Abstract
Biodegradable electrospun bandages represent a promising innovation in wound healing, offering advanced structural and biological benefits over conventional dressings. Electrospinning technology enables the fabrication of nano- and microfibrous scaffolds, closely resembling the extracellular matrix (ECM), thereby promoting cell adhesion, proliferation, and migration for faster tissue regeneration. These bandages are composed of biodegradable polymers such as polycaprolactone (PCL), polylactic acid (PLA), gelatin, chitosan, and sodium alginate, ensuring biocompatibility while reducing the need for frequent dressing changes. Functionalized electrospun bandages can be loaded with bioactive agents, including antibacterial nanoparticles, growth factors, and anti-inflammatory drugs, providing controlled drug release and reducing the risk of infection and inflammation. Their moisture-retaining properties create an optimal healing environment, especially beneficial for chronic wounds, diabetic ulcers, and burn injuries. Additionally, smart wound dressings incorporating stimuli-responsive polymers that react to environmental cues such as pH, temperature, or bacterial activity are being developed, further enhancing their therapeutic potential. With their ability to accelerate wound healing, prevent infections, and reduce patient discomfort, biodegradable electrospun bandages hold significant clinical value. Ongoing research into bioactive and responsive materials is expected to advance this technology, making electrospun dressings a gold standard for wound management in the near future.
Keywords
Plant Extract, Wound Healing Applications, electrospun bandages, dressing changes.
Introduction
Biodegradable electrospun bandages are emerging as a revolutionary approach to wound healing, offering an advanced alternative to traditional wound dressings. These bandages are fabricated using electrospinning, a technique that produces nano- and microfibrous scaffolds, mimicking the extracellular matrix (ECM) of natural skin. The high surface-area-to-volume ratio of electrospun fibers enhances cell adhesion, proliferation, and migration, accelerating tissue regeneration. A key advantage of biodegradable electrospun bandages is their ability to degrade naturally, eliminating the need for frequent dressing changes and minimizing patient discomfort. Common biodegradable polymers used include polycaprolactone (PCL), polylactic acid (PLA), gelatin, chitosan, and sodium alginate, which offer biocompatibility, antibacterial properties, and moisture retention—essential for an optimal wound-healing environment. Some formulations also incorporate growth factors, antimicrobial agents, or nanoparticles to further promote healing and prevent infections. These bandages are particularly beneficial for chronic wounds, diabetic ulcers, and burn injuries, where prolonged healing is a concern. Electrospun fibers loaded with antibacterial agents (e.g., silver nanoparticles) or bioactive molecules enhance wound repair by reducing infection risks. Additionally, drug-loaded electrospun fibers can provide controlled drug release, ensuring sustained therapeutic effects at the wound site. The future of biodegradable electrospun bandages lies in smart wound dressings, incorporating stimuli-responsive materials that change properties based on environmental factors like pH, temperature, or bacterial presence. With continued advancements, electrospun biodegradable bandages hold significant potential to transform wound management, reduce healing time, and improve patient outcomes in clinical settings.
MATERIALS AND METHODOLOGY:
Millipore water was collected from Ramnarain Ruia College, Sodium alginate was purchased from HIMedia Laboratories, Chitosan was purchased from HIMedia laboratories, Egg was purchased from local market and the albumin was separated out from it. Gelatin was procured from HIMedia laboratories. Centella Asiatica were purchased from plant nursery. The 4% w/v acetic acid was added in millipore water and then chitosan was added for dissolution of chitosan flakes by application of mild heating. After the chitosan gets mixed completely, the Ph was adjusted to 7.5 and 2% w/v egg albumin was added into the chitosan and mixed well, then 3%w/v sodium alginate was added in it and kept it for stirring overnight. After the mixture formulation got homogenised, addition of gelatin was done for clarification process. Once the formulation was prepared freeze-thaw cycles were given for further polymerization process and after that the gel was taken out and kept for electrospinning and the bandage was formed which was used for further characterization.
Electrospinning:
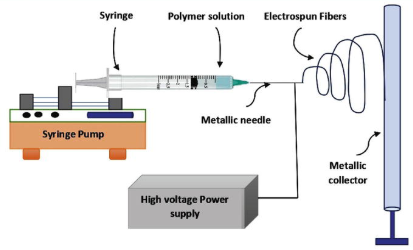
Fig.1
A widely used and successful method for creating nanofibers, electrospinning finds extensive use in textile, filtration, and medicinal applications. It creates ultrafine fibers with sizes ranging from nanometers to micrometers by applying a high-voltage electric field to a polymer melt or solution. The polymer is stretched into continuous fibers that are gathered on a grounded substrate by using electrostatic forces to overcome surface tension(1).
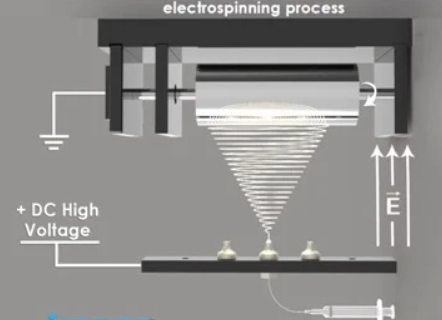
Fig. 2
Three essential parts make up the electrospinning process: a collector, a syringe pump that holds the polymer solution, and a high-voltage power source. The polymer droplet at the needle tip deforms into a Taylor cone when a high electric field is introduced. A charged jet is released when the electrostatic forces are greater than the surface tension. It quickly stretches and evaporates the solvent to form solid fibers. The resultant nanofibers are appropriate for a variety of applications due to their high surface area-to-volume ratios, adjustable porosity, and distinctive mechanical characteristics(2).
Fig.3
Preparation of plant extract:

Fig. 4
Centella asiatica were collected from local shop. The leaves were dried in aspetic condition and the moisture was allowed to get evaporated. The remained dried leaves were put it into Isopropyl alcohol (absolute) and it was kept in shaker at a speed of 40rpm for 14 h. After that the extract was filtered out with a cheesecloth and kept it in a glass container.
Preparation of plant loaded electrospun fibers:
Electrospinning is a straightforward yet highly effective method for fabricating ultrafine fibers. The preparation process involves several critical steps:
Polymer Solution Preparation
Selection of biodegradable polymers like Chitosan, egg albumin, sodium alginate and gelatin. And addition of Centella asiatica plant extract with a concentration of 10mg/ml. Dissolve the polymer in an appropriate solvent (e.g., millipore water) to create a homogenous solution with an optimized viscosity and conductivity. Stir the solution at a suitable temperature to ensure uniform polymer dispersion.
Electrospinning Setup
Load the polymer solution into a syringe fitted with a blunt needle or metallic spinneret. Connect the syringe to a syringe pump, which regulates the flow rate of the solution. Position a high-voltage power supply, typically in the range of 5–30 kV, to create an electric field. Set up a grounded collector (flat plate or rotating drum) to collect the electrospun fibers(3).
Electrospinning Process
Apply a high voltage to induce electrostatic repulsion within the polymer droplet at the needle tip. The polymer solution forms a Taylor cone and ejects a charged jet when the electrostatic forces overcome surface tension. As the polymer jet travels toward the collector, rapid solvent evaporation leads to solid fiber formation. The fiber deposition pattern depends on parameters like voltage, flow rate, humidity, and collector type(4).
Characterizations
Scanning electron microscopy:
Scanning Electron Microscopy (SEM) is a potent imaging method for examining the composition and morphology of surfaces at the nanoscale. In order to produce detailed images with high resolution and depth of field, a concentrated beam of high-energy electrons is scanned over a material, producing secondary and backscattered electrons. SEM is frequently used in biology, material science, and nanotechnology to examine topographical and structural characteristics and it is mainly used to assess the electrospun materials . To improve imaging quality, the sample is prepared by coating it with a conductive substance, such as carbon or gold. Energy-dispersive X-ray spectroscopy (EDS) and other advanced SEM techniques enable elemental composition measurement, which makes SEM indispensable for both industrial and research applications(5).
Spreadability test:
Spreadability refers to how effortlessly a cream or gel distributes when applied to the skin or wound surface. It is determined by measuring the time required for a gel to spread without resistance between two glass slides under a specified force. In the Spreadability test, a 2 cm radius is marked on the bottom of a petri plate, and 200 ?L of gel is placed at the center. Another petri plate is positioned on top in an inverted manner. A predetermined weight is applied for 5 minutes, allowing the gel to disperse. After removing the weight, the spread diameter is measured to evaluate spreadability(6).
Hemolysis test:
Hemolysis refers to the breakdown of red blood cells (RBCs). To assess hemolysis, the samples were rinsed with 0.9% saline solution before being immersed in 10 mL of saline inside a centrifuge tube and incubated at 37°C for 30 minutes. The samples were then introduced into the tube and further incubated at 37°C for one hour. After incubation, centrifugation was performed to separate the components. The supernatant was carefully collected, and its absorbance was measured at 541 nm using UV-visible spectroscopy to quantify hemoglobin release(7).
The Hemolysis rate was calculated by the given formula,
???????????????????????????????????? ???????????????? %=????.?????????????.????????????????.?????????????????????????.????
FT-IR:
Fourier Transform Infrared (FTIR) spectroscopy was utilized to analyze the physical and chemical composition of the formulated gel. This technique employs infrared spectroscopy and is advantageous as it requires minimal sample preparation for both liquid and solid states. The collected raw data is processed mathematically to generate a spectral output, which facilitates the identification of molecular bonds and chemical components present in the sample. The infrared absorption frequency spans from 600 to 4000 cm??1;, allowing for the detailed examination of functional groups. The spectral data is then analyzed to characterize the molecular interactions within the gel formulation(8).
Tensile stress:
A tensile stress tester is a mechanical device used to measure the tensile strength, elasticity, and deformation of materials under applied force. It operates by applying controlled tension to a sample until it breaks, recording key parameters such as ultimate tensile strength (UTS), elongation, and Young’s modulus. Commonly used in material science, biomedical research, and engineering, it evaluates the mechanical properties of polymers, metals, textiles, and biomaterials like wound dressings and scaffolds. Advanced models incorporate digital load cells and real-time data analysis for precise measurement, ensuring quality control and product reliability in research and industrial applications(9).
CAM assay:
We bought fertilized eggs from the Hatchery. The eggs were carefully delivered, cleaned, and incubated for three days at 37°C in an egg incubator. The assay was run on day four. To prevent contamination, the egg shell was cleaned with alcohol, and a syringe was used to remove any extra albumen. The CAM area window was left open for operation, and the upper section of the shell was fractured. The window was sealed using adhesive tape once the work was completed. Every two days, the infected eggs were monitored to assess the toxicity on the embryonic survival rate(10).
Histological studies:
Histological analysis is conducted to examine the microstructure of cells and tissues, aiding in the identification of anatomical details, functional properties, and pathological alterations. It plays a crucial role in disease diagnosis and medical research by providing insights into cellular changes. The hematoxylin and eosin (H&E) staining technique is widely used to highlight cellular components—nuclei appear blue, while the cytoplasm is stained pink. This contrast enhances the visualization of tissue abnormalities, aids in evaluating disease progression, and provides a deeper understanding of tissue organization in both healthy and diseased conditions(11).
In-vitro studies:
The MTT assay is a colorimetric method used to assess cell viability and proliferation during wound healing. It measures metabolic activity, where viable cells reduce MTT (yellow) to formazan (purple). This assay helps evaluate cell growth, toxicity, and the effectiveness of treatments promoting wound repair by quantifying cellular regeneration over time(12).
RESULTS:
Scanning electron microscopy:
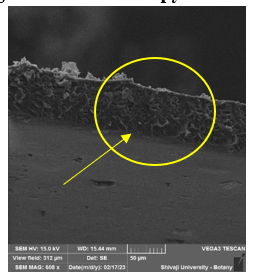
Fig. 5
By performing scanning electron microscopy we can clearly state that there is a mesh structure formed in the electrospun fiber which helps in cell proliferation and also can help to absorb and hold the moisture which will eventually help it for wound health and faster recovery.
Spreadability Test:
Fig. 6 & Table no. 6
The prepared topical cream exhibits spreading properties, demonstrating a measured sprea debility rate of 8 g.cm/sec. These characteristics highlight the formulation's rheological properties, contributing to its gritty, thick, and firm texture, which ensures even application over the skin. Such properties are essential for effective coverage and adherence, making the cream suitable for electrospinning applications, where controlled viscosity and spreading behaviour are critical for fiber formation and stability.
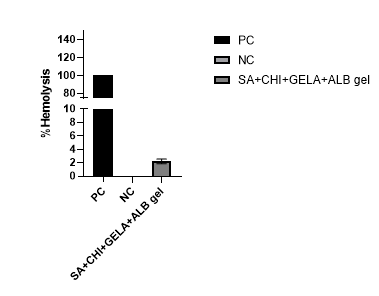
Fig.7
The haemolysis test is used to assess red blood cell (RBC) damage, providing insights into the toxicity of a sample. Based on the graphical analysis, the formulated gel exhibited only 2% hemolysis, whereas the positive control showed 100% hemolysis. These findings indicate that the cream is non-hemolytic, making it safe for further research and applications.
Tensile tester (Load displacement):

Fig.8
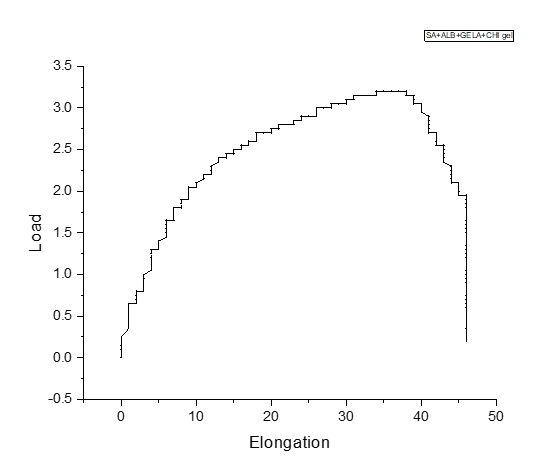
Fig. 9
According to studies the basic tensile strength of human skin is 1 to 32MPa. And the prepared electrospun bandages shows the tensile strength of 1 to 39MPa strength. This helps to state that the prepared electrospun bandages are the mechanically reliable alternative for wound healing purposes and can also replace the conventional wound dressing method.

Fig. 10
The chorioallantoic membrane (CAM) assay is an in-ovo technique used to assess cell toxicity and angiogenesis. On day 4, the sample was placed on the CAM region and incubated for 48 hours. In the control egg, no abnormal angiogenesis or mortality was observed, as no sample was introduced. In the sample egg, normal angiogenesis occurred without deformities, and the color and structural integrity of the CAM area remained unchanged, indicating the sample's biocompatibility and non-toxic nature.
Histological studies:
Histological studies of CAM area was done. Hematoxylin and eosin staining was carried out to examine the nucleus availability and cell structure. In comparison to topical cream sample, the control sample has less amount of nucleus present which was confirmed by hematoxylin staining and the there was also less staining of eosin seen as compared to topical cream sample. It concludes that the prepared topical cream is biocompatible and supports angiogenesis
FT-IR analysis:
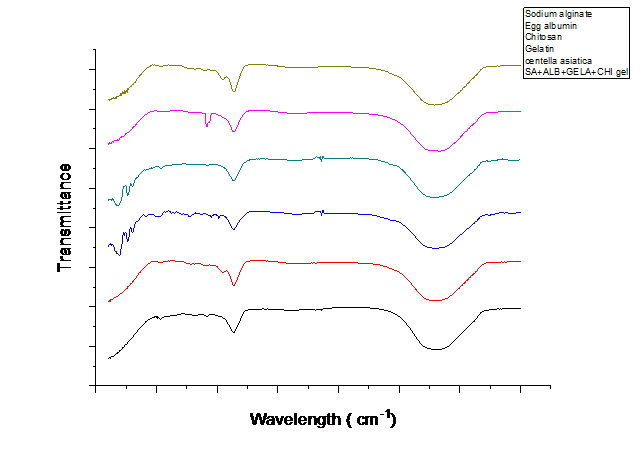
In FTIR spectroscopy, the 3303.17 cm??1; peak corresponds to O-H / N-H stretching, commonly associated with alcohols, phenols, or amines. The 2367 cm??1; peak represents C-H stretching, characteristic of aliphatic hydrocarbons. A peak at 1645 cm??1; is attributed to C=C stretching, typically found in aromatic rings or amides. Additionally, the 1406 cm??1; peak is linked to C-H bending (deformation) in alkanes or N-O symmetric stretching in nitro compounds. These spectral features indicate the presence of hydroxyl, alkyl, and aromatic functional groups in the sample. The FTIR spectra further confirm the uniform dispersion of sodium alginate, gelatin, chitosan, and egg albumin within the formulation, suggesting the successful incorporation of these biopolymers into the scaffold structure
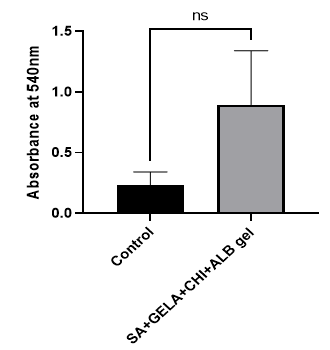
The MTT assay is a colorimetric method used to evaluate cell viability and metabolic activity. A higher absorbance value indicates greater cell viability. The absorbance of the topical cream sample was found to be higher than that of the control, suggesting that the formulation supports cell survival more effectively. These results confirm that the prepared topical cream is non-cytotoxic and biocompatible, making it a suitable candidate for further research and potential biomedical applications.
CONCLUSION:
The prepared electrospun bandages for wound healing purposes has shown excellent tensility. Fourier Transform Infrared (FTIR) spectroscopy was utilized to identify the chemical composition of the formulated topical cream. The hemolysis assay confirmed that the bandage is highly hemocompatible and non-hemotoxic, ensuring its safety for biomedical applications. The Spreadability test demonstrated that the formulated gel exhibits emulsion-like properties, enables easy application and coverage over a larger surface area. The chorioallantoic membrane (CAM) assay validated its role in angiogenesis promotion, with no adverse effects on living cells. SEM images showed the mesh like structures which clearly states that the formulated electrospun fibers can be a heathy alternative for wound health. Tensile testing of the prepared bandage showed a remarkable strength of 40MPa which meets the need of human skin tensility.
Furthermore, histological studies reinforced the CAM assay findings by revealing intact cell structures and nuclei, with no signs of necrosis. The adhesion test provided insights into the cream’s adhesive properties, a critical factor for wound healing applications.
Through extensive characterization, the electrospun bandage has been confirmed to be hemocompatible, biocompatible, non-cytotoxic, and capable of supporting cell metabolic activity and angiogenesis. These findings establish its potential for further investigation and possible future application as an effective wound healing alternative.
Conflict of Interest:
The authors have no conflicts of interest regarding this investigation.
ACKNOWLEDGEMENT:
We acknowledge profound gratitude to the Department, Chemistry of Ramnarain Ruia Autonomous College and ASK Solutions analytical and research laboratories for providing facilities and technical assistance for research work.
REFERENCES
- Brown, T. D., Dalton, P. D., & Hutmacher, D. W. (2016). Melt electrospinning today: An opportune time for an emerging polymer process. Progress in Polymer Science, 56, 116–166. https://doi.org/10.1016/j.progpolymsci.2016.01.001.
- Liu, Y., Zhang, X., Xia, Y., & Yang, H. (2010). Magnetic?Field?Assisted Electrospinning of Aligned Straight and Wavy Polymeric Nanofibers. Advanced Materials, 22(22), 2454–2457. https://doi.org/10.1002/adma.200903870.
- Reneker DH, Yarin AL. Electrospinning jets and polymer nanofibers. Polymer (Guildf) [Internet]. 2008;49(10):2387–425. Available from: http://dx.doi.org/10.1016/j.polymer.2008.02.00
- Vrbata P, Berka P, Stránská D, Dole P, Lázní M. Electrospinning of diosmin from aqueous solutions for improved dissolution and oral absorption. 2014;473:407–13.
- Sutunkova MP, Minigalieva IA, Shelomencev IG, Privalova LI, Ryabova Y V., Tazhigulova A V., et al. Electron microscopy study on the transport of lead oxide nanoparticles into brain structures following their subchronic intranasal administration in rats [Internet]. Vol. 12, Scientific Reports. Nature; 2022 [cited 2023 Jan 19]. Available from: https://www.nature.com/articles/s41598-022-24018-7
- Bakhrushina EO, Anurova MN, Zavalniy MS, Demina NB, Bardakov AI, Krasnyuk II. Dermatologic Gels Spreadability Measuring Methods Comparative Study. Int J Appl Pharm. 2022;14(1):164–8.
- Henkelman S, Rakhorst G, Blanton J, van Oeveren W. Standardization of incubation conditions for hemolysis testing of biomaterials. Mater Sci Eng C [Internet]. 2009;29(5):1650–4. Available from: http://dx.doi.org/10.1016/j.msec.2009.01.002
- Cleymand F, Poerio A, Mamanov A, Elkhoury K, Ikhelf L, Jehl JP, et al. Development of novel chitosan / guar gum inks for extrusion-based 3D bioprinting: Process, printability and properties. Bioprinting [Internet]. 2021;21:e00122. Available from: https://doi.org/10.1016/j.bprint.2020.e00122
- Cozzens D, Wei X, Faust R. Electrospinning of biostable polyisobutylene-based thermoplastic polyurethanes. J Polym Sci Part B Polym Phys. 2013;51(6):452–9.
- Talekar YP, Apte KG, Paygude S V., Tondare PR, Parab PB. Studies on wound healing potential of polyherbal formulation using in vitro and in vivo assays. J Ayurveda Integr Med. 2017 Apr 1;8(2):73–81.
- Beiki B, Zeynali B, Seyedjafari E. Fabrication of a three dimensional spongy scaffold using human Wharton’s jelly derived extra cellular matrix for wound healing. Mater Sci Eng C. 2017 Sep 1;78:627–38.
- Intini C, Elviri L, Cabral J, Mros S, Bergonzi C, Bianchera A, et al. 3D-printed chitosan-based scaffolds: An in vitro study of human skin cell growth and an in-vivo wound healing evaluation in experimental diabetes in rats. Carbohydr Polym [Internet]. 2018;199:593–602. Available from: https://doi.org/10.1016/j.carbpol.2018.07.057


 V. V. Pongade*
V. V. Pongade*











 10.5281/zenodo.14885552
10.5281/zenodo.14885552