectrophotometry analysis.
8) In vitro drug permeation
The in vitro buccal drug permeation study of Drugs through the buccal mucosa of sheep or rabbit is carried out at 37°C 0.2°C using Keshary-Chien or Franz type glass diffusion cells. It contains the donor and receptor compartments, both of which were tied with brand-new buccal mucosa. With its compartments clamped together, the buccal tablet's core side was facing the mucosa. [6, 16-19]. The methods currently used to study the dermato pharmacokinetics of drug substances may be broadly classified as in vitro models and in vivo studies. Considering the former category, results from in vitro permeation testing (IVPT) using human skin have been shown to correlate with clinical studies of skin uptake and bioequivalence.[22]
CONCLUSION:
There should be improvement in current treatment in case of safety and efficacy. Buccal drug delivery system bypasses the GI tract and hepatic portal system, increases bioavailability of drug, Patient compliance, though less permeable than the sublingual area, the buccal mucosa is well vascularised, and drug can rapidly be absorbed into the venous system underneath the oral mucosa, Lower inter subject variability than TDDS, the large contact surface of the oral cavity contributes to rapid and extensive drug absorption. Patches are gained importance in pharmaceutical areas due to novel, patient friendly and convenient product. Due to their small size and thickness, they have improved patient compliance, compared to tablets. Moreover, since mucoadhesion implies attachment to the buccal mucosa, patch can be formulated to exhibit systemic or local action. Due to the versatility of the manufacturing processes, the release can be oriented either towards the buccal mucosa or towards the oral cavity. Patch releasing drug towards the buccal mucosa exhibit the advantage of avoiding the first pass effect by directing absorption through the venous system that drains from the cheek. Buccal patch is a nondissolving thin matrix modified release dosage form composed of one or more polymer patch or layers, containing the drug and/or other excipients. The patch may contain a mucoadhesive polymer layer which bonds to the oral mucosa, for controlled release of the drug into the oral mucosa (unidirectional release), oral cavity (unidirectional release), or both (bidirectional release). The patch is removed from the mouth and disposed of after a specified time. [20,21].
REFERENCES
- Parmar HarshadG et al;" Buccal Patches : A Technical Note"; 2012; 029. Release , 1987;1(2):123– 131
- M.M.Veillard et al., “Preliminary Studies of Oral Mucosal Delivery of Peptide Drugs,” J. Controlled release , 1987;1(2):123– 131
- Y. Kurosaki et al., “Effects of Surfactants on the Absorption of Salicylic Acid from Hamster Cheek pouch as a Model of Keratinized Oral Mucosa,” Int. J. Pharm. 1988;4(7):13-19
- Shinde Pramod et al ;" Buccal Film : An innovative Dosage from Designed to Improve Patient complaince international journal of pharmaceutical and chemical sciences. 2012;1 (4):1
- Parmar HarshadG et al;" Buccal Patches : A Technical Note"; 2012; 029. Release , 1987;1(2):123– 131
- M.M.Veillard et al., “Preliminary Studies of Oral Mucosal Delivery of Peptide Drugs,” J. Controlled release , 1987;1(2):123– 131
- Y. Kurosaki et al., “Effects of Surfactants on the Absorption of Salicylic Acid from Hamster Cheek pouch as a Model of Keratinized Oral Mucosa,” Int. J. Pharm. 1988;4(7):13-19
- Shinde Pramod et al ;" Buccal Film : An innovative Dosage from Designed to Improve Patient complaince international journal of pharmaceutical and chemical sciences. 2012;1 (4):12
- Shinde Pramod et al ;" Buccal Film : An innovative Dosage from Designed to Improve Patient complaince, international journal of pharmaceutical and chemical sciences. 2012;1 (4):12
- Zhang.J et al, An In Vivo Dog Model for Studying Recovery Kinetics of the Buccal Mucosa Permeation Barrier after Exposure to Permeation Enhancers Apparent Evidence of Effective Enhancement without Tissue Damage, Int. J. Pharm, 1994:15–22.
- A.J. Hoogstraate et al., “Diffusion Rates and Transport Pathways of FITC-Labelled Model Compounds through Buccal Epithelium,” Proc. Int. Symp. Contr. Rel. Bioact.Mater. 1993;20:234–235
- Bobade Nishan N; "A Review on buccal drug delivery system, International Journal of Pharmacy and Pharmaceutical Science Research "; 2013 ; 3(1):21
- Bobade Nishan N; "A Review on buccal drug delivery system, International Journal of Pharmacy and Pharmaceutical Science Research "; 2013 ; 3(1):21
- A.H. Shojaei, Buccal mucosa as a route for systemic drug delivery: a review, J. Pharm. Pharmaceut. Sci., 1998;1(1): 15-30.
- SevdaSenel, Mary Kremer, Katalin Nagy and Christopher Squier, Delivery of Bioactive Peptides and Proteins Across Oral (Buccal) Mucosa, Current Pharmaceutical Biotechnology, 2001; 2: 175-186.
- S.K. Gupta, I.J. Singhavi, M. Shirsat, G. Karwani, A. Agrawal, A. Agrawal, Buccal adhesive drug delivery system: a review, Asian J. Biochemical and pharmaceutical research2011;2(1): 105-114.
- Amir H, et al, Systemic drug delivery via the buccal mucosal route, Pharmaceutical technology,2001:127
- Radha Madhavi et al;" Buccal film drug delivery system : An innovative and emerging technology-molecular pharmaceutics and organic process research";2013,1(3):19
- Chandra SK, Ramesh G, Vamshi VY, KishanV, Madhsudanrao Y. Development of mucoadhesive patches for buccal administration of prochlorperazine: Evaluation of in vitro release and mechanical properties: Int J Pharma Sci and Nanotech ;1(1): 64-70.
- P.K. Khobrgade et al ;" Literature studies on preparation and evaluation of buccal patches";2014;25(2):73.
- Naresh Kshirasagar et al;" Design and evaluation of chitosan containing mucco adhesive buccal patch of fluxotine hydrochloride";2012,2(6):23.


 Pratiksha Lonkar*
Pratiksha Lonkar*
 Pratik Bhange
Pratik Bhange

 10.5281/zenodo.14247271
10.5281/zenodo.14247271