Abstract
The drug discovery and development process is very lengthy, highly expensive, and extremely complex in nature. Considering the time and cost constrictions associated with conventional drug discovery, new methods must be found to enhance the declining efficiency of traditional approaches. AI is transformation in drug discovery, increasing interest from investors, industrial and academic scientists. Optimizing properties related to pharmacodynamics, pharmacokinetics, and clinical outcomes are required for Successful drug discovery. This review discusses about the drug discovery and development process, implementation and role of AI tools in drug development and challenges and future of AI. The combination of AI and drug discovery offers a promising strategy to overcome the challenges and complexities of the pharmaceutical industry. New research tools may need to be evaluated to explore new targets. The journey from initial discovery to marketed drug is a long and difficult task. From discovery to approved drug takes about 12 to 15 years and requires an investment of about $1 billion. On average, one million molecules are screened, but only one will be investigated in late-stage clinical trials and ultimately delivered to patients.
Keywords
Artificial intelligence, Machine learning, Computer-aided drug discovery, Drug discovery and development.
Introduction
Drug discovery is the process of identifying therapeutically useful compounds for the cure and treatment of disease. It includes drug identification, synthesis, characterization, validation, optimization, screening, and testing of therapeutic efficacy. Once these studies demonstrate the compound's importance, the drug development process start prior to clinical trials. The drug development process must go through several stages to produce a drug that is safe, effective and meets all regulatory requirements. (1) The development of new drugs is very complex, costly and risky. Its success is highly dependent on an intense collaboration and interaction between many departments within the drug development organization, external investigators and service providers, in constant dialogue with regulatory authorities, payers, academic experts, clinicians and patient organizations. Within the different phases of the drug life cycle, drug development is by far the most crucial part for the initial and continued success of a drug on the market. [2,3]
Drug Development Process- Key steps
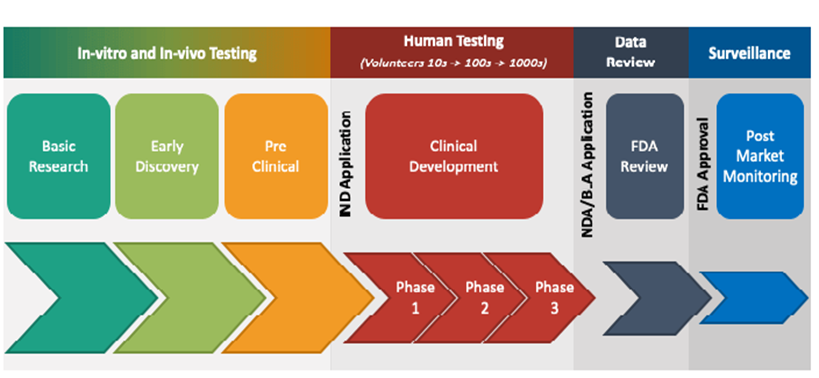
1. Discovery: The discovery stage starts with research and development in a laboratory setting. Researchers identify target molecules such as genes or proteins that are believed to have the potential to contribute to the development or aggravation of a disease.
2. Preclinical research: The discovery phase is followed by a pre-clinical research phase, where the lead compounds are tested both in vitro and in vivo – experimental models that comes as close as possible to resembling humans. Once fully characterized, the most promising compounds become lead candidates. The most important aspect of preclinical research is the rigorous safety tests with the purpose of ensuring that the candidate is not toxic before it can go through clinical studies in humans. Altogether, the discovery phase and the preclinical phase can take four to seven years. After completion of the preclinical tests, provided the results positively answer the researchers’ hypotheses, developers will apply for permission to proceed with clinical – in-human – studies. This is done either through an Investigational New Drug (IND) application in the US or a Clinical Trial Application (CTA) in the EU. The respective regulator authority then examines all available data and and decides whether to approve a move to the clinic.
3. Clinical development
Phase I – safety: Following regulatory approval and approval from ethics committees, the first clinical study, a phase I study – which constitutes the first study in humans, is initiated. Here, the candidate is generally tested on 20 to 80 healthy volunteers with the aim of determining whether the candidate behaves in the same way in the human body as the preclinical studies have indicated. A phase I study takes up to one year to perform.
Phase II – Proof-of-Concept: In the event of positive safety results from phase I, drug developers can apply for permission to take the next clinical development step – phase II. In this phase, the candidate is most often evaluated in 100 to 300 patients diagnosed with the disease that the candidate is intended to treat. Here, efficacy joins safety as minimum and maximum dosages of the drug are determined for use in the next phase of development. Phase II typically takes up to two years.
Phase III – regulatory evidence
In the case of positive safety and efficacy data from phase II, the next step is phase III. This is the last step in the evaluation of a drug before requesting market approval from pharmaceutical regulators. The number of patients enrolled in a phase III study is usually at least 1000 – this ensures that enough data is obtained to show that the drug is safe for humans and has the intended clinical efficacy. In connection with the phase III study, researchers document and report any side effects experienced by patients. This means that patients need to be exposed to the drug for long periods of time in order to make sure those side effects are properly assessed. Any side effects noted at this stage are those that are then listed in the package leaflet of the final product. Phase III takes on average one to four years.
Market approval & launch
The drug registration process
In the event of good results from phases I-III, an application for market approval is submitted, called New Drug Application (NDA)*/Biologics License Application (BLA) in the US and Marketing Authorisation Application (MAA) in the EU. These can include hundreds of thousands of pages of documentation summarising all collected data from the discovery phase onwards, and where the principal investigator argues for approval with the FDA and/or EMA Note that some new drugs are categorised as new molecular units (NMEs), i.e., active ingredients that have not yet been approved by the FDA or other regulatory authorities, and that these require an NME NDA application. Preparing the application documentation can take several months, followed by about 6-10 months for the authorities to process the application.
Market launch
If the regulatory authorities approve an application, the candidate – or medicine as it is now called – is ready for market launch. At this point, price negotiations begin between the principal and the potential buyers (government agencies or insurance companies, depending on the healthcare system). The price negotiation process can differ greatly from country to country.
Phase IV studies – monitoring marketing and safety
In some cases, regulatory authorities require follow-up phase IV studies after a drug has received market approval. This is done by collecting data from clinical practice – that is, real care units that treat patients. The aim is to increase pharmacovigilance. Phase IV studies evaluate whether the drug is interacting with other substances, and additional safety tests are conducted. This is especially important when it comes to drugs for complex medical conditions, or that are intended for the treatment of pregnant women who are unlikely to have been included in the studies in phase I-III. Additionally, phase IV studies may be relevant for drugs that will treat rare conditions, which tends to mean a limited number of patients in phases I-III. The results of the previous clinical studies thus have a weaker statistical certainty, which is why the authorities are asking for further confirmation of the safety and efficacy of the drug.
Challenges faced by Global Pharma Industry
- High attrition rates: Drug attrition is the high rate of failure that drug candidates experience during clinical development. The drug development process is known for its high attrition rate, with fewer than 10% of new drugs reaching the market. This can lead to significant financial losses for drug developers and delays in the availability of therapies for patients.
- Increased time to bring drug to the market: At present, bringing a single new drug to market costs around US$800 million, an amount that doubles every five years. According to the US Food and Drug Administration (FDA), it takes, on average, 12 years for an experimental drug to progress from bench to market.
- Dynamic regulatory requirement: Challenging economic conditions, the need for financial stability and operational resilience, changing consumer demands and behaviors, and environmental and social concerns are influencing regulatory agendas around the globe.
Artificial intelligence in drug discovery and development
Successful drug approval requires concurrently optimizing multiple properties related to pharmacodynamics, pharmacokinetics, and clinical outcomes. Pharmacodynamic properties are related to drug-target interactions and efficacy; appropriate pharmacokinetics include absorption, distribution, metabolism, excretion, toxicity (ADMET), and drug safety; and clinical outcomes include the therapeutic intent, as detailed on the list of drug indications and off-label uses, as well as undesired outcomes such as side effects or adverse drug reactions. Thus, a successful drug discovery based on three pillars: diseases, targets, and therapeutic modalities. AI impacts most therapeutic modalities, including targeted protein degradation, antibodies, gene therapy, and oligonucleotide and vaccine design. (4) The use of artificial intelligence (AI) has been increasing in various sectors of society, particularly the pharmaceutical industry. In this review, we highlight the use of AI in diverse sectors of the pharmaceutical industry, including drug discovery and development, drug repurposing, improving pharmaceutical productivity, and clinical trials, among others; such use reduces the human workload as well as achieving targets in a short period of time. We also discuss about the tools and techniques utilized in AI, ongoing challenges, and ways to overcome them, along with the future of AI in the pharmaceutical industry. (5)
Artificial intelligence: Things to know
Artificial intelligence AI, which is referred to as machine intelligence, is human intelligence simulation, by which a machine mimics cognitive behavior associated with the human brain during learning and problem-solving [7], using software and systems that, when learning and interpreting from input data, facilitate independent decisions-making for specific aims [8]. The lengthy drug discovery process is known to take around 12 years, starting from a preclinical study such as Hit and lead discovery and optimization all through clinical trials of phase l, ll, lll, until the final drug approval to be officially used in humans, with the need of high cost of around 1.2 billion dollars of the whole process, challenged by the drugs withdraw from the market due to their side and adverse effects on human. Consequently, a sophisticated system such as Artificial Intelligence (AI), including Machine Learning (ML) and Deep Learning (DL), has successfully decreased the cost and accelerated the drug discovery process [9,10].
As part of AI, machine learning models use data analysis in ML training algorithms to develop efficient ML models. These ML tools assist the 3-dimensional structure prediction of the target protein, which is crucial in drug discovery [11].
Benefits (12)
The impact of AI on drug development can be considered revolutionary, primarily because of its ability to significantly reduce the usual drug design time frames and expand the current drug discovery scale.
Here are four key benefits of AI in drug development:
1. Objectivity
AI is an objective science. It means the AI-based drug development process is not a subject of prejudice, existing knowledge, personal interests or anything else that can directly impact the development outputs.
2. Constant advancement
Besides assisting humans with mundane tasks, AI leverages the latest technologies in biology and computing. This process is ever-evolving, and with increasing powers of innovation and the reduction of costs of AI tools, AI plays a vital role in drug development advancements now and in future.
3. Higher predictivity
In drug screenings, the enhanced predictivity power of AI tools positively impacts the process of defining meaningful interactions. Therefore, a thorough and meticulous approach to parameters’ design can significantly reduce false positives.
4. Streamlined process and reduced human inefficiencies
With the help of a virtual lab, drug screening outputs can be streamlined, thus significantly reducing human resources hours.
Implementation of AI in drug discovery Process
1. Ensure access to reliable data
Improving drug discovery with AI requires access to large collections of relevant data. There are some commercial and public databases like PubChem, ChEMBL, Chem Bridge available with information on molecules, their properties, known targets, adverse effects, and relations between them. Besides that, you can establish partnerships with academia and research centers to get access to proprietary data and deep expertise.
2. Find external AI partners
Partnering with AI biotech startups and companies that deliver AI services is an effective way to accelerate AI adoption. The challenge here is to evaluate their work and the ready-made algorithms they provide. Many of those companies are quite small and new, so, in most cases, you’ll have limited information on the effectiveness of their solutions. Key indicators to watch out for are relevant scientific publications, collaborations with research centers and other biopharma companies, and the number of medicines being discovered or optimized with their technology.
3. Build AI talent in-house
The lack of relevant skills and talent in-house is one of the major barriers to successful AI implementation in the biopharma industry. You need to engage software engineers and create an internal data science team to make machine learning work for you. You may also enhance your existing talent pool with third-party experts to work on particular tasks. But AI-driven drug discovery needs not only IT and data specialists. The key people in this process are medical scientists familiar with machine learning and analytical approaches. They will participate in running AI projects and ensure the model interpretability (the ability to explain data and reasoning behind the prediction) to gain trust in outputs.
4. Pick up projects to evaluate AI impact
Clearly define where exactly to use AI in the first place and what you expect to reap — be it saving time, the discovery of several new targets, or progress in addressing previously incurable diseases. Prioritize several projects in which prediction tools could significantly enhance outcomes and test the AI impact on them for 12 to 24 months. (13)

Role of artificial intelligence (AI) in drug discovery. AI's applications in drug discovery are revolutionizing the field by enhancing various stages of the process, making it faster, more efficient, and cost-effective. Here’s a detailed look at how AI is being applied across different facets of drug discovery:
1. Molecular Design and Optimization
- De Novo Drug Design: AI algorithms, including generative models like Generative Adversarial Networks (GANs) and Variational Autoencoders (VAEs), create new molecular structures with desired properties by learning from existing data.
- Structure-Based Drug Design: AI helps in designing molecules that specifically interact with biological targets by analyzing protein structures and predicting binding affinities.
- Optimization: Machine learning models predict how modifications to a molecule will affect its activity, stability, and toxicity, aiding in optimizing drug candidates.
2. Predictive Modeling
- Pharmacokinetics and Toxicity Prediction: AI models predict how drugs will be absorbed, distributed, metabolized, and excreted in the body, as well as their potential for causing adverse effects. This helps in identifying promising candidates and reducing the risk of late-stage failures.
- Activity Prediction: AI can predict the biological activity of compounds against specific targets, streamlining the process of identifying effective drug candidates.
3. High-Throughput Screening
- Virtual Screening: AI can perform virtual screening of large chemical libraries to identify potential hits. This computational approach is faster and less expensive than traditional wet-lab screening.
- Data Integration: AI integrates data from high-throughput screening assays, combining results from multiple sources to refine the identification of promising compounds.
4. Target Identification and Validation
- Biological Data Analysis: AI analyzes omics data (genomics, proteomics, transcriptomics) to identify new drug targets and validate their role in disease processes.
- Network Analysis: AI helps in understanding biological networks and pathways, identifying key nodes and interactions that could be targeted by new drugs.
5. Biomarker Discovery
- Omics Data Integration: AI models analyze large-scale omics datasets to discover biomarkers that can be used for disease diagnosis, prognosis, or monitoring drug responses.
- Personalized Medicine: AI identifies biomarkers that can help in tailoring treatments to individual patients, improving efficacy and reducing adverse effects.
6. Drug Repurposing
- Existing Data Mining: AI analyzes existing drug databases and literature to find new uses for approved drugs or compounds in clinical trials. This approach can rapidly identify novel therapeutic indications.
- Drug Interaction Prediction: AI models predict potential interactions between existing drugs and new therapeutic targets, facilitating the identification of repurposing opportunities.
7. Clinical Trial Design and Management
- Patient Recruitment: AI analyzes patient data to identify suitable candidates for clinical trials, improving recruitment efficiency and ensuring that participants are likely to benefit from the treatment.
- Trial Design Optimization: AI helps design more efficient clinical trials by predicting optimal dosing regimens and identifying potential issues before trials begin.
- Real-Time Monitoring: AI systems monitor trial data in real-time to detect adverse effects or efficacy signals, allowing for dynamic adjustments to trial protocols.
8. Preclinical and Clinical Data Analysis
- Data Integration: AI integrates and analyzes data from preclinical studies and clinical trials, providing insights that guide further research and development.
- Pattern Recognition: Machine learning algorithms identify patterns and correlations in complex datasets, which can lead to new hypotheses and discoveries.
9. Synthetic Biology
- Gene Editing and Design: AI aids in designing gene editing experiments and synthetic biology applications by predicting outcomes and optimizing protocols.
10. Automated Laboratory Processes
- Robotics and Automation: AI-driven robotics and automation systems perform repetitive laboratory tasks, such as liquid handling and sample processing, with high precision and
Ai Tools Used In Drug Discovery And Development
Artificial intelligence (AI) tools are used in drug discovery to analyze large amounts of data and improve the process of finding and developing new medications. Some AI tools used in drug discovery include:
Table 1. Various AI tools used in drug discovery and development
|
Tools
|
Details
|
Website URL
|
Ref.
|
|
Deep Chem
|
MLP model that uses a python-based AI system to find a
suitable candidate in drug discovery
|
https://github.com/deepchem/deepchem
|
[14]
|
|
Deep Tox
|
Software that predicts the toxicity of total of 12 000 drugs
|
www.bioinf.jku.at/research/DeepTox
|
15
|
|
Deep Neural Net QSAR
|
Python-based system driven by computational tools that
aid detection of the molecular activity of compounds
|
https://github.com/Merck/DeepNeuralNet-QSAR
|
16
|
|
ORGANIC
|
A molecular generation tool that helps to create
molecules with desired properties
|
https://github.com/aspuru-guzik-group/ORGANIC
|
17
|
|
Potential Net
|
Uses NNs to predict binding affinity of ligands
|
https://pubs.acs.org/doi/full/10.1021/acscentsci.8b00507
|
18
|
|
Hit Dexter
|
ML technique to predict molecules that might respond to
biochemical assays
|
http://hitdexter2.zbh.uni-hamburg.de
|
|
|
Delta Vina
|
A scoring function for rescoring drug–ligand binding
affinity
|
https://github.com/chengwang88/deltavina
|
|
|
Neural graph fingerprint
|
Helps to predict properties of novel molecules
|
https://github.com/HIPS/neural-fingerprint
|
|
|
AlphaFold
|
Predicts 3D structures of proteins
|
https://deepmind.com/blog/alphafold
|
|
|
Computer
|
Helps to report procedure for chemical synthesis in
standardized format
|
https://zenodo.org/record/1481731
|
|
|
AIDDISON
|
An AI-powered drug discovery software that can visualize target molecule–protein interactions
|
https://www.sigmaaldrich.com/IN/en/services/software-and-digital-platforms/aiddison-ai-powered-drug-discovery
|
|
|
Autoencoders
|
An unsupervised learning model that can capture essential characteristics of molecules
|
https://pmc.ncbi.nlm.nih.gov/articles/PMC10385763
|
|
Challenges and Limitations of AI in Drug Discovery
The similarity-based methods employed in these technologies typically do not account for heterogeneous information defined in a relationship network. Combining feature-based and similarity-based methods can help overcome this restriction. The fact that AI/MLbased models need a lot of training and that the requirements vary depending on the application is another drawback. Due to the learning qualities ingrained in the modelling data, DL models produce “black boxes” that are challenging to interpret; however, their use is growing in popularity. Nevertheless, their use is limited by the need for large amounts of high-quality data that are expensive to produce and frequently kept private. The use of inadequate quantities of high-quality data also affects the performance and dependability of DL models.
While AI holds great promise in drug discovery, it also faces several challenges and limitations that can impact its effectiveness and integration into the drug development process. Here are some key challenges:
1. Data Quality and Availability
- Data Quality: AI models are heavily dependent on the quality of data they are trained on. Poor-quality, incomplete, or biased data can lead to inaccurate predictions and unreliable results.
- Data Scarcity: In some areas, high-quality datasets are limited, particularly for rare diseases or novel drug targets. This scarcity can hinder the development and training of robust AI models.
2. Interpreting AI Models
- Black Box Problem: Many AI models, especially deep learning algorithms, operate as "black boxes," making it difficult to understand how they arrive at specific conclusions. This lack of transparency can be problematic for scientific validation and regulatory approval.
- Complexity: The complexity of AI models can make it challenging to interpret their outputs, which is critical for understanding the underlying biological mechanisms and validating predictions.
3. Generalization and Overfitting
- Overfitting: AI models trained on specific datasets may perform well on those datasets but fail to generalize to new, unseen data. Overfitting occurs when the model becomes too tailored to the training data and loses its ability to predict accurately on other datasets.
- Bias: AI models can inherit biases present in the training data, leading to skewed results and potential disparities in drug efficacy or safety across different populations.
4. Integration with Existing Workflows
- Workflow Integration: Integrating AI tools into traditional drug discovery workflows can be complex and requires collaboration between data scientists, biologists, and chemists. Adapting existing processes and infrastructure to accommodate AI-driven approaches can be challenging.
- Resistance to Change: There may be resistance from researchers and industry professionals who are accustomed to traditional methods and may be hesitant to adopt new AI-driven approaches.
5. Regulatory and Ethical Issues
- Regulatory Approval: The use of AI in drug discovery raises regulatory challenges, including ensuring that AI-generated findings meet the rigorous standards for safety and efficacy required for drug approval.
- Ethical Concerns: AI applications must address ethical issues related to data privacy, consent, and the potential for unintended consequences. Ensuring responsible use of AI in drug discovery is crucial.
6. Validation and Reproducibility
- Model Validation: Validating AI models requires rigorous testing and independent verification to ensure that predictions are accurate and reliable. This validation process can be resource-intensive and time-consuming.
- Reproducibility: Ensuring that AI models produce consistent results across different studies and datasets is essential for scientific reproducibility. Variability in results can undermine confidence in AI-driven discoveries.
7. Complexity of Biological Systems
- Biological Complexity: Biological systems are highly complex and not fully understood. AI models may struggle to capture the full complexity of biological interactions and pathways, leading to incomplete or inaccurate predictions.
- Dynamic Nature: Biological systems are dynamic and can change over time, which can affect the accuracy of AI predictions if the models are not updated to reflect these changes.
8. Computational and Resource Constraints
- Resource Intensiveness: Training and deploying advanced AI models require significant computational resources and infrastructure, which can be costly and may limit access for smaller organizations or research groups.
- Scalability: Scaling AI solutions from research settings to large-scale drug discovery projects can be challenging and may require substantial adjustments and optimizations.
9. Interdisciplinary Collaboration
- Skill Gaps: Successful implementation of AI in drug discovery requires interdisciplinary collaboration, including expertise in data science, biology, chemistry, and pharmacology. Bridging these diverse skill sets can be difficult and may require additional training and coordination.
10. Ethical AI Development
- Bias and Fairness: Ensuring that AI systems are developed and trained in a way that minimizes bias and promotes fairness is critical. Addressing potential biases in training data and model outcomes is an ongoing challenge.
Despite these challenges, ongoing research and development efforts aim to address these limitations and improve the integration of AI in drug discovery. As technology evolves and best practices are established, many of these issues may be mitigated, leading to more effective and reliable AI-driven drug discovery processes.
Future Perspective (23)
The future of AI in drug discovery and development is promising and transformative. Here are some key perspectives:
- Enhanced Target Identification: AI algorithms can analyze vast datasets, including genomic, proteomic, and metabolomic information, to identify new biological targets for drug development more quickly and accurately.
- Predictive Modeling: Machine learning models can predict how different compounds will interact with targets, helping to prioritize candidates for further testing and reducing the number of compounds that need to be synthesized and tested in the lab.
- Optimized Clinical Trials: AI can assist in designing more efficient clinical trials by identifying suitable patient populations, predicting outcomes, and monitoring trial data in real-time, which can reduce costs and timelines.
- Personalized Medicine: AI can analyze patient data to tailor drug therapies to individual patients, improving efficacy and reducing adverse effects, thereby advancing the concept of precision medicine.
- Drug Repurposing: AI can facilitate the identification of new uses for existing drugs by analyzing existing data, which can lead to faster development timelines and lower costs compared to developing new compounds from scratch.
- Automated Drug Discovery: Robotics combined with AI can automate high-throughput screening processes, speeding up the discovery of viable drug candidates.
- Natural Language Processing (NLP): AI-driven NLP can help sift through the vast literature and clinical trial data to extract insights and trends that inform drug development strategies.
- Regulatory Support: AI can help streamline regulatory processes by providing robust data analysis and insights that support faster approval timelines, potentially improving the time-to-market for new therapies.
- Continuous Learning: AI systems can continuously learn from new data, enabling them to refine their predictions and models over time, which enhances their effectiveness in drug discovery.
- Collaborative Platforms: The future may see increased collaboration among academia, industry, and technology companies, leveraging AI to pool resources and expertise for drug discovery.
Overall, as AI technology continues to evolve, its integration into drug discovery and development processes is likely to lead to more efficient, innovative, and effective therapeutic solutions.
Ethical Issues & Regulations
The integration of AI in drug development brings several ethical issues and regulatory challenges. Here are some key considerations:
Ethical Issues
- Data Privacy and Security: AI systems often require access to vast amounts of patient data. Ensuring the privacy and security of this data is paramount to prevent breaches and misuse.
- Bias and Fairness: AI models can perpetuate or even exacerbate existing biases in healthcare data. If not carefully managed, this can lead to inequitable treatment outcomes across different demographic groups.
- Transparency and Accountability: The "black box" nature of some AI algorithms can make it difficult to understand how decisions are made. This lack of transparency raises questions about accountability, especially if an AI system leads to adverse patient outcomes.
- Informed Consent: Using AI in drug development may complicate the informed consent process, particularly regarding how patient data is used and the potential risks involved in AI-driven therapies.
- Intellectual Property: As AI generates new drug candidates and insights, questions arise about who owns these discoveries and how to protect intellectual property in an increasingly automated process.
- Human Oversight: The role of human oversight in decision-making is crucial. There is a risk that over-reliance on AI may diminish critical thinking and expert judgment in drug development processes.
Regulatory Challenges
- Lack of Clear Guidelines: Regulatory frameworks for AI in drug development are still evolving. There is a need for clear guidelines on how AI models should be validated and used in the drug approval process.
- Regulatory Approval Processes: Traditional drug approval processes may not adequately accommodate the rapid iteration and learning capabilities of AI. Regulatory bodies need to adapt to ensure that AI-driven drugs are safe and effective.
- Validation of AI Models: Ensuring that AI models are rigorously validated is critical. Regulatory agencies will need to establish standards for evaluating the performance and reliability of these models before they can be used in drug development.
- Interdisciplinary Collaboration: Effective regulation will require collaboration between AI experts, healthcare professionals, and regulatory bodies to understand the implications of AI in drug development fully.
- Post-Market Surveillance: Ongoing monitoring of AI-driven therapies will be essential to detect unforeseen issues and ensure long-term safety and efficacy.
Case Studies of Successful AI-Aided Drug Discovery Efforts
The potential of AI in the context of drug discovery has been demonstrated in several case studies. For example, the successful use of AI to identify novel compounds for the treatment of cancer has recently been reported by Gupta, R., et al. [24]. These authors trained a DL algorithm on a large dataset of known cancer-related compounds and their corresponding biological activity. As an output, novel compounds with high potential for future cancer treatment were obtained, demonstrating the ability of this method to discover new therapeutic candidates. The use of ML to identify small-molecule inhibitors of the protein MEK [25] has recently been described. MEK is also a possible target for the treatment of cancer, but the development of effective inhibitors has been challenging. The ML algorithm was able to identify novel inhibitors for this protein. Another example is the identification of novel inhibitors of beta-secretase (BACE1), a protein involved in the development of Alzheimer’s disease [26] by using an ML algorithm. AI has also been successfully applied in the discovery of new antibiotics [27]. A pioneering ML approach has identified powerful types of antibiotic from a pool of more than 100 million molecules, including one that works against a wide range of bacteria, such as tuberculosis and untreatable bacterial strains [28]. The use of AI in the discovery of drugs to combat COVID-19 has been a promising area of research during the last two years. ML algorithms have been used to analyze large datasets of potential compounds and identify those with the most potential for treating the virus. In some cases, these AI-powered approaches have been able to identify promising drug candidates in a fraction of the time that it would take when using traditional methods [29,30,31,32,33,34].
CONCLUSION
However, the successful application of AI in drug discovery is dependent on the availability of high-quality data, the addressing of ethical concerns, and the recognition of the limitations of AI-based approaches. The advancement of AI, along with its remarkable tools, continuously aims to reduce challenges faced by pharmaceutical companies, impacting the drug development process along with the overall lifecycle of the product, which could explain the increase in the number of start-ups in this sector AI can also make major contributions to the further incorporation of the developed drug in its correct dosage form as well as its optimization, in addition to aiding quick decision-making, leading to faster manufacturing of better-quality products along with assurance of batch-to-batch consistency. AI can also contribute to establishing the safety and efficacy of the product in clinical trials, as well as ensuring proper positioning and costing in the market through comprehensive market analysis and prediction.
REFERENCES
- Gupta D., Suryarao S. , “DRUG DEVELOPMENT: STAGES OF DRUG DISCOVERY AND DEVELOPMENT PROCESS”, JETIR August 2022, Volume 9, Issue 8.
- Hughes JP, Rees S, Kalindjian SB, Philpott KL. 2011,Principles of early drug discovery. Br J Pharmacol,162: 1239-1249.
- Pharmaceutical Research and Manufacturers Association (PREMA) Introduction to drug development process. 30th PREMA Anniversary.
- Catrin H. and Tudor I., “Artificial Intelligence for Drug Discovery: Are We There Yet?” Annual Review of Pharmacology and Toxicology, September 22, 2023: 528
- Debleena Paulz, Gaurav Sanap, “Artificial intelligence in drug discovery and development, elesvier, vol:26 January,2021.umber 1 January 2021. I
- Mohamad A.and Kaul. N, What are the applications of artificial intelligence in drug discovery & development? By prescouter, August,2018 p.g.8
- Niazi SK (2023) The Coming of Age of AI/ML in Drug Discovery, Development, Clinical Testing, and Manufacturing: The FDA Perspectives. Drug Des Devel Ther 17: 2691-2725.
- Paul D, Gaurav Sanap, Snehal Shenoy, Dnyaneshwar Kalyane, Kiran Kalia, et al. (2021) Artificial intelligence in drug discovery and development. Drug Discov Today 26(1): 80-93.
- Blanco González A, Alfonso Cabezón, Alejandro Seco González, Daniel Conde Torres, Paula Antelo Riveiro, et al. (2023) The Role of AI in Drug Discovery: Challenges, Opportunities, and Strategies. Pharmaceuticals (Basel) 16(6): 891.
- Askr H, Enas Elgeldawi, Heba Aboul Ella, Yaseen AM Elshaier, Mamdouh M Gomaa, et al. (2023) Deep learning in drug discovery: an integrative review and future challenges. Artif Intell Rev 56(7): 5975-6037.
- Gupta R, Devesh Srivastava, Mehar Sahu, Swati Tiwari, Rashmi K et al. (2021) Artificial intelligence to deep learning: machine intelligence approach for drug discovery. Mol Divers 25(3): 1315-1360.
- https://www.kandasoft.com/ai-and-its-impact-on-drug-development-benefits-challenges-and-use-cases/
- https://www.altexsoft.com/blog/ai-drug-discovery-repurposing/
- Zhu, H. (2020) Big data and artificial intelligence modeling for drug discovery. Annu. Rev. Pharmacol. Toxicol. 60, 573–589
- Ciallella, H.L. and Zhu, H. (2019) Advancing computational toxicology in the big data era by artificial intelligence: data-driven and mechanism-driven modeling for chemical toxicity. Chem. Res. Toxicol. 32, 536–547
- Chan, H.S. et al. (2019) Advancing drug discovery via artificial intelligence. Trends Pharmacol. Sci. 40 (8), 592–604
- Brown, N. (2015) Silico Medicinal Chemistry: Computational Methods to Support Drug Design. Royal Society of Chemistry
- Pereira, J.C. et al. (2016) Boosting docking-based virtual screening with deep learning. J. Chem. Inf. Model. 56, 2495–2506.
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 2019, 18, 463–477. [CrossRef] [PubMed
- Gómez-Bombarelli, R.;Wei, J.N.; Duvenaud,D.;Hernández-Lobato, J.M.; Sánchez-Lengeling, B.; Sheberla,D.;Aguilera-Iparraguirre, J.; Hirzel, T.D.; Adams, R.P.; Aspuru-Guzik, A. Automatic chemical design using a data-driven continuous representation of molecules. ACS Cent. Sci. 2018, 4, 268–276. [CrossRef]
- Tropsha, A. Best Practices for QSAR Model Development, Validation, and Exploitation. Mol Inf. 2010, 29, 476–488. [CrossRef] [PubMed]
- Schneider, G. Automating drug discovery. Nat. Rev. Drug Discov. 2018, 17, 97–113. [CrossRef] [PubMed]
- Rushikesh Dhudum 1, Ankit Ganeshpurkar 2 and Atmaram Pawar 3,* Revolutionizing Drug Discovery: A Comprehensive Review of AI Applications, 13 February 2024, 165.
- Gupta R., Srivastava D., Sahu M., Tiwari S., Ambasta R.K., Kumar P. Artificial intelligence to deep learning: Machine intelligence approach for drug discovery. Mol. Divers. 2021;25:1315–1360. doi: 10.1007/s11030-021-10217-3. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Zhu J., Wang J., Wang X., Gao M., Guo B., Gao M., Liu J., Yu Y., Wang L., Kong W., et al. Prediction of drug efficacy from transcriptional profiles with deep learning. Nat. Biotechnol. 2021;39:1444–1452. doi: 10.1038/s41587-021-00946-z. [PubMed] [CrossRef] [Google Scholar]
- Dhamodharan G., Mohan C.G. Machine learning models for predicting the activity of AChE and BACE1 dual inhibitors for the treatment of Alzheimer’s disease. Mol. Divers. 2022;26:1501–1517. doi: 10.1007/s11030-021-10282-8. [PubMed] [CrossRef] [Google Scholar]
- Melo M.C.R., Maasch J.R.M.A., de la Fuente-Nunez C. Accelerating antibiotic discovery through artificial intelligence. Commun. Biol. 2021;4:1050. doi: 10.1038/s42003-021-02586-0. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Marchant J. Powerful antibiotics discovered using AI. Nature. 2020. Online ahead of print . [PubMed] [CrossRef]
- Lv H., Shi L., Berkenpas J.W., Dao F.Y., Zulfiqar H., Ding H., Zhang Y., Yang L., Cao R. Application of artificial intelligence and machine learning for COVID-19 drug discovery and vaccine design. Brief. Bioinform. 2021;22:bbab320. doi: 10.1093/bib/bbab320. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Monteleone S., Kellici T.F., Southey M., Bodkin M.J., Heifetz A. Methods in Molecular Biology. Volume 2390. Humana Press Inc.; Totowa, NJ, USA: 2022. Fighting COVID-19 with Artificial Intelligence; pp. 103–112. [PubMed] [Google Scholar]
- Zhou Y., Wang F., Tang J., Nussinov R., Cheng F. Artificial intelligence in COVID-19 drug repurposing. Lancet Digit. Health. 2020;2:e667–e676. doi: 10.1016/S2589-7500(20)30192-8. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Verma N., Qu X., Trozzi F., Elsaied M., Karki N., Tao Y., Zoltowski B., Larson E.C., Kraka E. Predicting potential Sars-Cov-2 drugs-in depth drug database screening using deep neural network framework ssnet, classical virtual screening and docking. Int. J. Mol. Sci. 2021;22:1392. doi: 10.3390/ijms22031392. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Bung N., Krishnan S.R., Bulusu G., Roy A. De novo design of new chemical entities for SARS-CoV-2 using artificial intelligence. Future Med. Chem. 2021;13:575–585. doi: 10.4155/fmc-2020-0262. [PMC free article] [PubMed] [CrossRef] [Google Scholar]


 Sushila Chavan * 1
Sushila Chavan * 1
 Jain Monal 2
Jain Monal 2


 10.5281/zenodo.14031432
10.5281/zenodo.14031432