Abstract
Cyclic compounds are fundamental structures in organic chemistry. Several drugs are available on the market but they either lack specificity or have poor safety, severe side effects, and suffer from resistance. So, there is a dire need to develop safer and target-specific drugs. More than 85% of all physiologically active pharmaceuticals are heterocycles or contain at least one heteroatom. Nitrogen heterocycles constituting the most common heterocyclic framework. In this study, we have compiled the FDA approved heterocyclic drugs with nitrogen atoms and their pharmacological properties. Moreover, we have reported nitrogen containing heterocycles, including Pyrrole, Imidazole, Oxazole, Pyrazole, Pyradazine, Pyrimidines, Indole, Quinolones, Carbazole, Purines. Which are used in the treatment of different types of cancer, concurrently covering the biochemical mechanisms of action and cellular targets.
Keywords
Pyrrole, Imidazole, Oxazole, Pyrazole, Pyradazine, Pyrimidines, Indole, Quinolones, Carbazole, Purine, Antimalarial, Antifungal, Anticancer, Anti Convulsant, Antibacterial.
Introduction
The 5-membered ring compounds containing two heteroatoms, at least one of which is nitrogen, are collectively called the azoles. Thiazoles and isothiazoles contain a sulfur and a nitrogen atom in the ring. Dithiolanes have two sulfur atoms. 5 membered rings such as pyrole, imidazole, oxazole, pyrazole. Five-membered heterocycles are essential structural components in various antibacterial drugs; the physicochemical properties of a five-membered heterocycle can play a crucial role in determining the biological activity of an antibacterial drug. [1-2]

Six-membered rings are a fundamental class of cyclic compounds in organic chemistry, characterized by a ring structure consisting of six atoms. These rings can be saturated, as in cyclohexane, or unsaturated, as in benzene. The six-membered ring is a stable and versatile framework, exhibiting a range of chemical properties and reactivates. Six membered rings such as pyridazine, pyrimidines. They are important in organic chemistry due to their role in the formation of diverse molecules with potential pharmacological activities. [3-4]

Condensed rings, also known as fused rings or annealed rings, are polycyclic structures where two or more rings share a common bond. This leads to a complex molecular architecture with unique properties and reactivities. Condensed rings are prevalent in natural products, pharmaceuticals, and materials science, and understanding their properties and reactivities is crucial for advancing various fields. Condensed rings such as Indole, quinolones, Isoquinolones, carbazole, purine. [5-7]
LITERATURE OF REVIEW
Synthesis of Nitrogen Containing Hetero cycles:
Scheme -01 : An operationally simple, practical, and economical Paal-Knorr pyrrole condensation of 2,5-dimethoxytetrahydrofuran with various amines and sulfonamines in water in the presence of a catalytic amount of iron(III) chloride allows the synthesis of N-substituted pyrroles under very mild reaction conditions in good to excellent yields.[8]
Scheme-02:
The condensation of readily available O-substituted carbamates with 2, 5-dimethoxytetrahydrofuran gives N-alkoxycarbonyl pyrroles in good yield. N-alkoxycarbonyl protection can endow pyrrole with distinct reactivity in comparison with N-sulfonyl protection, for example, in a pyrrole acylation protocol employing carboxylic acids with a sulfonic acid anhydride activator. [9]
Scheme-03:
A general, selective, and atom economic metal-catalyzed conversion of primary diols and amines to highly valuable 2, 5-unsubstituted pyrroles is catalyzed by a stable manganese complex in the absence of organic solvents. Water and molecular hydrogen are the only side products. The reaction shows unprecedented selectivity, avoiding the formation of pyrrolidines, cyclic imides, and lactones. [10]
Scheme-04:
Starting from 1, 2-diketones and urotropine in the presence of ammonium acetate, a simple and efficient solventless microwave-assisted enabled the synthesis of 4,5-disubstituted imidazoles.[11]
Scheme-05:
A NHC-copper-catalyzed isocyanide insertion into alcohol to form an N-arylformimidate intermediate and subsequent base-promoted cycloaddition with benzyl isocyanide derivatives enables a straightforward and high-yielding synthesis of 1, 4-diaryl-1H-imidazoles. [12]
Scheme-06:
Mild and efficient protocols provide 1,4,5-trisubstituted and 1,4-/4,5-disubstituted imidazoles regioselectively in a single pot from aryl-substituted tosylmethyl isocyanide (TosMIC) reagents and imines generated in situ from virtually any aldehyde and amine. Mono- and disubstituted oxazoles can also prepared. [13]
Scheme–07:
Complementary methods for direct arylation of oxazoles with high regioselectivity at both C-5 and C-2 have been developed for a wide range of aryl and heteroaryl bromides, chlorides, iodides, and triflates. Using task-specific phosphine ligands, palladium-catalyzed C-5 arylation of oxazoles is preferred in polar solvents, whereas C-2 arylation is preferred in nonpolar solvents.[14]
Scheme – 08:
A quaternary ammonium hydroxide ion exchange resin catalyzes the reaction of p-tolylsulfonylmethyl isocyanide (TosMIC) with aromatic aldehydes to give 5-aryloxazoles. The base and the p-tolylsulfinic acid byproduct can be removed by simple filtration, resulting in oxazoles in high yield and purity.[15]
Scheme – 09:
Pd(PPh3)4 efficiently catalyses both direct arylation and alkenylation of oxazoles. The method is regio- and stereospecific with respect to bromoalkenes and tolerates a wide range of functional groups.[16]

Scheme – 10:
A phosphine-free [3+2] cycloaddition reaction of dialkyl azodicarboxylates with substituted propargylamines provides functionalized pyrazoles in good yields and high selectivity at room temperature. [17]
Scheme – 11:
A mild and convenient Cu-catalyzed aerobic oxidative cyclization of ?,?-unsaturated hydrazones provides a broad range of pyrazole derivatives. The reaction is initiated by the formation of a hydrazonyl radical, followed by cyclization and a concomitant C=C bond cleavage. [18]
Scheme -12:
An iron-catalyzed route for the regioselective synthesis of 1,3- and 1,3,5-substituted pyrazoles from the reaction of diarylhydrazones and vicinal diols allows the conversions of a broad range of substrates. [19]
Scheme – 13:
A Lewis acid-mediated inverse electron demand Diels-Alder reaction between 3-monosubstituted s-tetrazine and silyl enol ethers provides functionalized pyridazines, including 3-bromo-pyridazines, with high regiocontrol. [20]

Scheme – 14:
An unexpected C-C bond cleavage in the absence of metal enables an efficient approach toward 3, 6-diarylpyridazines and 6-arylpyridazin-3-ones from simple and commercially available 1, 3-dicarbonyl compounds and methyl ketones. [21]
Scheme–15:
Cu (II)-catalyzed aerobic 6-endo-trig cyclizations provide 1, 6-dihydropyridazines and pyridazines via the judicious choice of reaction solvent. Whereas 1, 6-dihydropyridazines were obtained in good yields with MeCN as the reaction solvent, employment of AcOH directly afforded pyridazines in good yields. [22]
Scheme – 16:
An oxidative annulation involving anilines, aryl ketones, and DMSO as a methine (=CH?) equivalent promoted by K2S2O8 provides 4-arylquinolines, whereas activation of acetophenone-formamide conjugates enables the synthesis of 4-arylpyrimidines. [23
Scheme – 17:
A ZnCl2-catalyzed three-component coupling reaction allows the synthesis of various 4, 5-disubstituted pyrimidine derivatives in a single step from functionalized enamines, triethyl orthoformate, and ammonium acetate. The procedure can be successfully applied to the efficient synthesis of mono- and disubstituted pyrimidine derivatives, using methyl ketone derivatives instead of enamines. [24]
Scheme – 18:
NH4I promotes a facile and practical three-component tandem reaction of ketones, NH4OAc, and N, N-dimethylformamide dimethyl acetal to provide a broad range of substituted pyrimidines in acceptable yields under metal- and solvent-free conditions. The method offers a broad substrate scope with good functional group tolerance, and gram-scale synthesis. [25]
Scheme –19:
A praseodymium-catalyzed aerobic dehydrogenative aromatization of saturated N-heterocycles shows high catalytic activity under mild reaction conditions to produce 7 classes of products with a broad substrate scope. [26]
Scheme – 20:
The polystyrene-cross-linking bisphosphine ligand PS-DPPBz was effective for the Ir-catalyzed reversible acceptorless dehydrogenation/hydrogenation of N-heterocycles. Notably, this protocol is applicable to the dehydrogenation of a broad range of indoline derivatives. [27]
Scheme –21:
Tetramethylammonium fluoride (TMAF) enables a direct and selective methylation of various amides, indoles, pyrroles, imidazoles, alcohols, and thiols. The method is characterized by operational simplicity, wide scope, and ease of purification. [28]
Scheme -22:
A heterogeneous cobalt oxide is an effective catalyst for aerobic dehydrogenation of various 1, 2, 3, 4-tetrahydroquinolines to the corresponding quinolines in good yields under mild conditions. Other N-heterocycles are also successfully oxidized to their aromatic counterparts. [29]
Scheme – 23:
A visible-light-mediated aerobic dehydrogenation reaction enables a simple and environmentally friendly method for synthesizing N-containing heterocycles using a nontoxic, stable, and inexpensive titanium dioxide catalyst and oxygen as green oxidant. The reaction provides a variety of substituted quinolines, indoles, quinoxalines, and 3, 4-dihydroisoquinolines. [30]
Scheme-24:
Visible-light mediates a scalable and operationally simple method for the chemoselective deoxygenation of a wide range of N-heterocyclic N-oxides at room temperature using only commercially available reagents. This protocol offers an unprecedented chemoselective removal of the oxygen atom in a quinoline N-oxide in the presence of a pyridine N-oxide through the judicious selection of the photocatalyst. [31]
Scheme – 25:
A palladium-catalyzed coupling of tert-butylimine of o-iodobenzaldehyde with aryl- and alkenyl-substituted terminal acetylenes followed by a copper-catalyzed cyclization provide isoquinolines in excellent yields and short reaction times, whereas 3-halo-2-alkenals can similarly be converted into pyridines. [32]
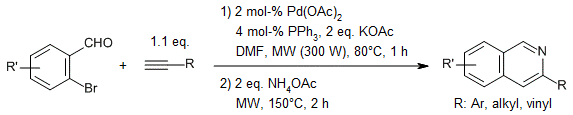
Scheme–26:
Sequential coupling-imination-annulation reactions of ortho-bromoarylaldehydes and terminal alkynes with ammonium acetate in the presence of a palladium catalyst under microwave irradiation gives various substituted isoquinolines, furopyridines, and thienopyridines in good yields.[33]
Scheme–27:
A copper(I)-catalyzed tandem reaction of 2-bromoaryl ketones, terminal alkynes, and CH3CN efficiently produces densely functionalized isoquinolines via N atom transfer and a three-component [3 + 2 + 1] cyclization in a facile, highly selective, and general manner.[34]

Scheme–28:
A palladium-catalyzed reaction sequence consisting of an intermolecular amination and an intramolecular direct arylation enabled highly regioselective syntheses of functionalized indoles or carbazoles and proved to be amenable to the use of inexpensive 1, 2-dichloroarenes as electrophiles. [35]
Scheme-29:
The iridium-catalyzed dehydrogenative cyclization of 2-aminobiphenyls proceeds smoothly in the presence of a copper cocatalyst under air as a terminal oxidant to yield N-H carbazoles. A similar catalytic system can also be used for a dimerization reaction of 2-aminobiphenyl involving 2-fold C-H/N-H couplings. [36]

Scheme–30:
Various carbazoles can be synthesized from substituted biaryl azides at 60°C using Rh2 (OCOC3F7)4 or Rh2(OCOC7H15)4 as catalysts.[37]

Scheme–31:
Purine is obtained in good yield when formamide is heated in an open vessel at 170 °C for 28 hours. [38]

Biological Activity of Nitrogen Containing Hetero Cycles:
Anti-proliferative agents
B. Parrino et al. described the synthesis of structurally diverse fused pyrroles such as pyrido[2?,3?:3,4]pyrrolo[1,2-a]quinoxalines (144), pyrido[3?,2?:3,4]pyrrolo[1,2-a]quinoxalines (145) and pyrido[2?,3?:5,6]pyrazino[2,1-a]isoindoles (146) (Fig. 45). The biological evaluation study indicated the anti-proliferative effect of these class of compounds (pGI50 = 7.09–7.27
Anti-allergic activity
Pyrrolo[2,3-d]pyrimidine derivative 185 is reported to be a potent Signal Transducers and Activators of Transcription 6 (STAT6) inhibitor96. STAT6 is an important transcription factor in interleukin (IL)-4 signaling pathway and a key regulator of the type 2 helper T (Th2) cell immune response. Therefore, STAT6 is considered as an excellent therapeutic target for allergic conditions, including asthma and atopic diseases. [40]
Anti-depressant activity
Both pyrrole derivatives 184a,b exhibit favorable in vitro and in vivo antidepressant activities as they are targeting serotonin 5-HT2A, 5-HT2C, and serotonin transporter. [41]
Antitubercular activity
Antitubercular activity Ramya V et al synthesized series of novel 5-(nitro/bromo)-styryl-2-benzimidazoles (1–12) derivatives and screened for in vitro anti-tubercular activity against Mycobacterium tuberculosis, and these compounds showed good antitubercular activities. Streptomycin was used as reference drug. [42]
Antilishmanial Activity
Kalpana bhandari et al synthesized a series of substituted aryloxy alkyl and aryloxy aryl alkyl imidazole and evaluated in vitro as antileishmanial against Leshmania donovani. Among all compounds exhibited 94–
100% inhibition. [43]
Antiviral Activity
Viruses are parasites that can cause serious infections for human such as HIV disease. The number of existing drug as antiviral is not enough and development of antiviral drugs is still in requirement, because of the viral resistance. The discovery and optimization of a novel class of quinolone small-molecules that inhibit NS5B polymerase, nonstructural protein 5B, a key enzyme of the hepatitis C virus viral life-cycle, is described by Kumar et al. The research led to the replacement of a hydrolytically labile ester functionality with bio-isosteric heterocycles such as oxazole and oxazdiazoles. Compound (50) was potent with IC50 (1.8 µM). [44]
The CDK inhibitors have garnered attention for their potential as anticancer agent.Pyrazolopyridazine is a npotent inhibitor of CDK1/cyclin B. Series of pyrazolo[3,4-c]pyridazines of in an attempt improve potency against the CDK family. Pyrazolopyridazine is also as a potent inhibitor of glycogen synthase kinase-3 (GSK-3). CDK2/cyclin A , showed good selectivity against the tested kinases. The GSK-3 has a major role in the regulation of the cell cycle, transcription, and insulin action. The kinase inhibitors could be useful in the treatment of cancer. The triazolo[4,3-b]pyridazinones, are used as adenine receptor ligands and in vitro antitumor activities was reported. Pyrazolopyridazine as a potent inhibitor of (Cyclin-Dependent Kinases) CDK1/cyclin B and these compounds have been revealed antitumor activities [87]. Pyrido[4,3-b]carbazole-type alkaloids (43a-c) represents a 3-aza analog of the antitumor natural product, olivacine. Structural modifications of this compound type will be carried out, aiming at an improvement of antineoplastic activity. [45]
Antituberculosis Activity
Some pyridazinone and phthalazinone derivatives (39,40) carrying N-(phenylsulfonyl) acetohydrazide moiety at position 2 of these rings were exhibited antitubercular activity against M. tuberculosis H37 Rv. Unsubstituted compounds were more active than after the substitution of chlorine at the para position in the phenyl ring. [46]
Anti-inflammatory and Analgesic Activities
Indole-based chalcone derivatives reported as COX-1 and COX-2 inhibitor by Ozdemir et al. Compound 3-(5Bromo-1H-indol-3-yl)-1-(4-cyanophenyl)prop-2-en-1one (21) and compound 3-(5-methoxy-1H-indol-3-yl)-1(4-(methylsulfonyl)phenyl)prop-2-en-1-one (22) were found to demonstrate a significant activity. [47] Antifungal and Antibacterial Activity
Ramya v et al synthesized a series of novel 5-(nitro/bromo)-styryl-2-benzimidazole derivatives and tested for the antibacterial activity against Staphylococcus aureus, Escherichia coli, Enterococcus faecalis, and Klebsiella pneumoniae and antifungal activity against Candida albicans and Aspergillus fumigates. This was comparable with ciprofloxacin. [48]
Anticonvulsant Activity
Zhe-Shan Quan et al. [99] reported a series of 5-alkoxy- [1, 2, 4]triazolo[4,3-a]quinoline derivative with anticonvulsant activity evaluated by the maximal electroshock test (MES) and their neurotoxicities were measured by the rotarod test. 5-hexyloxy-[1, 2, 4]triazolo[4,3-a] quinoline (Structure 34) was found to be most potent anticonvulsant, with median effective dose (ED50) of 19.0 mg/kg. They extended their work to synthesized a series of 7-alkoxy-4,5-dihydro- [1,2,4]triazolo[4,3-a]quinoline-1(2H)- one derivatives and compound 7-benzyloxyl- 4,5- dihydro-[1,2,4]thiazolo [4,3-a]quinoline-1(2H)-one (Structure 35) was among the most active with (ED50) of 12.3 mg/kg. Derivatives of 8-substituted quinoline were synthesized and tested against seizures induced by maximal electro shock (MES), pentylenetetrazole (scMet) and compound 8-(3'-(4"-phenylpiperazino)- 2'-hydroxypropyloxy) quinoline was potent in both model of seizure. [49]
CONCLUSION
The introduction of a new, convenient option that The scope of nitrogen-based compounds in medicine is growing daily and their diverse analogs provide a viable and important path for discover of drugs with various biological applications. The N-heterocyclic frameworks offer a high degree of structural diversity that has proven useful for the search of new therapeutic agents in improving the pharmacokinetics and other physicochemical features. Numerous drugs that are currently in clinical practice have fatal side-effects and have developed multidrug resistance, and have been extensively used in practice to treat various types of diseases with high therapeutic potency. Research and development of nitrogen-based compounds in medicinal chemistry has become a rapidly developing and increasingly active topic. A large amount of work has been made towards N-heterocyclic skeleton medicinal chemistry. The overwhelming advantages of nitrogen-containing drugs in the medicinal field, including easy preparation, low toxicity, less adverse effects, high bioavailability, lower drug resistance, good biocompatibility, etc., encourage efforts towards further research and development. Hence, the properties of these scaffolds are vital to the synthetic strategy in the current drug discovery and design system. In this review, we mainly covered and widely described the current trends in families of nitrogen-based heterocyclic molecules namely, oxazole, pyrazole, imidazole, pyrimidine, quinoline and its derivatives, with highly promising biological properties such as anticancer, anti-inflammatory, antibacterial, antifungal, antitubercular, antidiabetic, antioxidant, anti-HIV and other medicinal properties. These significant points confirm the enormous potential of various N-heterocyclic cores in pharmaceutical applications suggesting a massive scope for these promising moieties because of their diverse molecular targets. We believe that this review article will be valuable for encouraging the structural design and development of sustainable and effective nitrogen-based drugs against various diseases, with minimal side-effects.
REFERENCES
- Gumus, S. (2011). "A computational study on substituted diazabenzenes" (PDF). Turk J Chem. 35: 803–808. Archived from the original (PDF) on 2016-03-03. Retrieved 2014-04-10.
- Gilchrist, Thomas Lonsdale (1997). Heterocyclic chemistry. New York: Longman. ISBN 978-0-582-27843-1.
- Joule, John A.; Mills, Keith, eds. (2010). Heterocyclic Chemistry (5th ed.). Oxford: Wiley. ISBN 978-1-405-13300-5.
- Chisholm, Hugh, ed. (1911). "Quinoline" . Encyclopædia Britannica. Vol. 22 (11th ed.). Cambridge University Press. p. 759
- Gilchrist, T.L. (1997). Heterocyclic Chemistry (3rd ed.). Essex, UK: Addison Wesley Longman.
- N. Azizi, A. Khajeh-Amiri, H. Ghafuri, M. Bolourtchian, M. R. Saidi, Synlett, 2009, 2245-2248.
- J. L. Hann, C. L. Lyall, G. Kociok-Köhn, S. E. Lewis, J. Org. Chem., 2023, 88, 13584-13589.
- J. C. Borghs, Y. Lebedev, M. Rueping, O. El-Sepelgy, Org. Lett., 2019, 21, 70-74.
- W. Fu, L. Zhu, S. Tan, Z. Zhao, X. Yu, L. Wang, J. Org. Chem., 2022, 87, 13389-13395.
- Z.-G. Lea, Z.-C. Chen, Y. Hu, Q.-G. Zheng Synthesis, 2004, 1951-1954.
- G. Bratulescu, Synthesis, 2009, 2319-2320.
- B. Pooi, J. Lee, K. Choi, H. Hirao, S. H. Hong, J. Org. Chem., 2014, 79, 9231-9545.
- J. Sisko, A. J. Kassick, M. Mellinger, J. J. Filan, A. Allen, M. A. Olsen, J. Org. Chem., 2000, 65, 1516-1524.
- S. Shi, K. Xu, C. Jiang, Z. Ding, J. Org. Chem., 2018, 83, 14791-14796.
- J. Li, X. Jia, J. Qiu, M. Wang, J. Chen, M. Jing, Y. Xu, X. Zheng, H. Dai, J. Org. Chem., 2022, 87, 13945-13954.
- N. A. Strotman, H. R. Chobanian, Y. Guo, J. He, J. E. Wilson, Org. Lett., 2010, 12, 3578-3581.
- B. A. Kulkarnia, A. Ganesan, Tetrahedron Lett., 1999, 40, 5637-5638.
- F. Besselièvre, S. Lebrequier, F. Mahuteau-Betzer, S. Piguel, Synthesis, 2009, 3511-3512.
- J. Wang, Y. Cheng, J. Xiang, A. Wu, Synlett, 2019, 30, 743-747.
- X. Zhang, Y. He, J. Li, R. Wang, L. Gu, G. Li, J. Org. Chem., 2019, 84, 8225-8231.
- Y. Zhang, J. Liu, X. Jia, Synthesis, 2018, 50, 3499-3505.
- Z. Fan, J. Feng, Y. Hou, M. Rao, J. Cheng, Org. Lett., 2020, 22, 7981-7985.
- S. Onodera, T. Kochi, F. Kakiuchi, J. Org. Chem., 2019, 84, 6508-6515.
- N. Panda, A. K. Jena, J. Org. Chem., 2012, 77, 9401-9406.
- D. C. Schmitt, A. P. Taylor, A. C. Flick, R. E. Kyne, Jr., Org. Lett., 2015, 17, 1405-1408.
- S. D. Schnell, J. A. González, J. Sklyaruk, A. Linden, K. Gademann, J. Org. Chem., 2021, 86, 12008-12023.
- Q. Gao, Y. Zhu, M. Lian, M. Liu, J. Yuan, G. Yin, A. Wu, J. Org. Chem., 2012, 77, 9865-9870
- Z. Fan, Z. Pan, L. Huang, J. Cheng, J. Org. Chem., 2019, 84, 4236-4245.
- Z. Liu, J. Lou, J. Xiao, Org. Lett., 2021, 23, 1606-1610.
- T. Kodama, I. Sasaki, H. Sugimura, J. Org. Chem., 2021, 86, 8926-8932.
- S. D. Jadhav, A. Singh, Org. Lett., 2017, 19, 5673-5676.
- T. Sasada, F. Kobayashi, N. Sakai, T. Konakahara, Org. Lett., 2009, 11, 2161-2164.
- F. Fang, J. Xia, S. Quan, S. Chen, G.-J. Deng, J. Org. Chem., 2023, 88, 14697-14707.
- J.-L. Zhang, M.-W. Wu, F. Chen, B. Han, J. Org. Chem., 2016, 81, 11994-12000.
- L. Su, K. Sun, N. Pan, L. Liu, M. Sun, J. Dong, Y. Zhou, S.-F. Yin, Org. Lett., 2018, 20, 3399-3402.
- Y. Sun, K. Gao, J. Org. Chem., 2023, 88, 7463-7468.
- T. Zhang, Y. Lv, Z. Zhang, Z. Jia, T.-P. Loh, Org. Lett., 2023, 25, 4468-4472.
- D. Zhang, T. Iwai, M. Sawamura, Org. Lett., 2020, 22, 5240-5245.
- H.-G. Cheng, M. Pu, G. Kundu, F. Schoenebeck, Org. Lett., 2020, 22, 331-334.
- K. Maeda, R. Matsubara, M. Hayashi, Org. Lett., 2021, 23, 1530-1534.
- V. Iosub, S. S. Stahl, Org. Lett., 2015, 17, 4404-4407.
- J. Noh, J.-Y. Cho, M. Park, B. Y. Park, J. Org. Chem., 2023, 88, 10682-10692.
- K. D. Kim, J. H. Lee, Org. Lett., 2018, 20, 7712-7716.
- W. Jo, S.-y. Baek, C. Hwang, J. Heo, M.-H. Baik, S. H. Cho, J. Am. Chem. Soc., 2020, 142, 13235-13245.
- J. Li, J. Zhang, H. Yang, G. Jiang, J. Org. Chem., 2017, 82, 3245-3251.
- K. R. Roesch, R. C. Larock, Org. Lett., 1999, 1, 553-556.
- D. Yang, S. Burugupalli, D. Daniel, Y. Chen, J. Org. Chem., 2012, 77, 4466-4472.
- L. Su, S. Xie, J. Dong, F. Liu, S.-F. Yin, Y. Zhou, Org. Lett., 2022, 24, 5994-5999.
- Y. N. Aher, A. B. Pawar, J. Org. Chem., 2022, 87, 12608-12621.
- C. Suzuki, K. Hirano, T. Satoh, M. Miura, Org. Lett., 2015, 17, 1597-1600.


 Annabhathula Srilaya*
Annabhathula Srilaya*

























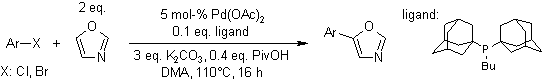






 10.5281/zenodo.13774655
10.5281/zenodo.13774655