Abstract
Electrophoresis is an electrokinetic process which separates charged particles in a fluid using a field of electrical charge.An ampholytes in acidic medium becomes positively charged hence moved towards the cathode while in alkaline medium, it becomes negatively charged hence moved towards the anode based on the migration of charged particles in an electric field the sample gets separated.It depends upon the type and size of the molecules, electrical field strength,net electrical charge of the molecules and properties of the supporting medium like pH ,ionic strength and temperature and viscosity. The procedures differ in some ways but all need a source for the electrical charge, a support medium and a buffer solution. The differenttypesof electrophoresis includes capillary electrophoresis,gel electrophoresis paper electrophoresis etc based on the presence or absence of supporting medium. Electrophoresis is used in laboratories for the separation of molecules based on size, density and purity and finds its applications in in molecular biology, biochemistry and medicine forthe separation of DNA,RNA and Proteins.[1].
Keywords
Electrophoresis, Charged particles, Electric field, Ampholytes, Migration, Molecule size, Electric field strength, Net electrical charge, Supporting medium, Ph ,Ionic strength, Temperature ,Viscosity, Molecular biology ,Biochemistry, Medicine, DNA, RNA, Proteins.[2][3].
Introduction
Rophoresis as an analytical tool was introduced by the Swedish chemist arne Tiselius first in his doctoral thesis in 1930, then later in a modified and improved form Tiselius 1937 Tiselius main interest was in the chemistry of the serum proteins his investigations resulted in the development of specialized apparatus and methodology for electrophoresis from which have been derived the techniques we use today For his pioneer work in this field Tiselius was awarded the nobel prize in 1948 Electrophoresis is used in laboratories to separate macromolecules based on their charges. The technique normally applies a negative charge called cathode so protein molecules move towards a positive charge called anode Therefore, electrophoresis of positively charged particles or molecules cations is sometimes called cataphoresis, while electrophoresis of negatively charged particles or molecules anions is sometimes called anaphoresis
Electrophoresis is the basis for analytical techniques used in biochemistry for separating particles, molecules, or ions by size, charge, or binding affinity either freely or through a supportive medium using a one-directional flowof electrical charge. It is used extensively in DNA RNA and protein analysis. Liquid droplet electrophoresis is significantly different from the classic particle electrophoresis because of droplet characteristics such as a mobile surface charge and the nonrigidity of the interface.Also the liquid–liquid system where there is an interplay between the hydrodynamic and electrokinetic forces in both phases, adds to the complexity of electrophoretic motion[4] Electrophoresis is a technique used to separate macromolecules in a fluid or gel based on their charge binding affinity and size under an electric field. In the year 1807 Ferdinand Frederic Reuss was the first person to observe electrophoresis. He was from Moscow State University. Anaphoresis is the electrophoresis of negative charge particles or anions whereas cataphoresis is electrophoresis of positive charge ions or cations. Electrophoresis has a wide application in separating and analysing biomolecules such as proteins, plasmids, RNA, DNA, nucleic acids. of electrophoresis have become the leading methods of the analysis of biomolecules in biochemistry and molecular biology, including genetic materials such as DNA or RNA, proteins, and polysaccharides. The different forms of electrophoresis have been modified into several types of electrophoresis, which are used to separate various types of biomolecules, analyze their characteristics, and study their interaction with a molecule of interest. The following are selected electrophoresis methods, based on different formats of electrophoresis Electrophoresis is a technique that separates molecules in their liquid state, based on their ability to move in an electric field.[5]
Principle Of Electrophoresis
Active fractions isolated by a preparative fractionation procedure may be subjected to a number of analytical fractionation procedures to determine their purity. Analytical fractionations are distinguished from preparative fractionations by the criteria shown in. The difference between preparative and analytical fractionations. Analytical Preparative Scale Small Large Fate of sample Destroyed Preserved Product Information Active fraction In an analytical fractionation, therefore, a small amount of sample is sacrificed in order to gain information about the state of purity of the material being analysed. Of the many physico-chemical techniques which have contributed to our knowledge of proteins and nucleic acids, electrophoretictechniques occupy a position of primary importance. Electrophoresis finds its greatest usefulness in the analysis of mixtures and in the determination of purity, although certainformsofelectrophoresis may be applied on a preparative scale Electrophoresis may be defined as the migration of charged ions in an electric field. In metal conductors, electric current flows between electrodes and is carried by ions. The negative electrode - the cathode - donates electrons and the positive electrode - the anode - takes up electrons to complete the circuit. The ions that result from the take up of electrons from the cathode will be negatively charged and will thus migrate towards the positive anode. Because oftheir anodic migration, negative ions are called anions 115 electric current is carried by the movement of electrons, largely along the surface of the metal. In solutions, the PA NIC Positive Anode Negative Cathode NI Not the Ions 116 5 Ions which result from the donation of an electron to the electrondeficient positively charged anode will themselves be electron deficient, and thus positively charged. These will migrate to the cathode and are thus called cations.. Electrophoresis: the movement of ions in an electric field. There is a potential difference voltage between the anode and the cathode and if the solution between these is of constant composition and constant cross-section the voltage gradient between them (dV/dx) will be linear, with units of volts cm-1 the effects of non-linear voltage gradients will be explored An ion placed in such an electric field will experience a force Where F = electrophoretic force K = a constant (embodying the Faraday constant and q = net charge on the protein (atomic charges/protein Avogadro's number) molecule) This force will cause the protein to accelerate towards either the cathode or the anode, depending on the sign of its charge.[6]
ABSORPTION OF ELECTROPHORESIS
Electrophoresis is a laboratory technique that works on the principle of electromagnetism.
• The macromolecules placed in an electric field move in the direction of the negative or positive pole as per their charge.
• As nucleic acid is a negatively charged particle, it moves toward the anode.
• If molecules are positively charged then they will move toward the cathode.
• If the molecules are negatively charged then they will move toward the anode.
• harged macromolecules are placed in the electric field move towards the negative or positive pole based on their charge. Nucleic acid has a negative charge and therefore it migrates towards the anode.[7]
• This technique is divided into two types viz slab electrophoresis and capillary electrophoresis.
• Charged particles under the influence of a liquid media placed in an electric field will migrate to the electrode of the opposite charge. Positive ions cations will migrate to the cathode, the negative electrode. Negative ions anions will migrate to the anode, the positive electrode.
• To get started, we will review terminology related to the charge characteristics of molecules.
• Gel electrophoresis is a technique used to separate DNAfragments according to their sizeDNA samples are loaded into wells indentations at one end of a gel, and an electric current is applied to pull them through the gel. DNA fragments are negatively charged, so they move towards the positive electrode.[8]
Instrumentation
The instrumentation of a typical system is composed of electrolyte reservoirs, a capillary tube electrodes, a voltage power supply, and a detector Sarker and Nahar, 2012 A capillary tube mostly made of silica, is 25–75 cm long with a diameter of 300–400 ?m outer and 25–75 ?m inner The most widely used detector in capillary electrophoresis for the detection of secondary metabolites is a UV spectrophotometer Tomas-Barberan, 1995 A number of factors such as concentrations and pHs of electrolytes, voltage, temperature, capillary dimension and sample loading methods affect the analysis of phytochemicals analysis by CE Sun and Chen, 1998. Samples are injected into CE by hydrodynamic, electrokinetic injection, and on-capillary sample concentration. Hydrodynamic injection is carried out by applying pressure at the inlet, applying vacuum at the outlet, or by elevating the inlet siphon effect. This is based on differences of pressure at the inlet and the outlet. Electrokinetic injection is done by applying a low voltage of 5–10 kV, which is typically 3–5 times lower than the separating voltage (Weston and Brown, On-capillary sample concentration is an injection method of isotachophoresim mode in CE, while samples are simply concentrated prior the separation.
capillary electrophoresis.
Capillary electrophoresis is a highly effective analytical technique for the separation and analysis of a wide range of substances in the lab. Having the proper equipment and environment is essential for a successful . In this article, we’ll provide an overview of the standard capillary electrophoresis equipment set.
• Power supply: A high-voltage variable-current power supply is the foundational component of CE equipment. The electric field used in sample separation is produced by the power source.
• Buffer reservoirs: CE requires two identical buffer reservoirs filled with anodic and cathodic solutions. The stability of the electric field depends heavily on these buffer reservoirs.
• Electrodes: A cathode and an anode, both of which need to be submerged in the buffer reservoirs, are necessary for the CE process. The electric field is produced by the electrodes, which are linked to the power source and are an essential component in the process.
• Capillary: Fused silica is used to construct the capillary that is utilized in CE, and its diameter is typically less than one hundred microns. The capillary functions as a route for the samples to pass through on their way to being separated.
• Optical viewing window: CE involves the utilization of an optical viewing window that is in proper alignment with the detector. The viewing window makes it possible to observe the separation process while it is taking place.
• Injection system: An appropriate injection system is needed for loading sampless and buffers into the capillary during CE. The injection can be automated for increased accuracy. Samples are often injected using one of three methods: gravity pressure or vacuum, or electrokinetics.
• Detector: Conductimetric fluorimetric absorption spectrophotometry amperometric or mass spectroscopic detectors are all suitable for monitoring the amount of substance passing through the capillary at any given time in CE.
• Thermostatic system: Thermostatic control of the capillary environment is essential for The reliability and precision of the outcomes are guaranteed by the controlled temperature.
• Recorder: In the data collected during electrophoresis must be recorded for further analysis. The data must be recorded so that it can be analyzed later.
• Computer or suitable integration: In addition requires a suitable integrator or computer for digital data conversion. It is easier to analyze and evaluate the digital data.[9]
Working
Electrophoresis uses an electric field applied across a gel matrix to separate large molecules such as DNA RNA and proteins by charge and size. Samples are loaded into the wells of a gel matrix that can separate molecules by size and an electrical field is applied across the gel. This field causes negatively charged molecules to move towards the positive electrode. The gel matrix, itself, acts as a sieve through which the smallest molecules pass rapidly while longer molecules are slower-moving. For DNA and RNA, sorting molecules by size in this way is trivial, because of the uniform negative charge on the phosphate backbone. For proteins, which vary in their charges, a clever trick must be employed to make them mimic nucleic acidssee polyacrylamide gel electrophoresis Different kinds of gels have different pore sizes. Like sieves with finer or coarser meshes some gels do a better job of separating smaller molecules while others work better for larger one Gel electrophoresis may be used as a preparative technique that is when purifying proteins or nucleic acids, but most often it is used as an analytical tool. Agarose gel electrophoresis is a technique used to separate nucleic acids primarily by size. Agarose is a polysaccharide obtained from seaweeds It can be dissolved in boiling buffer and poured into a tray, where it sets up as it coolsto form a slab. Agarose gels are poured with a comb in place to make wells into which DNA or RNA samples are placed after the gel has solidified. The gel is immersed in a buffer and a current is applied across the slab. Double-stranded DNA has a uniform negative charge that is independent of the sequence composition of the molecule. Therefore, if DNA fragments are placed in an electric field they will migrate from the cathode towards the anode The rate of migration is directly dependent on the ability of each DNA molecule to worm or wiggle its way through the sieving gel. The agarose matrix provides openings for macromolecules to move through. The largest macromolecules have the most difficult time navigating through the gel, whereas the smallest macromolecules slip through it the fastest. All fragments of a given size will migrate the same distance on the gel, forming the so-called bands on the gel. Visualization of the DNA fragments in the gel is made possible by addition of a dye, such as ethidium bromide, which intercalates between the bases and fluoresces when viewed under ultraviolet light By running reference DNAs of known sizes alongside the samples it is possible to determine the sizes of the DNA fragments in the sample. It is useful to note that by convention, DNA fragments are not described by their molecular weights unlike proteins but by their length in base-pairs( bp) or kilobases (kb). Agarose gel electrophoresis separation of DNA - orange bands are DNA fragments Polyacrylamide gel electrophoresis Like DNA and RNA, proteins are large macromolecules, but unlike nucleic acids, proteins are not necessarily negatively charged. The charge on each protein depends on its unique amino acid sequence. Thus, the proteins in a mixture will not necessarily all move towards the anode. Additionally, whereas double-stranded DNA is rod-shaped, most proteins are globular (folded). Further, proteins are considerably smaller than nucleic acids, so the openings of the matrix of the agarose gel are simply too large to effectively provide separation. Consequently, unaltered (native) proteins are not very good prospects for electrophoresis on agarose gels. To separate proteins by mass using electrophoresis, one must make several modifications. Gel matrix First, a matrix made by polymerizing and cross-linking acrylamide units is employed. A monomeric acrylamide is polymerized and the polymers are cross-linked using N,N-Methylene-bisacrylamide to create a mesh-like structure. One can adjust the size of the openings of the matrix/mesh readily by changing the percentage of acrylamide in the reaction. Higher percentages of acrylamide give smaller openings and are more effective for separating smaller molecules, whereas lower percentages of acrylamide are used when resolving mixtures of larger molecules. (Note: polyacrylamide gels are also used to separate small nucleic acid fragments, with some acrylamide gels capable of separating pieces of DNA that differ in length by just one nucleotide.) Charge alteration by SDS A second consideration is that proteins must be physically altered to “present” themselves to the matrix like the negatively charged rods of DNA. This is accomplished by treating the proteins with the anionic detergent, SDS (sodium dodecyl sulfate). SDS denatures the proteins so they assume a rod-like shape and the SDS molecules coat the proteins such that the exterior surface is loaded with negative charges, masking the original charges on the proteins and making the charge on the proteins more proportional to their mass, like the backbone of DNA.
Stacking Gel
A third consideration is that a “stacking gel” may be employed at the top of a polyacrylamide gel to provide a way of compressing the samples into a tight band before they enter the main polyacrylamide gel (called the resolving gel). Just like DNA fragments in agarose gel electrophoresis get sorted on the basis of size largest move slowest and smallest move fastest), the proteins migrate through the gel matrix at velocities inversely related to their size. Upon completion of the electrophoresis, proteins may be visualized by staining with compounds that bind to proteins, like Coomassie Brilliant Blue or silver nitrate.
Isoelectric focusing Proteins vary considerably in their charges and, consequently, in their pI values (pH at which their charge is zero). This can be exploited to separate proteins in a mixture. Separating proteins by isoelectric focusing requires establishment of a pH gradient in a tube containing an acrylamide gel matrix. The pore size of the gel is adjusted to be large, to reduce the effect of sieving based on size. Molecules to be separated are applied to the gel containing the pH gradient and an electric field is applied. Under these conditions, proteins will move according to their charge. 2D gel electrophoresis Both SDS-PAGE and isoelectric focusing are powerful techniques, but a clever combination of the two is a powerful tool of proteomics - the science of studying all of the proteins of a cell/tissue simultaneously. In 2-D gel electrophoresis, a lysate is first prepared from the cells of interest. The proteins in the lysate are separated first by their pI, through isoelectric focusing and then by size by SDS-PAGE.[10]
Types of Electrophoresis
1. Capillary electrophoresis
1. Gel electrophoresis
2. Paper electrophoresis
2. Slab electrophoresis
1. Zone electrophoresis
2. Immunoelectrophoresis
3. Isoelectrofocusing
Capillary Electrophoresis:
Principle:
Capillary electrophoresis separates molecules due to their electrophoretic mobilities. A molecule's electrophoretic mobility depends on its charge and how much it is attracted or repelled by the voltage as well as the frictional drag force that resists movement. Friction is proportional to the radius of the molecule. Thus, electrophoretic mobility is based on size and charge. The velocity a charged molecule travels down a capillary is the product of its electrophoretic mobility and the applied electric field. Higher voltages therefore lead to faster velocities and faster separations Capillary electrophoresis Cpillary electrophoresisand negative ions towards the anode while neutral molecules do not move under applied current. Capillary electrophoresis is thus the technique of performing electrophoresis in buffer-filled capillary tubes across which high voltage is applied to achieve the separation of molecules Capillary electrophoresis is a method of separation of the components of a mixture by using an electrical field. Electrophoresis Capillary electrophoresis is an analytical technique performed in a thin diameter glass tube that separates molecules and ions based on their mobility under the influence of an applied voltage. In this technique, a mixture of molecules and ions is separated based on their charge and size. The term capillary electrophoresis consists of two terms: capillary and electrophoresis. Capillary refers to a very thin glass tube of submillimetre diameter. Electrophoresis is a separation technique that sorts the pool of ions based on their size and charges using electric current. The electrophoretic system consists of two electrodes a buffer as a carrier or vehicle for the ions, and an electricity source. Anode and cathode constitute the two electrodes denoting positive and negative electrodes, respectively. The molecules that will be separated can be positively charged ions, negatively charged ions, or neutral without any charges. Neutral molecules and ions have differential mobility when an external electric current is applied. Positive ions move towards the cathode in a capillary differs from other forms of electrophoresis in that it is performed within confined narrow tube. Molecules can be either positively or negatively charged. When an electric field is applied, such charged molecules start to move towards the electrode of opposite charge. Therefore, cations move toward the cathode, while anions move toward the anode in CE.Basic principle: Capillary electrophoresis is performed in narrow-bore capillaries filled with electrolytewhere separation of components of a mixture is achieved with the differential Migration of solutes in an electric field by two forces, the electrophoretic migration and the Electro-osmotic flow (EOF). Silanol groups present in the fused silica capillary are readily Ionized in the majority of conditions to form the negatively charged of the inner wall Camilleri, 1998 which attract the positively charged components of the mixture to be separated. Upon the Application of voltage in such an electric double layer, there is a net flow of cationic components Towards cathode. Samples to be separated using a typical CE instrument are incorporated at the Anode and are detected at the cathode. Combined forces of the electrophoretic migration and the Electro-osmotic flow favour the migration of electrolytes first to the cathode with the highest Velocities, whereas anions are migrated last by the force difference of the electro-osmotic flow Toward the cathode and the electrophoretic flowfrom the anode in the opposite direction. Neutral Compounds are not well-separated Weston and Brown, 1997 as these are migrated out from the Capillary by the effect of electro-osmotic flow.[11]

Fig.1 Capillary electrophoresis
Advantages:
1.High Resolution:
• Capillaries can provide exceptional separation efficiency and resolution.
2. Speed:
• Faster runs due to high electric fields being applied without much heat generation.
3. Low Sample Requirement:
• Requires a very small amount of sample.
4. Automation:
• Automated sample injection and detection are possible.
Here is a colored diagram illustrating the advantages of Capillary Electrophoresis
Disadvantages
1. Cost:
• Initial setup and equipment are relatively more expensive.
2. Maintenance:
• Capillaries can become clogged and might require regular maintenance.
3. Expertise:
• Higher skill level required for operation and troubleshooting.[12]
Gel Electrophoresis:
Principle:
In the agrose gel electrophoresis the potential difference is applied across the electrodes in a horizontal electrophoretic tank containing agarose gel and biomolecules (such as nucleic acid or proteins) is loaded, then molecules migrated to their respective electrodes. The rate of migration of charged particles depends on the size, shape, molecular mass etc. In this process, larger molecules have difficulty in moving through the pore size of the supporting media, whereas the smaller molecules has more mobility through it. The bands of protein or nucleic acid is visualized by using intercalating dye, i.e., ethidium bromide (Etbr), they are visualized by fluorescence when illuminated with ultraviolet lights.[12]
Gel Electrophoresis:
• Gel electrophoresis is a technique commonly used in laboratories to separate charged molecules like DNA, RNA and proteins according to their size.
• By comparing the bands of the DNA samples with those from the DNA marker, you can work out the approximate length of the DNA fragments in the samp

Fig.2 GEL Electrophoresis
How Does Gel Electrophoresis Work
• Electrophoresis enables you to distinguish DNA fragments of different lengths.
• The gel consists of a permeable matrix, a bit like a sieve, through which molecules can travel when an electric current is passed across it.
• An electric current is applied across the gel so that one end of the gel has a positive charge and the other end has a negative charge.
• The movement of charged molecules is called migration. Molecules migrate towards the opposite charge. A molecule with a negative charge will therefore be pulled towards the positive end
• DNA is negatively charged, therefore, when an electric current is applied to the gel, DNA will migrate towards the positively charged electrode.
• Shorter strands of DNA move more quickly through the gel than longer strands resulting in the fragments being arranged in order of size.
• The use of dyes, fluorescent tags or radioactive labels enables the DNA on the gel to be seen after they have been separated. They will appear as bands on the gel.
• A DNA marker with fragments of known lengths is usually run through the gel at the same time as the samples.
• By comparing the bands of the DNA samples with those from the DNA marker, you can work out the approximate length of the DNA fragments in the samples.
• Gel electrophorisis is simple, rapid and sensitive analytical technique for the separation of charged particle.
• The gels, however, are porous and the size of the pores relative to that of the molecule determines whether the molecule will enter the pore and be retarded or will bypass it. The separation thus not only depends on the charge on the molecule but also on its size. Needless to say, that resolution of a sample is sharper and better in a gel than in any other type of medium.
• Agrose gel is used as a supporting media for the separation of DNA, RNA or protein under the influence of electric charge.
• Most of the biomolecules has a net charge at any pH other than at their isoelectric point.
• There is difference in the electrophoretic mobility of these charged molecules due to their difference in size, shape, and charge
There are basically two types of materials are used to make gels
• It is linear polysaccharide.
• It is made up of repeating units of agarobiose, comprises alternating units of 3,6-anhydrolactose and galactose.
• This gel has generally larger pore size, which makes them suitable to separate larger molecules having molecular mass more than 200 kDa.
• It is most commonly used for the electrophoresis of both protein and nucleic acids.
• Agarose is used in concentration between 1% and 3%.
Polyacrylamide Gel
• Polyacrylamide gel is consisting of chains of acrylamide monomers crosslinked with N, N’-methylenebisacrylamide units, which is commonly termed as bisacrylamide.
• In this gel, pore size and resolving power is totally depends upon the concentration of acrylamide and bisacrylamide.
• The concentration of the gel normally varies from 5% to 25%.
• This gel is used in electrophoresis for the separation of proteins ranging from molecular weight <5000>200,000, and polynucleotides ranges from <5>
APPARATUS OF GEL ELECTROPHORESIS
1. Vertical gel apparatus: It is commonly used inSDS PAGE for the separation of proteins.
2. Horizontal gel apparatus: It is used for immune electrophoresis, iso-electric focusing and electrophoresis of DNA and RNA in the agarose gel.
Type Of Gel Electrophoresis
1. Agarose gel electrophoresis
2. SDS-PAGE
3. Pulse field gel electrophoresis (PFGE)
4. 2D gel electrophoresis
Agarose Gel Electrophoresis
• Agarose gel the supporting media in the electrophoresis.
• For the electrophoresis of DNA, RNA and Protein agrose gel is used.
In the agrose gel electrophoresis the potential difference is applied across the electrodes in a horizontal electrophoretic tank containing agarose gel and biomolecules (such as nucleic acid or proteins) is loaded, then molecules migrated to their respective electrodes. The rate of migration of charged particles depends on the size, shape, molecular mass etc. In this process, larger molecules have difficulty in moving through the pore size of the supporting media, whereas the smaller molecules has more mobility through it. The bands of protein or nucleic acid is visualized by using intercalating dyeethidium bromide they are visualized by fluorescence when illuminated with ultraviolet lights.
Requirement/ instrumentation:
• An electrophoretic unit,
• A power supply,
• Gel casting trays
• Combs
• Agarose gel or media
Electrophoresis buffer
Composition and ionic strength of electrophoresis buffer is most important factor for the separation of nucleic acids (DNA or RNA)
Most routinely used buffers are:
• TAE- (Tris-acetate-EDTA), it has lower buffering capacity and generally used to separate larger nucleic acid fragments (>12kb).
• TBE- (Tris-borate-EDTA), it has high buffering capacity and higher ionic strength and generally used for the separation of low molecular weight compound (<1kb>
• Loading buffer: Nucleic acid is before loading on to a gel is first mixed with the gel loading buffer, which usually consists of:-
• Salts: It creates environment with favorable ionic strength and pH of the sample, e.g., Tris-HCl.
• Metal chelator: It prevents nucleases to degrade the nucleic acid such as EDTA.
• Loading dyes: It provides color for tracking and easy monitoring of sample. Such as, bromophenol blue, xylene cyanol.
• Transilluminator: (An ultraviolet light box), which is used to visualize bands in gels.
Applications
• In molecular genetic diagnosis or genetic fingerprinting for analysis of PCR products.
• For the estimation of size of DNA molecule.
• In the separation of restricted DNA and RNA.
• In addition to providing an excellent medium for fragment size analyses, agarose gels allow purification of DNA fragments.
Page Electrophoresis
• Sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis is routinely used for the separation of proteins on the basis of their mass.
• It involves the use of vertical gel apparatus to separate proteins. This technique uses anionic detergent sodium dodecyl sulfate (SDS) which disassociates proteins into their individual polypeptide subunits and gives a uniform negative charge along each denatured polypeptide. When these denatured polypeptides are loaded at the cathode end of an electric field, then we get clear bands of proteins arranged in decreasing order of their molecular mass from the cathode to anode.
Requirement/ instrumentation
• Gel or media acrylamide solutions.
• Buffer system the separation and migration patterns of proteins in gel electrophoresis are determined by the chemical composition and pH of the buffer system.
Three basic types of buffers are required:
• Gel casting buffer to cast gel
• Sample buffer to prepare the sample
• Running buffer to fill the electrode reservoirs
• lower reservoir amine buffer with HCl (running gel)
• Upper reservoir amine buffer with glycine (stack gel)
• Staining or tracking dye
• Protein samples
• Molecular weight markers
• An electrophoresis chamber and power supply
• Glass plates (a short and a top plate)
• Casting frame
• Casting stand
• Combs
Applications
• For measuring molecular weight.
• In Peptide mapping.
• For Estimation of protein size.
• For Determination of protein sub-units or aggregation structures.
• For Estimation of protein purity.
• In Protein quantitation.
• In monitoring protein integrity.
Pulse Field Gel Electrophoresis (Pfge)
• Conventional agarose gel electrophoresis cannot separate linear double stranded DNA molecules that have radius of gyration, which is larger than the pore size of the gel.
• However, with certain changes in the orientation of electric field with respect to the gel, large DNA fragments can be resolved.
• It is used to separate large DNA molecule by applying gel matrix as electric field that periodically changes direction.
• This technique is invented by Schwartz and Cantor in 1984.
• DNA fragments up to 10 mb can be separated by this technique.
As opposed to the continuous unidirectional electric fields applied in conventional gel electrophoresis, pulsed-field gel electrophoresis uses pulsed, alternating, orthogonal electric fields. When such a field is applied to a gel, large DNA molecules become trapped into their reptation tubes every time the direction of the electric field is changed. These molecules remain immobile till they reorient t themselves along the direction of the new electric field. It is here that different DNA molecules adapt a behavior consonant with their respective sizes; large DNA molecules take a longer time to reorient themselves and are consequently retarded more in the new electric field as compared to the smaller DNA molecules.
Thus, all those molecules of DNA whose reorientation times are less than the period of the electric pulse can be fractionated in a size dependent manner. Factors, which are of extreme importance for determining the limit of resolution of pulsed-field gel electrophoresis are given below:
1. The absolute periods of the electric pulses.
2. The angles at which the two electric fields are applied to the gel.
3. The relative field strengths of the two electric fields and the degree of uniformity of the two electric fields.
4. The ratio of the periods of the electric pulses employed to generate the two electric fields.[13]

Fig.3Paper Electrophoresis
Paper Electrophoresis:
Low-voltage paper electrophoresis is the simplest and cheapest form of electrophoresis. A strip of commercially available chromatography paper is soaked in buffer and placed with one end in each buffer reservoir (connecting wicks may be used). It is important that the paper is saturated with the buffer since it is the buffer that conducts the majority of the current. A spot of sample is placed in the center of the strip. When the current is applied ions separate out and migrate toward the attractive electrodes . Paper electrophoresis has several limitations. Only small charged molecules can be reliably separated since many macromolecules adsorb on to the paper, although it is possible to reduce the adsorption by using a buffer that is more alkaline than the isoelectric point of the sample. Paper systems are associated with high electrical resistance causing heating that dries out the paper. Although this method has been largely superseded by gel methods for many samples it is still useful for separation of small molecules such as amino acids, small peptides, nucleotides, and inorganic ions. An important limitation to this technique is that considerable diffusion of small molecules occurs at low voltage[14]
• Paper electrophoresis involves the use of a strip or sheet of filter paper as the stationary or supporting phase.
• This technique is commonly applied for the separation of small molecules like amino acids, peptides, and nucleotides.
1. Preparation of the Paper:
• The filter paper strip is cut to the required size and soaked in a buffer solution to maintain a stable pH throughout the experiment.
2. Sample Application:
• A small aliquot of the sample is applied onto the paper, generally some distance away from one end.
• Precise application is crucial to prevent excessive spreading, which can degrade resolution.
3. Electrophoresis Chamber Setup:
• The paper strip is positioned in a chamber filled with a buffer solution. Both ends of the strip should be in contact with this buffer to ensure electrical conductivity.
• Electrodes (anode and cathode) are then placed at each end of the chamber.
4. Application of Electric Field:
• Upon connection to a power supply, an electric field is established across the paper strip.
• Charged molecules in the sample migrate toward the electrode of opposite charge.
5. Detection and Analysis:
• After electrophoresis, the paper strip is removed.
• The separated molecules can be detected using suitable staining or detection techniques, and their migration distance can be utilized for analysis.
Type of paper electrophoresis
• Paper electrophoresis can be classified based on the voltage applied during the process.
Here's a brief differentiation between high voltage and low voltage paper electrophoresis:
1. High Voltage Paper Electrophoresis:
1. Voltage:Uses higher voltages, typically above 2000V.
2. Duration:Faster separation due to the higher voltage, so the process is relatively shorter.
3. Resolution:Offers better resolution and sharper bands because of rapid movement.
4. Applications:Especially useful when rapid results are required or for separating smaller molecules.
2. Low Voltage Paper Electrophoresis:
1. Voltage: Uses lower voltages, typically below 300V.
2. Duration: Takes a longer time for separation due to the lower voltage.
3. Resolution:Bands may not be as sharp as in high voltage electrophoresis, but the process is gentler.
4. Applications: Suitable for separating larger molecules or when samples are sensitive to higher voltage conditions
Advantages of paper electrophoresis:
1. Simplicity:
• Straightforward setup and minimal equipment required.
2. Low Cost:
• Filter paper and buffers are relatively inexpensive.
Disadvantages of paper electrophoresis:
1. Resolution:
• Lower resolution compared to gel and capillary electrophoresis.
2. Fragility:
• Wet paper can tear easily during handling.
3. Capacity:
• Limited amount of sample can be loaded.[15]
Slab Gel Electrophoresis:

Fig.4 Slab Gel Electrophoresis
Slab gel electrophoresis has become a ubiquitous tool in the bioscience laboratory for the analysis of polynucleotides in such applications as sequencing, restriction mapping, PCR and mutational analysis. The most common media for slab gel electrophoresis are polyacrylamide and agarose Polyacrylamide is typically used for smaller molecules while agarose is used for larger molecules because of its larger pore size. For many separations involving polynucleotides, polyacrylamide gel electrophoresis is the standard methodology. In a typical PAGE experiment a gel is cast by polymerizing acrylamide and N,N?-methylenebisacrylamide, a crosslinking agent, in the presence of ammonium persulfate and tetramethlyethylenediamine (TEMED). The total monomer concentration (acrylamide and bisacrylamide) is commonly denoted by T and the amount of crosslinking agent (bisacrylamide) by C (weight percent of T). After polymerization, the analyte is entered into wells formed in the gel, and the electric field is applied through a buffer solution. After electromigration the analyte can be visualized by staining, UV shadowing, or by using fluorescent or radiolabels. In the case of fluorescence detection, the analyte can have a covalently-attached fluorescent label or the gel can be stained with a dye such as ethidium bromide which associates with the analyte and then de-stained to remove excess dye. The dye reveals the position of the analyte as bands. In the case of radioactive tracers, the analyte is tagged before electrophoresis and then detected by exposure to radioactive sensitive film. Some typical techniques using slab gel electrophoresis include Southern blotting whereby analytes are transferred from the slab gel to an immobilizing membrane allowing them to be more easily accessible.[16]
For larger DNA (50-100 kb) separation is difficult using constant electric fields; however, by using periodic or pulsed electric fields, separation can be accomplished A typical application of pulsed field gel electrophoresis (PFGE) is chromosomal DNA separation Although PFGE has been performed in capillariesit is still mostly used in the slab gel format. A recent advancement in the area of slab gel electrophoresis is the use of ultrathin gels To reduce Joule heating, the geometry of the slab gel is changed such that the thickness of the gel is small, typically on the order of 100 ?m. These ultrathin gels posses the advantage that higher electric field strengths can be applied while still minimizing Joule heating resulting in more rapid separations. When is compared to conventional slab gel electrophoresis techniques, either vertical or horizontal, one can observe significant differences First, the interpretation of the results is spatial in slab gel electrophoresis and time based in CGE as shown in CGE also offers a lot of variability in the separation parameters as depicted in While slab gel electrophoresis is still a labor-intensive manual approach requiring long preparation and separation times (up to hours), CGE is automated and offers rapid separation of the analyte mixtures (as low as seconds). Albeit the overall amount of sample required by both techniques is comparable (5–20 µL), while in slab gel mode the entire sample volume is injected for a single run, in CGE hundreds of runs can be made from the same volume as in that case the injection volume is in the nanoliter range. The capillaries with internal diameters of 25–100 µm usually applied in CGE allow the use of significantly higher voltages compared to slab gel electrophoresis owing to better Joule heat dissipation In addition, there is an interesting difference in peak/band capacity in these methods. While CGE offers very high peak capacity, it is still a one-dimensional separation tool and cannot accommodate the two-dimensional option of the slab gel technique. On the other hand, the multilane feature of the slab gel systems that increases sample throughput can be countered by the use of multicapillary systems, such as manifested in DNA sequencing devices . Regarding equipment cost, commercially available automated CE systems are significantly more expensive than simple slab gel electrophoresis units, even considering the documentation part involved for this latter. Also, important to note that capillary electrophoresis can be directly interfaced with ESI slab gel electrophoresiS is used to separate large biomolecules based on their molecular sizes This size separation is achieved by electrophoresis of the solute through a suitable polymer matrix that acts as a molecular sieve.Capillary gel electrophorer(CGE) combines the principles of slab gel electrophoresis and CZE separation.As CGE uses a smaller capillary diameter that is much better at dissipating heat compared to slab gels, higher electric fields can be used for faster separation in CGE. Resolution and efficiency in CGE are comparable to CZE up to efficiencies of 106–107 theoretical plates per meter He two materials used most commonly for DNA slab gel elecrophoresis are polyacrylamide and agarose. Polyacrylamide gels are formed with pore sizes that depend on the percentage of acrylamide used as well as the proportion of cross-linker, N,N?-methylene bisacrylamide. Acrylamide monomer concentrations range from 3.5% to 20%. The polymerization reaction is generally carried out directly to form a slab in a mold. Details and specific protocols are provided in the volume by Sambrook. Excellent – down to single base – resolution of nucleic acids is achieved using polyacrylamide gels, and as a result they are used for DNA sequencing. Agarose is a polymer of d-galactose and 3,6-anhydrogalactose and is used in concentrations ranging from 0.3% to 2.0% The agarose gel is formed when the constituents are heated and then cooled under ambient conditions to room temperature. Agarose is used for higher molecular weight separations due to the larger pore sizes and the ability to maintain mechanical stability at low weight percentages. An additional advantage of agarose is that it can be easily dried to a thin, transparent film, which makes it quite suitable for autoradiography.[17]
Zone electrophoresis (ZE):
Principles:
Zone electrophoresis is the simplest form and is what is often meant when CE and ME are discussed. The capillary/channel is filled with a BGE and on applying voltage, analytes migrate in discrete zones and at different velocities based on their charge to hydrodynamic radius ratio. Both cations and anions are separated simultaneously due to the difference in their electrophoretic mobility and the EOF. However, it is not applicable for neutral molecules which co-migrate as one unresolved peak[18]
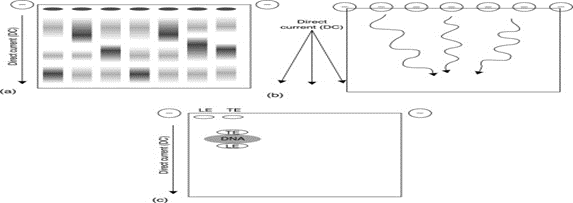
Fig.5 Zone electrophoresis
Zone Electrophoresis
This mode is characterized by two features the whole separation system together with electrode vessels is filled with a homogeneous solution of electrolytes, which is called the background electrolyte (BGE) the mixture of analyzed species to be separated is introduced or injected in a rather low amount as a short plug at one end of the separation column The mode of zone electrophoresis is often used in capillary columns, when it is called capillary zone electrophoresis (CZE). The BGE is mostly formed by a suitable buffer to maintain a certain pH, which is optimal for the separation of a given mixture. This mode is also almost exclusively used for gel electrophoresis: here the whole separation system is formed by a buffer-soaked hydrophilic slab gel and the mixture to be separated is introduced in little amounts at one end. After applying the driving electric field the analytes migrate in the column or in the slab gel with mutually different velocities so that they are spatially separated. After some time they are detected with a suitable detector located near the second end of the separation column; in the case of the slab gel electrophoresis, the plate with the gel is removed from electricity and the separated analytes are visualized as spots by a suitable reagent. The conductivity of the BGE in the separation system is only slightly influenced by the presence of the separated analytes, if their amount is low enough. Then, the ? in the denominator of the second term in eqn [8] is practically constant in time and homogeneous across the column length so that the equations can be regarded as linear. The individual zones of analytes, although narrow at the beginning, become broader when moving in the column as diffusion causes their dispersion and their longitudinal profile attains a Gaussian shape. If, however, the amount of an analyte is rather high, it influences conductivity in its own zone, so it is no longer constant. Then, the nonlinearity arising in this way causes the electromigration dispersion – another type of broadening of the zones that further deforms zones to the triangular shape The quantitative determination of a particular analyte is performed by integration of its detector signal.Abstract Capillary zone electrophoresis (CZE) is emerging as a useful tool in proteomic analysis. Interest arises from dramatic improvements in performance that result from improvements in the background electrolyte used for the separation, the incorporation of advanced sample injection methods, the development of robust and sensitive electrospray interfaces, and the coupling with Orbitrap mass spectrometers with high resolution and sensitivity. The combination of these technologies produces performance that is rapidly approaching the performance of UPLC-based methods for microgram samples and exceeds the performance of UPLC-based methods for mid- to low nanogram samples. These systems now produce over 10,000 peptide IDs in a single 100-minute analysis of the HeLa proteome. [19]
Immunoelectrophoresis
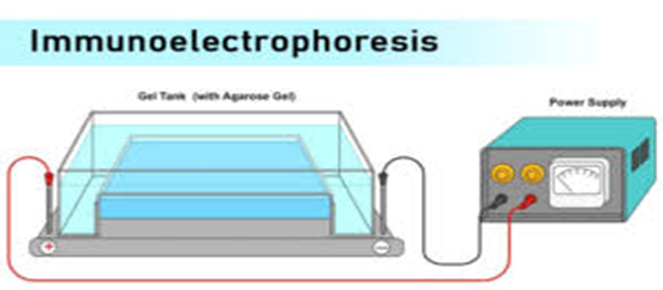
Fig.6 Immunoelectrophoresis
Iunoelectrophoresis is a technique used for the characterization of antibodies. The term “immunoelectrophoresis” was coined by Grabar and Williams in 1953.The principle behind this technique is the electrophoresis of antigen for immunodiffusion with a poly-specific antiserum to form precipitinbands. The molecules possessing the charge move toward the appropriate electrode. The migration of the molecules depends on a number of factors such as net charge, size, pH of the buffer, and ionic strength of the buffer. Therefore, when an antigen is subjected to electrophoresis in an agarose gel, it gets separated according to the properties it possesses. The antigen thus resolved is subjected to immunodiffusion with antiserum added in the agarose gel. The antibodies diffuse laterally to meet diffusing antigens, and lattice formation and precipitation occur permitting determination of the nature of the antigens. The precipitin line indicates the presence of the antibody, specific to antigen. Immunoelectrophoresis is a general name for a number of biochemical methods for separation and characterization of proteins based on electrophoresis and reaction with antibodies. All variants of immunoelectrophoresis require immunoglobulins, also known as antibodies, reacting with the proteins to be separated or characterized. The methods were developed and used extensively during the second half of the 20th century. In somewhat chronological order: Immunoelectrophoretic analysis (one-dimensional immunoelectrophoresis ad modum Grabar), crossed immunoelectrophoresis (two-dimensional quantitative immunoelectrophoresis ad modum Clarke and Freeman or ad modum Laurell), rocket-immunoelectrophoresis one-dimensional quantitative immunoelectrophoresis ad modum Laurell fused rocket immunoelectrophoresis ad modum Svendsen and Harboe, affinity immunoelectrophoresis Immunoelectrophoresis is a general term describing many combinations of the principles of electrophoresis and reaction of antibodies, also known as immunodiffusion
• Immunoelectrophoresis refers to precipitation in agar under an electric field.
• It is a process of a combination of immuno-diffusion and electrophoresis.
• An antigen mixture is first separated into its component parts by electrophoresis and then tested by double immuno-diffusion.
• Antigens are placed into wells cut in a gel (without antibody) and electrophoresed. A trough is then cut in the gel into which antibodies are placed.
• The antibodies diffuse laterally to meet diffusing antigens, and lattice formation and precipitation occur permitting determination of the nature of the antigens.
• The term “immunoelectrophoresis” was first coined by Grabar. [20]
Isoelectric Focusing Electrophoresis:
The separation of ampholytic components according to isoelectric point has played an important role in isolating, reducing complexity and improving peptide and protein detection. This brief review outlines the basics of isoelectric focusing, including a summary of the historical achievements and considerations in experimental design. Derivative methodologies of isoelectric focusing are also discussed including common detection methods used. Applications in a variety of fields using isoelectric point based separations are provided as well as an outlook on the field.

Fig.7 Isoelectric Focusing Electrophoresis
Isoelectric focusing is another technique which distinguishes isoelectric proteins depending upon their isoelectric points (pH at which proteins carry no net charge). Every protein has a characteristic isoelectric point. In this technique, pH gradient is applied and proteins movement is based on charge in the applied electric field region, until reaches isoelectric point (Pergande and Cologna, 2017). When the pH is increased, loss of proton by the protein happens, so carrying negative charge and this decisively provokes their movement toward anode. Subsequently, when pH falls below the isoelectric point, causing a positive charge on protein and ultimately movements toward cathode. Finally, at isoelectric point there is no movements and proteins start concentrating as shown in . Also considering that, amphoteric compounds require a limited pH range to be concentrated. Moreover, the molecular weight greater than 2 kDa are preferable, this is because lower molecular weight causes expansion of analyte to nearby segments, making diffusion more prominent, leading toward deterioration (Smoluch et al The isoelectric focusing mechanism is also adopted by living cells for the purpose of providing the proper environment for biochemical reactions. This purely biological system can cause a few orders of magnitude difference in enzyme concentrations between cell compartments. In 1984, Slavik and Kotyk demonstrated the presence of a continuous pH gradient ranging from pH 7.2 in the center of the cell to 6.4 in the cell periphery. Because the efficiency of IEF is directly proportional to strengthand pH gradient, it can be calculated that the resolution in living cells is more than four orders of magnitude higher than in any manmade apparatus The large diversity in pI values among natural proteins suggest that this parameter has biological meaning and that it is controlled by natural selection. Studies show that this parameter is controlling the entry of a protein into the nucleus. Posttranslational modifications like phosphorylation or dephosphorylation regulate a molecule's location within the cell, where they can act as regulators of diverse biochemical functions.[21]
Factors affecting Electrophoresis:
Electrophoresis refers to the migration of charged particles under the influence of an electric field. These are the main factors that affect electrophoresis:
1. Sample: The size, shape, and charge of the sample influence its rate of migration rate. The migration rate is inversely proportional to the size of the molecule. It is also affected by the shape of the sample. An increase in net charge speeds up migration.
2. Buffer solution: The buffer stabilizes the pH of the supporting medium and affects the compound migration rate. Zwitterionic buffers are found to offer higher resistance to continuous electrolysis compared to standard buffers, particularly in capillary zone electrophoresis.
3. Applied voltage: The migration rate of the molecules being separated is affected by the applied voltages. DNA migrates faster through the gel when higher voltage is applied, but excessive voltage can cause the gel to melt or lead to smearing and distortion of DNA bands.
4. Frictional force: A frictional force hampers the mobility of charged molecules and slows them down. This frictional force is the measure of multiple factors including the hydrodynamic size and shape of the molecule, the viscosity of the butter, and the pore size of the medium in which electrophoresis occurs.
5. Supporting media: The type of supporting media used impacts the migration rate of compounds. Inert media are preferable as the electrophoretic rate can be affected by molecular sieving, adsorption, and electro-osmosis processes that occur in the medium. Adsorption can cause tailing, leading to a comet-like movement rather than a band, reducing both the rate and resolution of separation.
6. Electroendosmosis: Electroendosmosis, or electro-osmotic flow, can alter electrophoretic separation. This occurs due to charged groups on the surface of the support medium, such as sulfate groups in agarose, carboxyl groups in paper, and silanol (Si-OH) groups on glass capillary surfaces. These ionized groups create an electrical double layer at the capillary wall/electrolyte interface. When a voltage is applied, cations in the electrolyte migrate toward the cathode, dragging the electrolyte solution with them, resulting in a net electroosmotic flow toward the cathode.[22][23]
Advantages Of Electrophresis
• It has a high efficiency of separation.
• It provides sample analysis in a short period of time.
• It produces fewer waste products.
• It is a simple strategy to use.
• The experiment can be performed with a small amount of sample[24]
Disadvantages Of Electrophoresis
• During electrophoresis, gels can melt, the buffer can run out, and various genetic materials can run in unanticipated manners.
• Heat is dissipated by the capillary tube’s narrow diameter, resulting in greater diffusion. As a result, the resolution is not always accurate.[25]
Applications:
1. To separate complex molecules .many complex biological molecules like vitamins b12.antibiotics proteins can be separated efficiently by electrophoresis.this is possible due to charge difference among the mixtures.
2. For analysis of nucleic acid molecules like rna and dna studies.these long chain molecules can be analyzed only after separation after electrophoresis .this helps to determine the size or breaks in the dna or rna molecule.
3.In forensic science is the development of methods for amplification and detection of dna fragments uising polymerase chain which has to lead to rapid and advances technique in dnatyping in forensic .
4. Capllaey electrophoresis used for the detection of specific mrna fragments to help identify the biological fluid or tissue origin of a forensic sample .
5. Capillary electrophoresis may be used for the simultaneous determination of the ions nh4+. Na+,k+,mg2+ and ca2+ in saliva
6.Paper electrophoresis used for lipoproteind in serum enzymes in blood.
7.Electrophoresis used for separation of alkaloid antibiotics in different samples can be carried out .
8.Gel electrophoresis used for analysis of a sample of glutamic acid to check its contamination with glutamine.
9.Accurate determination of the relavtive molecular masses of the two polypeptide chains of human insulin can be carried out by gel electrophoresis.
10. Gel electrophoresis can be used for separation of the isoenzymes of lactic dehydrogenase.
11. Electrophoresis can be used for determination of the number of different coat proteins of a virus.[26].
REFERENCES
-
-
-
- Landers, J. P. (1993). Capillary Electrophoresis: Theory and Practice.
- Camilleri, P. (1998). Capillary Electrophoresis: Theory and Applications.
- Righetti, P. G. (1983). Isoelectric Focusing: Theory, Methodology, and Applications.
- Tiselius, A. (1937). "Electrophoresis of Serum Proteins."
- Righetti, P. G. (1983). Isoelectric Focusing: Theory, Methodology, and Applications.
- Li, S. F. Y. (1992). "Capillary Electrophoresis: Principles, Practice, and Applications." Journal of Chromatography A, 624(1-2), 1-20.
- Camilleri, P. (1998). Capillary Electrophoresis: Theory and Applications.
- Righetti, P. G. (1983). Isoelectric Focusing: Theory, Methodology, and Applications.
- Sarker, S. D., & Nahar, L. (2012). "Hyphenated Techniques in Natural Product Analysis." Journal of Pharmaceutical and Biomedical Analysis, 66, 107-115.
- Hames, B. D. (1998). "One-dimensional polyacrylamide gel electrophoresis." Methods in Molecular Biology, 80, 1-13.
- Weston, A., & Brown, P. R. (1997). "High-Performance Capillary Electrophoresis." Journal of Chromatography A, 792(1-2) 1-20.
- Jorgenson, J. W., & Lukacs, K. D. (1983). "Capillary zone electrophoresis." Science, 222(4621), 266-272.
- Camilleri, P. (1998). "Capillary Electrophoresis: Theory and Applications." Journal of Chromatography A, 792(1-2), 13-25.
- Smith, I. (1968). "Chromatography and Electrophoresis." Journal of Chromatography, 36(2), 255-266.
- Righetti, P. G. (1983). Isoelectric Focusing: Theory, Methodology, and Applications.
- Laemmli, U. K. (1970). "Cleavage of structural proteins during the assembly of the head of bacteriophage T4." Nature, 227(5259), 680-685.
- Sambrook, J., & Russell, D. W. (2001). "Molecular Cloning: A Laboratory Manual." Cold Spring Harbor Laboratory Press.
- Jorgenson, J. W., & Lukacs, K. D. (1983). "Capillary zone electrophoresis." Science, 222(4621), 266-272.
- Landers, J. P. (1993). "Capillary Electrophoresis: Theory and Practice." Journal of Chromatography A, 652(1), 3-15.
- Clarke, H. G., & Freeman, T. (1968). "Quantitative immunoelectrophoresis of human serum proteins." Clinical Science, 35(3), 403-413.
- Westermeier, R. (2005). Electrophoresis in Practice: A Guide to Methods and Applications.
- Pergande, M. R., & Cologna, S. M. (2017). "Isoelectric Focusing: A Review of the Fundamentals and Applications." Analytical Chemistry, 89(1), 32-44.
- Smoluch, M. W., et al. (2017). "Isoelectric Focusing of Proteins: A Review." Journal of Chromatography B, 1044, 115-125.
- Rickwood, D., & Hames, B. D. (1990). Gel Electrophoresis of Nucleic Acids: A Practical Approach.
- Westermeier, R. (2005). Electrophoresis in Practice: A Guide to Methods and Applications.
- Hames, B. D. (1998). Gel Electrophoresis of Proteins: A Practical Approach.m.


 Shaik Rakiul Islam*
Shaik Rakiul Islam*
 Elluru Naga Deepthi
Elluru Naga Deepthi







 10.5281/zenodo.14293603
10.5281/zenodo.14293603