Abstract
As nanocarriers, liposomes have long been studied for their ability to deliver medications to their sites of action while lowering toxicity.Nanotechnology is among the most exciting new technologies of the twenty-first century.Phospholipids give liposomes their form. Anticancer medications, among other medications, can be delivered by liposome encapsulation. Liposomes can include both hydrophilic and hydrophobic medicines. Once closed lipid bilayer vesicles were discovered, the name liposomes was first used in 1960. As medication carriers, liposomes are helpful, but making them using organic solvents calls for sophisticated techniques. Although destroying every cancer cell is a challenge, that can be utilised in cancer treatment. The majority of the time, liposomes are nanosized lipid particles. Although they need sophisticated fabrication techniques involving organic solvents, liposomes are helpful as medication carriers. Given that cancer causes millions of deaths annually, it remains a serious public health concern. These advantages have led to the licensing and effective usage of several liposomal medications in clinics over the last few decades. Liposomes are a promising option for drug delivery and cancer treatment in the realm of nanomedicine because of their ability to retain drugs with different physical and chemical properties. Furthermore, the potential, challenges, and prospective improvements to liposomal medicines for cancer treatment were investigated. This review describes liposome, history, structure, composition, types, preparation methods, evaluation parameters and clinical applications.
Keywords
Liposomes, Nanomedicine, Phospholipids, Cancer.
Introduction
Cancer is a disease that causes healthy body cells to become abnormally divided and is acknowledged as one of the major medical challenges of the twenty-first century. A major medical concern of the twenty-first century, this complication is brought on by the accumulation of environmental toxins or genetic abnormalities. Cancer claims millions of lives each year, and both the death rate and the number of new cases increase each. Cancer was estimated to have killed 9.6 million people worldwide in 2018, making it the second most common cause of death, according to World Health Organization (WHO) data. Developing and low-income nations account for over 70% of cancer-related deaths. On the other hand, it's also important to take wealthy nations' cancer incidence and mortality rates into account. Antitumor agent-infused chemotherapy is well recognized as a crucial cancer treatment. The poor sensitivity and specificity of free drug chemotherapy makes it difficult to use. Consequently, this constraint has hindered sufficient anticancer effect exertion and precluded accurate treatment due to side effects. Chemoimmunotherapy, a concurrently administered combination treatment, has also been proposed as a viable and effective approach to cancer therapy, specifically targeting tumour cells resistant to traditional pharmaceuticals. Many traditional and cutting-edge cancer treatments have been developed and used in recent years. For example, different nanomedicines, such as virus nanoparticles (VNPs), quantum dots, polymer nanomaterials, and liposomes, have been used to lessen the negative effects of conventional anticancer treatments, notably chemotherapy agents. Liposomes, which are spherical nanoparticles (NPs), have a unique structure among several nanomedicines. The liposome exhibits a notable advantage over numerous other nanocarriers due to its two aqueous and organic phases, which facilitate the trapping of hydrophilic and hydrophobic substances. Encasing anticancer medications within various types of liposomes is one method to improve their specificity, bioavailability, and biocompatibility. Considerable efforts have been undertaken in the last 20 years to use liposomes for medicinal applications. While some of these medications—like DaunoXome® and Caelyx®—have received approval for both clinical and general use, others are nearing the end of production and approval processes. Therapeutic liposomes come in a variety of forms, including pH-sensitive liposomes and immunoliposomes. Targeted drug delivery systems (DDSs), another name for immunoliposomes, are a broad class of nanomedical devices that have demonstrated strong anti-malignant benefits in studies and research. Known by another name, pH-sensitive liposomes are a class of polymorphic liposomes in which a change in pH causes the structure and constituent molecules to change, releasing the medicine within. In addition to being used as drug delivery systems, liposomes, like other nanomedicines, may also be used for imaging, diagnostics, and tissue regeneration. The identification, management, and treatment of illnesses and malignancies are made easier by the use of liposomes in many different contexts. [1]

Fig: -1 Liposomes.
Advantages of Liposomes: -
1) Liposomes are able to form complexes with molecules that are negatively or positively charged.
2) The DNA is partially shielded from deteriorative processes by liposomes.
3) Large DNA fragments—possibly even larger than a chromosome—can be carried by liposomes.
4) Targeting particular cells or tissues with liposomes is possible.
5) Increase of efficacy and therapeutic index of drug.
6) Increase of drug/molecule stability thanks to the encapsulation.
7) Non-toxic, flexible, biocompatible, biodegradable, and nonimmunogenic
8) Reduction in toxicity of the encapsulated agents.
9) Reduction of the exposure of sensitive tissues to toxic drugs.
10) Improved pharmacokinetic.
Disadvantages of Liposomes: -
1) The expense of production is large.
2) Drug or molecule fusion and leakage through encapsulation.
3) Sometimes processes resembling hydrolysis and oxidation occur in phospholipids.
4) Limited half-life.
5) Minimal solubility.
History of Liposomes: -
The name liposome is derived from two Greek Word ‘Lipos’ meaning fat and and ‘soma’ meaning body. Liposomes were first described by British hematologist Dr Alec D Bangham FRS in 1961, At the Babraham institute, in Cambridge. A liposome is a tiny bubble, made out of the same material as a cell membrane. Since the first introduction of nanotechnology in 1959 by Richard Feynman in his Caltech address, "There is plenty of room at the bottom," nanomedicine in its current form has been viewed as a possibility. [2] From the first investigations into the behavior and structure of tiny lipid particles in an aqueous environment to the first lipid-based drug delivery nanoparticle authorized by the US FDA, around fifty years passed. Pliny the Elder's initial observations, made about 2000 years ago marked the beginning of the process of examining the behavior of lipids and fat particles in aqua. Anthonie Van Hook's discovery of the cell in the late seventeenth century led to a number of inquiries concerning the composition of cells.[3]
Structures of Liposomes: -Depending on the compartment structure and lamellarity, liposomes can be categorized as unilamellar vesicles (ULVs), oligo lamellar vesicles (OLVs)multilamellar vesicles (MLVs), or multivesicular liposomes (MVLs) (Figure 1). Although OLVs and MLVs have a structure similar to that of an onion, they also have two to five or more concentric lipid bilayer. There have been reports of ULVs in a variety of sizes, including SUVs under 200 nm and LUVs between 200 and 500 nm. A lot of work has gone into defining liposomal NPs and understanding their rational features. These days, liposomes are described as spherical, spontaneously forming pieces with a hydrophilic center and a lipid bilayer membrane. [4] The size of liposomes varies, ranging from around 10 nm to 2500 nm (or 2.5 µm). Nonetheless, the majority of liposomes used to administer drugs usually range in size from 50 to 450 nm.

Fig:-2 Structure Of Liposomes.
Liposomal drug delivery system classifications and architectures. (a) A structural diagram showing the makeup of liposomes. The size of the inner aqueous core is significantly larger than that of a normal phospholipid bilayer, which has a diameter of 4.5 nm. (b) Liposomal vesicles are categorized based on their particle size, lamellarity, and compartment; (c) Liposome sizes and lamellarities vary; (d,e) Doxil and Vyxeos' cryo-transmission electron microscopy (f,g) Electron micrographs of DepoFoam particles, which often have a diameter of 1–100 ?m (DepoCyt, for example), and MLVs, which typically have a diameter of 0.2–5 ?m (Mepact, for example).
[5]
LIPOSOMES COMPOSITIONS: -
1. Phospholipids Used For Liposomes: -
The various programs encompass every aspect of phospholipid research, from fundamental to practical, covering everything from the utilization of phospholipids in various forms of administration—such as liposomes, mixed micelles, emulsions, and extrudates—to research focused on industrial applications. Additionally, parenteral, oral, and topical delivery routes are all included in these programs. With this analysis, we hope to shed light on potential avenues for future investigation and provide a brief overview of the field of phospholipids. Phospholipids are special and multifunctional compounds. They are the primary constituents of cellular membranes and are found naturally. Arranged as a lipid bilayer, phospholipids play a crucial role in the construction and functionality of cellular membranes. They have a lipophilic/hydrophobic tail and a hydrophilic head group, making them amphiphilic. amphiphilic The chemical structure of phospholipids is demonstrated by 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and its various alternative headgroups, which differ in the type of alcohol they contain: phosphatidylethanolamine (PE) with ethanolamine, phosphatidylglycerol (PG) with glycerol, phosphatidylserine (PS) with serine, and phosphatidylinositol (PI) with inositol.[6]
2. Natural Lipids: -
Normal cell membrane bilayers are mostly made of glycerophospholipids. A glycerol unit is joined to two fatty acid molecules and a phosphate group (PO42-) to form phospholipids. Additionally, the phosphate group can form a connection with the small, vital chemical molecule choline (Figure 2A). Egg yolks and soybeans are two common sources of natural phospholipids. With reference to the polar head groups, phospholipids are categorized as phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI), phosphatidylglycerol (PG), and phosphatidic acid (PA). Because the hydrocarbon chain is unsaturated, natural phospholipids are less stable than synthetic phospholipids when liposomes are being prepared. Natural phospholipids are made up of different types of fatty acids: oleic acid, also known as 9Z-octadecenoic acid, which is found in egg yolk lecithin; margaric acid, also known as heptadecanoic acid, H3C-(CH2)15-COOH); and palmitic acid, also known as hexadecanoic acid, which is a saturated fatty acid. These fatty acid patterns make up the PCs and phospholipids formed from eggs: arachidonic acid (C20:4), oleic acid (C18:1), linoleic acid (C18:2), stearic acid (C18:0), and palmitic acid (C16:0). [7]
3.Steroids: -
A subset of immune-related, potentially blinding inflammatory disorders known as non-infectious anterior uveitis (AU) makes up 60% of all uveitis patients encountered in eye centers 1,2,3,4,5. Prolonged uncontrolled inflammation can lead to sight-threatening disorders in the eyes, such as glaucoma, cataract development, and central retinal edema. In as many as 25% of patients, these problems result in blindness 6, 7 Topical eye drop therapy is currently the gold standard treatment for anterior uveitis, with corticosteroids being the primary choice8. On the other hand, topical eye drop application has a few drawbacks: 1. The drug's short precorneal drug residence time, small conjunctival sac capacity (25 ?l), and nasolacrimal duct drainage all contribute to the low bioavailability of the drug. Less than 5% of medications administered topically are thought to be absorbed by the eyes10; 2. Many drug delivery methods, including those based on polymers and lipids, have been investigated for the treatment of ocular illnesses in an effort to circumvent the issues of low bioavailability and side effects,12,13,14. Because they are biocompatible and biodegradable, liposomes are the most researched nanocarriers in ocular diseases14. Clinical trials for eye disorders have already included a few liposomal formulations 15,16.[8]
4. Surfactants: -
Therapeutic treatments are frequently hampered by adverse side effects, poor drug metabolism, inadequate patient compliance, or rejection of invasive therapy. Numerous drug carriers have been created to address the aforementioned issues; nevertheless, certain carriers, including liposomes, niosomes, or microemulsions, are mostly restricted to the skin's surface and do not effectively transfer medications through the skin.Many strategies have been used to get around this stratum corneum barrier-related issue. These include the use of forces that do not depend on concentration gradients (iontophoresis, electroporation, phonophoresis, microneedles, jet injectors, etc.) to increase skin permeability, and, more recently, modifications to the drug carrier itself (e.g., using vesicles).With an architecture made up of both hydrophobic and hydrophilic moieties, elastic vesicle scaffolds have been designed and tested as innovative topical and transdermal delivery systems. As a result, these scaffolds can hold drug molecules with a broad range of solubility. Better penetration of intact vesicles is made possible by these vesicles' high deformability. In terms of both quantity and depth, this method is far more effective in delivering both high- and low-molecular-weight medications to the skin. [9]
TYPES OF LIPOSOMES: -
1.Conventional Liposomes: -
2.Charged Liposomes: -
3.Stealth Stabilised Liposomes: -
4.Actively-targeted Liposomes: -
5.Stimuli-responsive Liposomes: -
1.Conventional Liposomes: -
These liposomes, which were the first generation of liposomes, were created using synthetic or natural phospholipids, either with or without cholesterol.To increase the liposomes' fluidity, cholesterol was introduced, which changed the liposomes' stability and bilayer rigidity. According to Wu et al., the addition of cholesterol to liposomes made of DSPE-PEG2000 and hydrogenated soybean phospholipids (HSPC) resulted in a decrease in liposomal membrane stiffness. Furthermore, somewhat stiff liposomes demonstrated superior tumor penetration and heightened anti-tumor efficacy. Kaddah et al. looked into the effects of cholesterol on the DPPC liposome membrane's permeability and fluidity. A high dose of cholesterol caused the average liposome to grow in size and change in shape from irregular to regular, spherical, and nanoscale vesicles. Furthermore, cholesterol decreased the fluidity of the bilayer and altered the release of hydrophilic molecules from lipid vesicles. Since conventional liposomes were easily eliminated by the mononuclear phagocyte system and quickly accumulated in the liver and spleen, they demonstrated a short blood circulation period. As a result, MPS limits the dispersion of traditional liposomes to other bodily tissues and prevents them from reaching the intended site. In vitro, conventional liposomes likewise demonstrated comparatively little stability. Thus, to improve in vivo liposome stability and blood circulation, stealth stable liposomes were developed. [10]
Applications Of Conventional Liposomes: -
As most conventional liposomes are readily engulfed by macrophages, they can be employed to treat disorders affecting the macrophage system. Furthermore, a variety of fungi and parasites reside in macrophages, and pathogenic conditions such visceral Leishmaniasis can be treated by using traditional liposomes that specifically target them. In addition, traditional liposomes are mostly employed as adjuvants in vaccines to deliver antigenic materials to the bloodstream and boost immunity. [11]
2.Charged Liposomes: -
Differentially charged liposomes have been thoroughly investigated for their ability to activate dendritic cells (DCs), which are important antigen-presenting cells and are essential in bridging the gap between innate and adaptive immunity. It is unclear, therefore, how the differentially charged liposomes affect DC activation. In this work, we have examined the effects of neutral, anionic, and cationic liposomes based on 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) on the uptake, immunostimulation, and intracellular fate in DCs obtained from mouse bone marrow. The results of fluorometric analysis using a pH-sensitive dye and flow-cytometry-based pathway inhibition assays revealed that cationic liposomes were taken up by DCs more efficiently through endocytosis and transported to neutral compartments for further processing, whereas anionic and neutral liposomes were inclined to accumulate in acidic compartments. These findings therefore endorse the use of cationic DSPC liposomes as a preferred option for vaccine delivery vehicles over neutral and negatively charged liposomes, especially for the preferential activation of DCs. The creation of powerful adjuvants and efficient delivery methods is essential to the development of subunit vaccines. These vaccines work only when antigens are delivered, if they are well absorbed by antigen-presenting cells (APCs) and subsequently trigger immune responses.[12]
3.Stealth Stabilised Liposomes:-
Liposomes are one of the more promising new drug-delivery technologies. Several formulations of liposomes are now being used in clinical settings. Liposomes are an advanced method for delivering active compounds to the site of action. The field of liposome technology has advanced from studying typical vesicles, often known as "first-generation liposomes," to studying "second-generation liposomes," which produce long-circulating liposomes by adjusting the vesicle's size, charge, and lipid content. Additionally, liposomes with altered surfaces have been created employing a variety of compounds, including glycolipids and sialic acid. Adding the synthetic polymer poly-(ethylene glycol) (PEG) to liposome composition was a major advancement toward the production of long-circulating liposomes. Many liposome formulations including active compounds with excellent target efficiency and activity have been produced as a result of this technology. Additionally, stealth liposomes can be actively targeted with monoclonal antibodies or ligands through synthetic alteration of the terminal PEG molecule. This study addresses developing advances in this promising technology, with a particular focus on stealth technology. It also summarizes pre-clinical and clinical data related to the main liposome formulations.[13]
4. Actively-Targeted Liposomes: -
It is now approved to use many liposome nanocarriers for the passively targeted delivery of anticancer medications. But passive targeting can't tell the difference between healthy and unhealthy cells. In order to improve the location and accumulation of liposome medications in tumor tissues, tailored liposomes have been produced. Targeting the overexpressed surface receptors of cancer cells and the tumor microenvironment (TME) are two ways that active targeting liposomes can increase therapeutic efficacy and decrease adverse effects. Proteins, peptides, growth factors, aptamers, antibodies, and vitamins are examples of ligands for actively targeted liposomes. Antitumor medications typically cause substantial side effects, which has limited their clinical use. Liposomes have been extensively utilized and researched in tumors due to their remarkable qualities, which allow them to be an excellent medication carrier in the field of tumor therapy. Numerous pharmacological preparations based on liposomes have been effectively converted into clinical applications in recent years due to the technology's quick advancement. [14]
5.Stimuli-Responsive Liposomes: -
By distributing the active pharmaceutical component (API) to a particular location of action, medication delivery ultimately aims to maximize the API's bioavailability and decrease its hazardous side effects. While limiting systemic release and encouraging prolonged circulation to enhance uptake at the cancerous site due to the enhanced permeation and retention effect (EPR), the association of the therapeutic agent with a carrier system can minimize damage to healthy, non-target tissues in the case of antitumor therapy. Carrier systems have been developed from a wide range of materials, including inorganic nanoparticles, lipids, and polymers that have been endowed with stimuli-sensitive properties to achieve triggered release. Stimuli-responsive systems have emerged as a promising means of delivering and releasing payloads in a site-selective manner. Tumor microenvironment characteristics, such as pH, redox potential, or distinct enzymatic activity, can operate as endogenous stimuli. In order to effect the release of medications encapsulated in liposomes, triggered release is typically achieved through the membrane instability resulting from local flaws within bilayer membranes. The literature published between November 2008 and February 2016 that describes novel advancements in stimuli-sensitive liposomal drug delivery methods utilizing redox processes, pH shift, enzyme transformation, and photochemical activation mechanisms is the main emphasis of this study.[15]
6.Bubble Liposomes Method: -
Anticancer medications that are encapsulated in liposomes have a better therapeutic window due to increased antitumor activity and less adverse effects. Many research groups have attempted to create tumor-targeting liposomes with increased drug release in an attempt to create more potent liposomal formulations for anticancer therapy. Our previous work included the development of AG73-Dox, doxorubicin-encapsulated AG73 peptide-modified liposomes that targeted endothelial cells and cancer, as well as "Bubble liposomes," or gas-entrapping liposomes for ultrasound imaging (BLs). In this work, we coupled AG73-Dox with BLs and US to increase its anticancer efficacy. First, we assessed the cytotoxicity of AG73-Dox in conjunction with BLs and US to see if adding these two factors may increase AG73-Dox's cytotoxicity. In order to ascertain whether BLs and US application could augment AG73-Dox's cytotoxicity, we first assessed the cytotoxicity of AG73-Dox in conjunction with BLs. More so than non-targeted Dox-encapsulated liposomes, BLs and US increased the cytotoxicity of AG73-Dox. After treating Dox with BLs and US, we next looked at its intracellular behavior. While AG73-Dox plus BLs and US did not improve Dox's cellular absorption, it did encourage drug release in the cytoplasm. We inhibited cellular absorption via endosomes at a low temperature in order to better understand the release of Dox in the cytoplasm. Because of this, until AG73-Dox was absorbed into cells, BLs and US did not exhibit an improved drug-release impact. As a result, AG73-Dox combined with BLs and US may be helpful for cancer treatment as a dual-purpose drug delivery mechanism with regulated and targeted release.[16]
METHODS OF LIPOSOMES PREPARATION :-
1.Thin Film Hydration Method :-
An advantageous approach for loading the lipophilic medication is the thin-film hydration method. Lipid-solvent solution evaporation occurs during flask rotation under vacuum, resulting in a thin film. You can obtain MLVs suspension by hydrating the lipid film with the aqueous solution. The drug material can be passively or actively injected during or after the liposome production, respectively, and the particle size can be further lowered to produce SUVs. This manufacturing process is used for the commercial products of AmBisome, Visudyne, and Shingrix (Adjuvant systemAS01B) . A lactose solution is used to hydrate the ingredients after they are evaporated, and filtering, lyophilization, homogenization, and size reduction are the processes used to make Visudyne, for instance. System adjuvantAS01B, a single vial in the Shingrix product line, is a liposome-based adjuvant that contains two immune enhancers: MPL (3-O'Desacyl-4?-monophosphoryl lipid A) and QS21 (a triterpene glycoside that has been isolated from the bark of the tree Quillajasaponaria Molina). After dissolving in the organic solution, the MPL and additional lipids are dried. For formulation, the QS21 aqueous solution is added after hydration and size reduction. Preparing liposomes using thin-film hydration and extrusion is one of the most straightforward and popular techniques. The process creates a thin layer of lipids by letting an organic solvent evaporate, hydrating the dry lipid layer with an aqueous phase, and then extruding the mixture through a series of polycarbonate filters to create unilamellar vesicles.

Fig: -3 Thin Film Method.
The following are some benefits of using the thin film hydration process to create liposomes.
- The method is simple to perform and widely accepted in preparation liposomes.
- It utilizes common organic solvents (chloroform, methanol) for dissolution of lipids.
- Homogenous unilamellar vesicles are formed after extrusion of hydrated lipid phase.
- Formulation of single and dual drug-loaded liposomes by passive loading is possible using thin film hydration method.[17]
2. Double-Emulsification Method: -
The interior aqueous phase (W1) of water-in-oil-in-water (W1/O/W2) double-emulsion globules contained tubular liposomes carrying a hydrophilic model molecule (fluorescein sodium salt, FSS). Our theory was that liposomes and their contents may be better controlled in terms of release by acting as a layer of defense provided by the oil membrane of double emulsions. Bulk liposomes were produced, and individual double-emulsion globules' release of liposomes was seen under a microscope. External coalescence was used to release the liposomes containing FSS, and capillary video microscopy was used to visually observe how this system behaved. The water-soluble surfactant Tween 80 and the oil-soluble surfactant Span 80 were used to stabilize the double-emulsion globules, with n-hexadecane serving as the oil phase (O). The tubular liposomes are composed of Ceramide-VI and l-?-phosphatidylcholine as lipids. The release of liposomes from the W1 phase into the W2 phase was regulated by changes in the concentrations of Span 80 in the O phase and Tween 80 in the external aqueous phase (W2). The main discovery of this work is that double-emulsion globules can be stabilized just by the liposomes' presence in the W1 phase.[18]
Formulated Double Emulsion Based Dispersion: -
Double emulsion solvent evaporation is the most widely utilized double emulsion approach for active molecule encapsulation and micro- and nanoparticle production. This method was first applied to microencapsulation (Pisani et al., 2008; Alex and Bodmeier, 1990). This approach involves two steps for homogenization: first, water soluble medications are added to the oil phase (O) and polymer/lipophilic pharmaceuticals are introduced to the inner aqueous phase (W1). Finally, both steps are combined.[19]
3. Detergent Removal (Depletion) Method: -
Lipids are hydrated (and solubilized) using a detergent solution in the detergent removal process. Mixed (detergent/lipids) micelles are created when the detergent combines with the phospholipids, preventing the hydrophobic parts from coming into contact with the aqueous phase. The mixture of micelles becomes richer in lipids when the detergent is removed gradually, leading to the creation of unilamellar vesicles. High CMC detergents including sodium cholate Triton X-100, sodium deoxycholate, and alkyl glycoside are frequently used. There are various ways to achieve detergent elimination. The dilution procedure (by 10- to 100-fold) using a buffer is the most straightforward way to remove detergent. The first micelles in a mixed lipid–detergent system become larger and more polydisperse when diluted with a buffer of the aqueous solution. Finally, once the system is diluted past the mixed micellar phase boundary, a spontaneous transition from polydisperse (elongated) micelles to vesicles takes place. As the dilution factor increased in the aqueous solution of a mixed system made up of detergent and lecithin, the aggregates gradually changed from spherical micelles to longer, flexible cylindrical micelles to nearly monodisperse unilamellar vesicles (at the higher dilution factors).
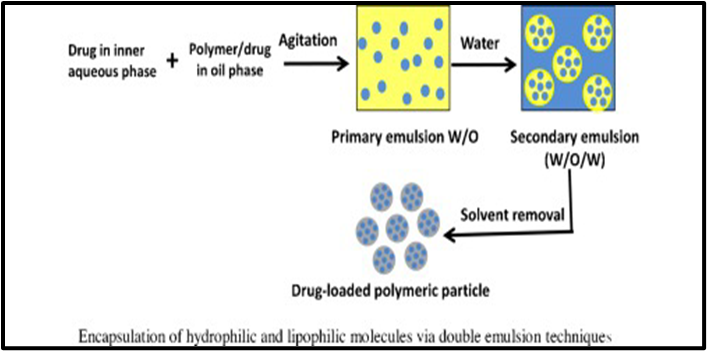
Fig: - 4 Detergent Removal Method
The idea of spontaneous curvature (and the crucial packing factor) can be used to explain this sequence. Bile salt alone creates highly curved (spherical) micelles, whereas lecithin alone forms aggregates of low spontaneous curvatures. Therefore, in the mixed lecithin-bile salt system, spherical (or elongated) mixed micelles develop at high bile salt levels. Due to the fact that bile salt is significantly more soluble in water than lecithin, dilution after dilution reduces the amount of bile salt in the aggregates, which in turn reduces the spontaneous monolayer curvature and promotes the production of liposomes. Detergent dialysis is an alternate method for removing detergent that offers good repeatability and produces liposome populations of uniform size in the end. Nevertheless, this method leaves residues of the detergent(s) in the liposomal nanoformulation. Finally, other effective methods for removing detergent have included centrifugation, column gel chromatography, and adsorption onto hydrophobic resin beads.[20]
4. Freeze-Drying (Lyophilization) Method: -
In order to produce a solid dosage form meant for various routes of administration (parenteral, oral, nasal, and/or pulmonary), liposome dispersions must still be freeze-dried nowadays. Years of research have not, however, improved process condition optimization because drug leakage occurs when freezing and dehydration upset vesicle structure. The primary formulation and process parameters that can provide a product with the right qualities and boost production efficiency are reviewed in this article, which starts with the thermal properties of phospholipids. Specifically, an outline of the methods by which certain excipients are cryo- and/or lyo-protective is given, together with information on the potential application of co-solvent combinations. The imaging techniques that have recently been suggested to describe the look of freeze-dried goods and liposome dispersions upon reconstitution are likewise the subject of attention. Combining this data would enable a more thorough understanding of the variables influencing inter-vial variability in an effort to raise the standard of the finished pharmaceutical product. Primary drying, secondary drying, and freezing are the three main stages of a standard freeze-drying procedure. Ice crystals occur during the freezing phase, a chilling process in which the majority of the solvent separates from the liposomes and excipients. Because annealing causes the size of the ice crystals to expand, it can also be used to decrease sample heterogeneity and lower the drying rate. When the chamber pressure is lowered to a few millibars and the shelf temperature is raised to provide the heat needed for sublimation, the major drying process begins. To enable water desorption, the temperature is increased during the secondary drying process. The retention of the entrapped chemicals and the integrity of the liposome may depend on each of these processes. The preservation of liposome structure is significantly influenced by the freezing rate as well. The creation of fine ice crystals and a uniform distribution of the protectant are the outcomes of ultrafast chilling (e.g., submerging small volume suspensions in liquid nitrogen), which may lessen the disruption of the liposomal bilayer structure. Conversely, a gradual freezing rate lowers the super. [ 21]
5. In-Situ Preparation Of Liposomes: -
The development of nanomedicines and their successful clinical application have been slowed by methodological limitations that have restricted our capacity to investigate protein corona creation. In an unlabeled process, we identified the hard and soft corona structural characteristics and the associated proteomic compositions on liposomes using surface plasmon resonance, a customized biosensor for in situ structure determination on liposomes and corona separation, and proteomics using sensitive nano liquid chromatography tandem mass spectrometry with an open-source bioinformatics platform.For in vivo relevance, undiluted human plasma under dynamic flow conditions was employed. Two light-triggered indocyanine green (ICG) liposome formulations in preclinical development and a standard liposome formulation are used to demonstrate proof-of-concept. Protein enrichment and corona structure (thickness, protein-to-lipid ratio, and surface mass density) varied depending on the formulation. Regardless of pegylation, liposomal lipids induced the enrichment of stealth-mediating apolipoproteins in the hard coronas; the pegylated liposome formulation with ICG showed their preferred enrichment in the soft corona.As a component of the biological identity influenced by the surface properties of nanomaterials, this implies that the soft corona of loosely connected proteins adds to the stealth properties. Providing for the first time genuinely complementary hard and soft corona compositions with matching in situ structural data, the methodology fills up important methodological gaps in biocorona research. It is ideal for the preclinical development of lipid nanomedicines since it has been created in a simple, repeatable single-experiment format.The safety and efficacy profile of nanoparticles (NPs) is determined by their biological identity, the protein corona, which they acquire by assimilation with their physiological milieu.1-3 A dynamic soft corona (SC) of high exchange rate proteins and a slower exchanging hard corona (HC) of tightly-bound proteins with greater surface selectivity for the nanomaterial are common descriptions of corona structure.Possible conformational changes in the bound proteins caused by nanomaterials complicate these attempts because they can only be seen in vivo and may evade the standard preclinical toxicological procedures.8. Pre-coating with artificial coronas made in vitro15 or in vivo16 can be used to lengthen their blood circulation periods and regulate biodistribution, as it was recently shown that the corona regulates NP interactions with immune cells. Similar nanoparticles can also form coronas in distinct persons, owing to the impact of disease and individual variance on plasma composition or protein structure.[22]
EVALUATION OF LIPOSOMES: -
1.Particle Size: -
Transmission electron microscopy (TEM) techniques such as cryogenic microscopy, freeze structure, and negative stain allow for the direct visualization of particle morphology and size. Negative-stain TEM has the disadvantage of requiring sample drying prior to staining, which could lead to liposome collapse, shrinkage, or aggregation. Cryo-TEM has the advantage of requiring the least amount of work for sample preparation, whereas the other two types do not require drugs. [23]
2. Percent Drug Encapsulated: -
Estimating the drug's behavior in a biological system is made easier by the amount of pharmaceuticals entrapped within the liposomes. To calculate the percentage of drug encapsulation, first separate the drug portion that is encapsulated from the free drug friction. After that, appropriate detergents are used to dissolve the liposomes in an aqueous solution containing the encapsulated drug fraction. To extract the free drug from the sample, the following techniques are used: Mini column centrifugation technique (a)b) Using protease aggregates.
3. Phase Behavior: -
Liposomes experience an irreversible phase shift at the changeover. The TC serves as a marker of drug entrapment region as well as stability and permeability. DSC is in charge of it.
4. Drug Release Rate: -
In vivo tests, which aid in the prediction of the medication's pharmacokinetics and bioavailability, can be used to measure the rate at which the drug releases from the liposomes. On the other hand, in vivo research is thought to be more thorough. For the investigation, liposomes containing the tracer insulin are used. This particular insulin is favored because of its exclusive release inside the embryonic circulation and swift elimination by the kidneys, which is correlated with the rate of degradation of the released liposome-derived tracer.
5. Sterility: -
Aerobic or anaerobic cultures are used to perform sterility tests.[24]
MARKETED CLINICAL LIPOSOMES: -
1. Cancer Treatment: -
Doxil is a doxorubicin (DOX) liposome coated in polyethylene glycol that was created with the intention of treating Kaposi's sarcoma. Sun Pharma produced LipoDox®, another FDA-approved PEGylated liposomal product encapsulating DOX, in 2012. The second anthracycline antitumor medication incorporated into liposomes for the treatment of acute myeloid leukemia (AML) was daunorubicin.[25]
2. Fungal Treatment: -
Fungizone® and Ambisome® are two of the main anti-fungal liposome formulations that have been approved. Amphotericin B, an antifungal medication, is encapsulated and has several benefits over the free form. These saline-stabilized Amphotericin B liposomes have a greater bioavailability, less toxicity, and fewer adverse effects.[26]
3. Photodynamic Therapy :-
The only liposomal medication delivery agent authorized for treating age-related macular degeneration is Visudyne®, which works by preventing the development of new blood vessels in the eye. [27]
4 .Pain Management :-
DepoDur is a sustained-release morphine formulation that extends the clinical impact duration through the use of DepoFoam Technology. Additionally, Exparel® releases Bupivacaine gradually for long-lasting pain relief via the use of DepoFoam technology.[28]
Provides an overview of the various formulations of clinically authorized liposomes, including information on their active components, lipid composition, delivery route, and intended use.
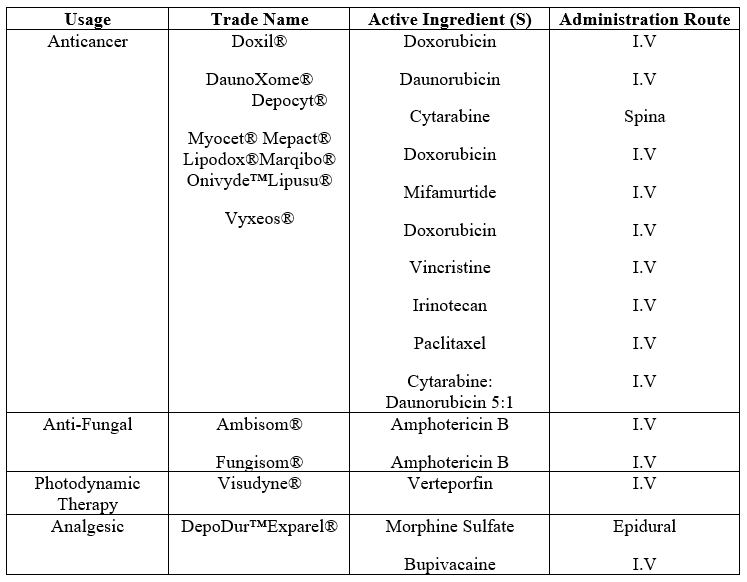
Table: -1 Clinically Used Liposomes Grouped By Therapeutic Usage: -
Application Of Liposomes In Nanomedicine: -
The encapsulated drug molecules' temporal and spatial distribution inside the body can be changed via liposome encapsulation, potentially reducing hazardous side effects and improving treatment efficacy. The uses of liposomes in pharmacology and medicine can be categorized into two main categories: the use of liposomes containing drugs or other substances for therapeutic or diagnostic purposes, and the use of liposomes as a form, instrument, or reagent in basic research on cell interfaces, recognition techniques, and the mechanism of action of specific materials. The way liposomes interact with cells and what happens to them in vivo after injection is a key factor in determining the advantages and disadvantages of liposomal drug carriers.Studies of liposomes' interactions with cells both in vitro and in vivo have revealed that simple adsorption or subsequent endocytosis is the main way in which liposomes interact with cells. Fusion involving cell membranes is far less common. The interchange of bilayer components, such as lipid and cholesterol—molecules bound to membranes—with elements of cell membranes constitutes the fourth potential interaction. The fate of liposomes in vivo is likewise determined by these interactions. The body has an intricate defense mechanism to keep itself safe. Larger things that enter the body induce thrombus development, and finally their surface is passivated by coating with bio macromolecules; in contrast, immune system cells consume smaller particles, bacteria, and colloids. This immune system reaction has led to significant efforts in the creation of biocompatible and unidentifiable surfaces. However, it has also limited the range of applications for microparticulate drug carriers to solely targeting immune system cells. Liposomes are made entirely of natural materials; this is not an anomaly. The macrophages, primarily found in the spleen, liver, and bone marrow, promptly eliminate them from the bloodstream. [29]
ACKNOWLEDGMENT: -
We are grateful to the college, friends,and teaching staff for Supporting us in preparing the review.
REFERENCES
- Foad Rommasi and Neda Esfiandri, ``A Review on Liposomal Nanomedicine: Applications for Drug Delivery in Cancer Therapy”, National Library Of Medicine, 2021;95(16).
- M. Joyce Nirmala,corresponding authors, Uma Kizhuv etil, Athira Johnson, Balaji G, Ramamurthy Nagarajan, and Vignesh Muthuvijayanb,``A Review On Cancer nanomedicine: a review of nano-therapeutics and challenges ahead'’, National Library Of Medicine, 2023;13(13)
- Eichman P. From the lipid bilayer to the fluid mosaic: a brief history of membrane models. Resource Center Social Hist Philos Sci Teach Teachers Netw News. 1999;9(2):1.
- Peng Liu, Guiliang Chen, and Jingchen Zhang, “A Review on Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives,” National Library Of Medicine, 2022;27(4) .
- Etheridge ML, Campbell SA, Erdman AG, Haynes CL, Wolf SM, McCullough J. The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomed Nanotechnol Biol Med. 2013;9(1):1–14. doi: 10.1016/j.nano.2012.05.013.
- Simon Dresher and Peter Van Hoogvest, “A Review On The Phospholipid Research Center: Current Research in Phospholipids and Their Use in Drug Delivery,”National Library Of Medicine, 2022;12(12) .
- Khater D., Nsairat H., Odeh F., Saleh M., Jaber A., Alshaer W., Al Bawab A., Mubarak M.S. Design, preparation, and characterization of effective dermal and transdermal lipid nanoparticles: a review. Cosmetics. 2021;8.
- Dandona, L., Dandona, R., John, R. K., McCarty, C. A. & Rao, G. N. Population based assessment of uveitis in an urban population in southern India. Br. J. Ophthalmol. 84, (2000).
- Sima Sing, Harsh Vardhan, Niranjan G Kotla, Balaji Maddiboyina , Dinesh Sharma and Thomas J Webster, “A Review On The role of surfactants in the formulation of elastic liposomal gels containing a synthetic opioid analgesic,”International Journal Of Nanomedicine, 2016;11 .
- Hamdi Nsairat , Dima Khater, Usama sayed, Fadwa Odeh, Abeer Al Bawab, Walhan Alshaer, “A Review On Liposomes: structure, composition, types, and clinical applications,”Heliyon Volume 8,Issue 5,(2022)
- Lee, M. K. Liposomes for Enhanced Bioavailability of Water-Insoluble Drugs: In Vivo Evidence and Recent Approaches. Pharmaceutics. 2020, 12(3):
- Mithun Maji, Sneha Ghosh, Nicky Didwania, and Nahid Ali, “A Review On Differentially Charged Liposomes Stimulate Dendritic Cells with Varying Effects on Uptake and Processing When Used Alone or in Combination with an Adjuvant,”Acs PublicationPublished June 25, (2024) ;
- Maria Laura Immordino,Franco Dosio, and Luigi Cattle, “A Review On Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential,”International Journal Of Nanomedicine, 2006; 1(3).
- Shile Wang, Yangu Chen, Qinqin Huang, Jiancheng Guo, “A Review On Liposomes for Tumor Targeted Therapy: A Review,”International Journal of Molecular Sciences, 2023;24(3)
- Y.Lee and D. H. Thompson, “A Review On Stimuli-Responsive Liposomes for Drug Delivery,”Author Manuscript Peer reviewed and accepted for publication by a journal,”2017;9(5) .
- Nobuhito Hamano, Yoichi Negishi, Daiki Omata, Yoko Takahashi, Maya Manandhar, Ryo Suzuki, Kazuo Maruyama, Motoyoshi Nomizu,and Yukihiko Aramaki, “A Review On Bubble Liposomes and Ultrasound Enhance the Antitumor Effects of AG73 Liposomes Encapsulating Antitumor Agents,” Molecular Pharmaceutics, 2013;10(2).
- Adler-Moore J., Gamble R.C., Proffitt R.T. Treatment of Systemic Fungal Infections with Phospholipid Particles Encapsulating Polyene Antibiotics. 5,874,104. U.S. Patent. 1999 February 23;
- Godin B, Sakamoto JH, Serda RE, et al. Emerging applications of nanomedicine for the diagnosis and treatment of cardiovascular diseases. Trends Pharmacol Sci. 2010;31(5):199–205.
- Muhammad Iqbal, Nadiah Zafar, Hatem Fessi, Abdelhamid Elaissari, “A Review On Double emulsion solvent evaporation techniques used for drug encapsulation,”International Journal Of Pharmaceutics, (Volume 496, Issue 2 ),2015.
- Jang H, Hu PC, Jung S, Kim WY, Kim SM, Malmstadt N. et al. Automated formation of multicomponent-encapuslating vesosomes using continuous flow microcentrifugation. Biotechnol J. 2013;8(11):1341–6. doi: 10.1002/biot.201200388.
- Silvia Franzé, Francesca Selmin, Elena Samaritani, Paola Minghetti, and Francesco Cilurzo, “A Review On Lyophilization of Liposomal Formulations: Still Necessary, Still Challenging,”Pharmaceutics Journal, 2018;10(3) .
- Otto K. Kari, Joseph Ndika, Petteri Parkkila, Antti Louna, Tatu Lajunen, Anne Puustinen, Tapani Viitala, Harri Alenius, Arto Urtti, “A Review On In situ analysis of liposome hard and soft protein corona structure and composition in a single label-free workflow,”Nanoscale, 2020;9(9)
- Stefano Giordani , Valentina Marasai, Andrea Zattoni, Barbara Roda, Pierluigi Reschiglian. “A Review On Liposomes characterization for market approval as pharmaceutical products: Analytical methods, guidelines and standardized protocols,”Journal of Pharmaceutical and Biomedical Analysis, 2023;
- Gustafsson J, Arvidson G, Karlsson G, Almgren M. 1995. Complexes between cationic liposomes and DNA visualized by cryo-TEM. Biochim Biophy Acta. 1235:305–312.
- Cabanes A., Even-Chen S., Zimberoff J., Barenholz Y., Kedar E., Gabizon A. Enhancement of antitumor activity of polyethylene glycol-coated liposomal doxorubicin with soluble and liposomal interleukin 2, Clinical cancer research. Off. J. Am. Assoc. Canc. Res. 1999;5:687–693.
- Azanza J.R., Sádada B., Reis J. Liposomal formulations of amphotericin B: differences according to the scientific evidence. Rev. Española Quimioter. : Pub. Ofic. Soc. Espan. Quimioter. 2015;28:275–281.
- Chang H.I., Yeh M.K. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 2012;7:49–60.
- Angst M.S., Drover D.R. Pharmacology of drugs formulated with DepoFoam: a sustained release drug delivery system for parenteral administration using multivesicular liposome technology. Clin. Pharmacokinet. 2006;45:1153–1176.
- Hadis Daraee,Ali Etemadi,Mohammad Kouhi,Samira Alimirzalu &Abolfazl Akbarzadeh, “A Review On Application of liposomes in medicine and drug delivery,”Artificial Cells, Nanomedicine, and Biotechnology , 2014;381-391.


 Karishma Yakub Maneri* 1
Karishma Yakub Maneri* 1
 Inagle Sanjay 2
Inagle Sanjay 2





 10.5281/zenodo.13997934
10.5281/zenodo.13997934