Abstract
Virosomes are viral envelopes that can be packaged as vaccines and carriers for cellular transfer of various macromolecules. Since virosomes are biologically active, they are non-toxic and non-autoimmune. Attempts have been made to use them as antibiotics or as adjuncts and delivery systems for drugs and organic compounds for medical purposes. Studies have shown that the virosome can carry a variety of biological molecules such as nucleic acids, peptides, proteins and small organic molecules. Virosome is a revolutionary hybrid drug delivery system that combines the advantages of viral and non-viral carriers. Abstract: Virosomes are spreadable envelopes that can be used as vaccines and carriers for the cellular delivery of various macromolecules. Since virosomes are bioactive, they are non-toxic and non-autoimmune. Attempts have been made to use them as antibiotics, food additives and delivery systems for drugs and organic compounds for medical purposes. Studies have shown that virosomes can carry a variety of biomolecules such as nucleic acids, peptides, proteins and small molecules. Virosome is a revolutionary hybrid drug delivery system that combines the advantages of viral and non-viral carriers. Therefore, this review provides a new perspective on the production, materials and application of virosome-based nanovaccines in viral diseases, especially for SARS-CoV-2 vaccine discovery. To increase the efficiency of gene transfer by introducing molecules directly into cells, virosomes have been created by combining liposomes with viral envelope proteins. Currently, a lot of research has been done on new methods of vaccine delivery, including microparticles, liposomes and nanoparticles, among other things for infectious diseases. In contrast to conventional methods of vaccine production, virosome-based vaccines represent the next generation of vaccines due to the balance between potency and tolerance in terms of stimulation prevent. The liposome surface is modified with antibodies and ligands that are recognized by specific cell types to improve tissue localization. Liposomes and fungal protein have been combined to form virosomes, which deliver molecules directly into the cell, thereby improving the efficiency of gene transfer
Keywords
Virosomes, Hybrid, Drug
Introduction
New therapies for neurological or cancer diseases include delivery systems that facilitate the delivery of drugs to host tissues and specific cell types with controlled delivery and uptake of mediators. Successful therapies require delivery systems to deliver drugs to their specific targets, and new drugs are sometimes hindered by a lack of proper delivery methods(1). Elliptical therapy involves targeted delivery of the drug by controlling the release and cross-linking into specific cells and tissues. Targeted drug delivery, sometimes called smart drug delivery, is a method of delivering drugs to a patient by increasing the concentration of the drug in certain parts of the body compared to others(2). Delivery systems that allow drug administration to treat specific tissues and cell types through the controlled release and uptake of mediators have been incorporated into innovative treatments for neurological or cancer diseases. Several drug delivery systems have been developed so far. They are usually grouped into viral vectors and non-viral vectors(3). Viruses such as retroviral, adenoviral or lentiviral are more useful than other vectors in gene transfer, but they are not suitable for transducing agents such as synthetic oligonucleotides, proteins, small compounds or Currently, various non-viral carriers such as liposomes, polymeric micelles and composite carriers have also been developed for gene and drug delivery. Compared to viral strains, non-viral strains are safer but less effective in drug delivery(4). To overcome the limitations of viral and non-viral agents and their uses, virosomes were developed, a hybrid drug delivery system containing viral and non-viral components. Virosomes were first prepared by Almeida and colleagues, who incorporated purified influenza protein into liposomes. A virosome is a drug or vaccine delivery system that consists of a single (single or double) phospholipid membrane that contains virus-derived proteins for assembly so that the virosomes can interact with the target cell. Virosomes are complex replication derived virus particles without nucleic acid. Virosomes do not replicate, but vesicles have a complex fusion function, thereby delivering target compounds such as drugs, antigens and genes to the target cell(5). Virosomes also have additional properties that can stimulate the immune system. In addition, the lipid bilayer of virosomes protects drugs from degradation. Therefore, their stability and biocompatibility will be improved. Virosomes also have additional properties that can stimulate the immune system. Virosomes are vesicles with a sterile fusion function that deliver an internal substance such as a drug, antigen, or gene to a target cell(6). The viral envelope of the virosome contains a lipid membrane and the viral spike glycoprotein, but no viral genetic material. Virosomes were first prepared by Almeida et al. by loading purified non-expressing proteins into preformed liposomes. After that, several viruses were created, including the Sendai virus, the Semliki forest virus and the Sindbis virus. Virosomes protect active pharmaceutical ingredients from proteolytic degradation and the low pH in endosomes, so that the contents remain intact after reaching the cytoplasm(7). Therefore, viruses derived from influenza viruses are already marketed as influenza vaccines. In one study, after hepatitis A virus antigens were assembled on the surface of influenza-like virosomes, an immune response to hepatitis A virus was induced in mice that was higher than the immune response produced by the vaccine. Currently, many studies have been conducted on new vaccine delivery methods such as microparticles, liposomes, and nanoparticles, among others, for infectious diseases(8). Unlike conventional methods of vaccine production, virosome-based vaccines represent the next generation in vaccination due to the balance between potency and tolerance due to meaning of inhibitory stimulation. The surface of the liposome is modified with antibodies and ligands recognized by specific cell types to improve tissue localization. Liposomes and fungal protein have been combined to form virosomes, which deliver molecules directly into the cell, thereby improving the efficiency of gene transfer(9).
ADVANTAGES OF VIROSOMAL DRUG DELIVERY (10-12)
- Activate the transport of a substance into the cytoplasm of the target cell
- Viruses are biological.
- Medicines are protected from corruption.
- Biocidal and non-lethal
- No risk of transmission
- No autoimmunity
- Materials widely used and essential drugs (antibiotics tumors, proteins, peptides, nucleic acids, anti-infectives, antifungal funds)
- It enhances the movement of the compound in the endolysosomal pathway.
- Deliver antigens to a specific target and enhance the immune response.
- Absorption, distribution and elimination of drugs in the body.
- Increase the scale according to the standard method.
- The Virosome gives the patient a unique vaccine.
- It can be fixed by injection or through the nose.
- Release of the active substance in the cytosol of the selected cell. The antigen is a protective factor against other cell damage, and because of the deposition, the immune system is significantly improved.
- Stimulate the delivery of antigens and enhance the immune response.
- Virosomes can interfere with specific vaccination mechanisms.
DISADVANTAGES OF VIROSOMES (13, 14)
- They can stimulate immune responses due to the presence of viral glycoprotein on the surface.
- The problem with virosomes is that they break down quickly in the bloodstream and have a short life span.
- Production problems
- Insufficient raw materials
- Slow delivery.
- There is no evidence that virosomes can be used indefinitely.
- Rapid degradation can be avoided by improving virosome stability or ensuring that virosomes reach their target sites immediately after injection.
- Using high quality products that have proven to be the most effective cleaning methods.
- Quality control can be tested using the latest technology, and batch-to-batch variations can be measured.
- External loading methods are used to reduce loading problems.
- To extend the life of the floor, use ice with appropriate skin protection.
- Choosing the right method of preparation and sterilization, such as autoclaving or membrane filtration, with a Lal-free test, validated for pyrogen removal, will help increase.
STRUCTURE OF VIROSOMES
Virosomes are spherical, monolayer phospholipid bilayer vesicles with a typical diameter of 120-180 nm. Influenza virus is the most commonly used virus to synthesize virosomes and genetic material. Virosomes cannot replicate without active fusion vesicles. Regenerative-stimulated neurons (IRIVs) contain high concentrations of phosphatidylcholine (PC) and phospholipids (PL). More than 70% of the virosomal composition is made of PC(15). The remaining 30% of the membrane components are influenza virus envelope phospholipids, which help to release the glycoproteins hemagglutinin (HA) and neuraminidase (NA). By changing the number or type of membrane lips used, virosomes can be modified for better drug absorption or greater physiological effect. Depending on the type of positive or negative phospholipids present in the membrane, carriers for antisense oligonucleotides and other genetic molecules can be produced. HA is the main antigen of influenza and consists of two polypeptides HA1 and HA2, which are involved in receptor binding and membrane fusion(16). The HA1 global head contains a receptor site with high affinity for sialic acid on the surface of antigen presenting cells (APC) such as lymphocytes and macrophages. HA2 Integration of IRIV with the endosomal membrane is mediated by HA2 polypeptide(17). Peptides, cytokines and monoclonal antibodies are all antibodies that can bind to virosomes (MAbs). It was also found on the surface of the virosome core. Viruses can attach to tumor-specific monoclonal antibody (Fab) fragments to direct the receptor to specific tumor cells(18).
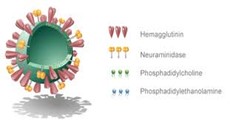
Figure 1 Structure Of Virosomes
METHOD OF PREPARATION OF VIROSOMES (19, 20)
Virus selection for virosomes:
Virosomes are non-enveloped envelopes that can be derived from a variety of viruses. The influenza virus envelope is usually used to produce virosomes, but it can also be produced from other viruses such as Sendai virus, Epstein-Bohr virus, HIV, Sandbis, Semliki forest virus, murine leukemia virus, herpes simplex virus. , Newcastle disease virus.
Selection of antigens:
Antigens are selected according to need. Antigens are used as antigens such as bacterial parasite, cancer cell or whole cell. Cellular components including RNA, DNA or plasmids can also be used as antigens. This antigen is attached to a lipid anchor so that the antigen is ready to be loaded onto virosomes.
Reconstruction of Virosomes:
Virosome dissolved with detergents such as octaglucoside, Triton x-100, free p-40 are dissolved with the viral material inside, the detergent and material are retained genes, then the detergents are removed by various methods such as dialysis and resin is extracted from the supernatant. Using ultracentrifugation, the viral matrix protein and nucleocapsid are removed. Viral phospholipid (82%) and viral protein are recycled in the supernatant. The antigen attached to the lipid anchor is now mixed with a polymeric solution or surfactant, and this solution is introduced into the virosome carrier to obtain the antigen-binding virosome.
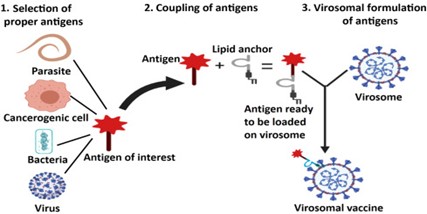
Figure 2 Method Of Preparation Of Virosomes
MECHANISM OF ACTION OF VIROSOMES (21-23)
Virosomes work together as carriers and act as helpers with various functions to induce immune responses They are pathogenic and produce antibodies. The inclusion of antigen on the surface, by the formation of B cells, in the form of a complex, is the main purpose for the response of immune cells, which is important for the introduction of the disease. The adjuvant function is related to the stimulation of the protective properties of virosomes and components of the immune system without causing non-specific inflammation, the adjuvant function is based on the presence of hemagglutin antibodies in advance with influenza, it binds to virosomes and leads to rapidity. uptake and processing by antigen presenting cells. APC). The common function of antibody to bind virus and prevent infection is one of the factors that make virosomal particles suitable for uptake and processing by the immune cell, size and surface area are important. virosomes to initiate an immune response. Helper APCs activate influenza-specific helper T cells and secrete cytokines to stimulate immune cells.
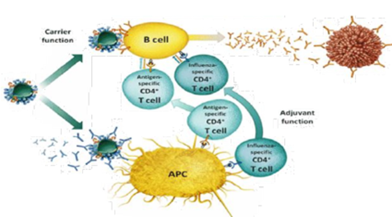
Figure 3 Mechanism Of Action Of Virosomes
ROUTE OF ADMINISTRATION OF VIROSOMES (24-26)
Transporting drugs to different parts of the body is the main goal of every scientist. Prepared virosomes are often resuspended in saline (150-135 mmol/L NaCl) containing other excipients (eg, calcium chloride, potassium chloride, sodium acetate, sodium lactate) to model physical activity. These compounds must be sterilized using liposome sterilization techniques such as membrane filtration. Generally, the concentration of the virosome carrier is between 20 and 200 mg/ml, although it can be increased depending on the characteristics of the virosome component and a specific project. The concentration of virosomes is usually in the range between 20 and 200 mg/ml in their buffer, depending on the characteristics of the virosome components and specific targets. Importantly, they must be purified or sterilized before handling, by the classic method of liposome sterilization, i.e. membrane filtration. These concentrations vary to optimize treatment with different virosomes or for specific purposes. Viruses can be administered by a number of routes such as intravenous (IV), subcutaneous (SC), intramuscular (IM), intravesticular, oral, topical or inhaled, and dermal. For long-term distribution, virosomes can also be inserted into injection devices.
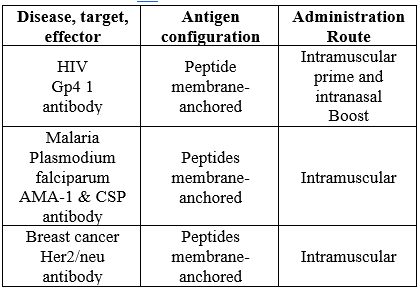
Table 1 Route Of Administration Of Virosomes
PHARMACOKINETICS OF VIROSOMES (27, 28)
Pharmacokinetic data can be used to interpret differences in the therapeutic effect of liposome-encapsulated drugs and free drugs, and are therefore used for dosage planning. Pharmacokinetics governs the duration of retention, spread and degradation of viral vectors in vivo. The treatment of virosomes requires areas that can be considered after internal administration, because this is the most popular time for various virosome details that are used for clinical treatments except for topical designs. Virosomes alter the tissue distribution and rate of drug release by affecting pharmacokinetic parameters. Ideally, the drug is transported in the aqueous phase by the midstream virosome and released at a sufficient rate to be completely free from accumulation in tissues and other specialized sites. Bioavailability in the case of viral vectors can be defined as the amount of free drug that leaves the carrier and is available for redistribution to nearby tissues.
The effect of the pharmacokinetic parameter on the virosomes is presented, the following results-
- A more clinical indication.
- Targeted websites are more targeted.
- Maintenance of drug in plasma.
- Reduction of toxicity and non-specific reaction.
- Decreasing the non-exclusive location.
Table 2 Virosomes as a vesicular medication delivery mechanism is being studied
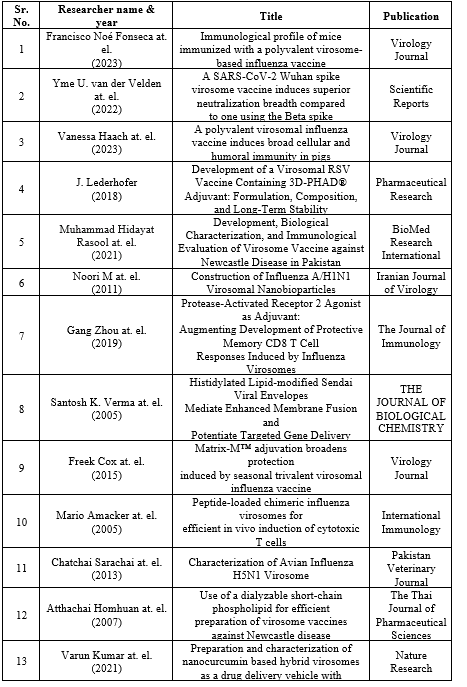
VIROSOME-CELL INTERACTION (29-31)
The innovative ability of the virosome to suppress the infected expression in vivo, which can help the immune players and organize macromolecules in a functional area, is its main advantage. Virosomes recognize and bind to the same receptors that enter when a person is infected with a virus. For example, influenza virosomes use phagocytic sialic receptors. When an infection finds a cellular host, the structure of viral and endosomal membranes is examined. When influenza virosomes emerge, for example, the viral HA protein uses its doublet form for a purpose. The NA enzyme is also involved in the process of virosome assembly as it increases immunogenicity and concentration in the specific tissue. The interaction between virosomes and receptors has been investigated for the treatment of many diseases, including viral infections, neurological diseases and many other diseases. The main goal in each case is to deliver a nanosized protein, harmful nucleic acid or drug particle to the site of action. Glycoproteins on the surface of virosomes bind well to peptides and proteins. Respiratory syncytial (RSV) vaccines use influenza virosomes engineered by fusing hepatitis C virus surveillance proteins to virosomal proteins. In response, influenza virosomes were used to create B epitope sites that are highly resistant to intestinal infections. In addition, the use of virosome architecture has been studied in several diseases. In this respect, this approach benefits from maintaining strategic spacing between multiple vaccine doses.
CHARACTERIZATION OF VIROSOMES (32-34)
Virosome preparation usually results in a protein-to-lipid ratio. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) is used to confirm the presence of HA protein in virosomes.
Protein detection - Virosome preparation should result in a similar protein to lipid ratio, the presence of hemagglutinin protein on the virions can be confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
Conjugation activity - In general, virosomes exhibit pH-dependent film-induced motion similar to local influenza, similar to natural influenza, and the resonance energy transfer method is used to Viewing the virosomal fusion and the fusion of the membrane with the tissue and the organism, in The laboratory methods, by combining the excimer virus with the biological film or synthesis, can be examined in the laboratory method to measure the use of a lipid called pyrene, where the surface migration of pyrene phosphatidylcholine is labeled in combination with an unlabeled membrane lead to a decrease in fluorescence.
Structure and Size –
Color negative electron microscopy is used to determine the ultra structure and size of virosomes. The dye is pH neutral to avoid structural changes of HA caused by acid.
EVALUATION (35-40)
Virosomes are viruses designed for vaccine delivery. Some of the parameters used to evaluate virosomes are:
- Vesicle size and size distribution: dynamic light transmission, transmission electron microscopy, zetasizer, photoluminescence spectroscopy, laser fluorescence, diffusion, gel saturation and avoid the sun. In general, negative color electron microscopy can be used to determine the ultrastructure and size of virosomes. Dyes are pH neutral to prevent HA structural changes.
- Vesicle morphology and surface morphology: mechanical electron emission, mechanical electron freezing.
- Surface pH and surface electrical potential: estimation of zeta potential and the tactile pH test.
- Surface charge: free flow of electricity. Front: electron beam scattering, scanning electron microscopy. Layers: X-ray surface scattering, ice-breaking electron spectroscopy, 13P-NMR.
- Drug delivery: Cell diffusion/dialysis.
- Percent free drug: Small-chamber centrifugation, ion exclusion and ion exchange chromatography, protamine concentration, radiolabeling, drug therapy.
- Animal toxicity: observation of life, genetics and pathology. Fever: rabbit fever reaction test or Limulus Embocyte Lysate (LAL) test.
- Chemical surface research: statically assisted particle mass.
- Protein recruitment: Virosome regulation usually ends with the protein-to-fat ratio. Sodium Dodecyl sulfate-polyacrylamide gel electrophoresis (SDSPAGE) can substantiate the pervasiveness of HA protein the virosomes.
- Synergistic activity: Virosomes are pH-dependent membrane proteins, similar to the original influenza virus. Fluorescence resonance energy transfer (FRET) experiments can be used to visualize virosomal fusion with biological or synthetic membranes.
- Protein content: The Bradford protein estimation method can be used to measure the protein content of completed virosomes.
NANOVACCINES
Nanotechnology has created the most important way to create new generation vaccines. Currently, many types of nanocarriers including polymers, peptides, virus-like particles (VLPs) and lipids have been used in vaccine design. However, polymeric and lipid-based nanocarriers are the most studied devices. Nanovaccines based on virosomes are an advanced approach for vaccine production. Virosomes are artificial vesicles that mimic viruses and allow them to present antigens to the immune system without being killed(41).
Nanovaccine virosomes have many advantages, including:
- Strengthening the immune response: They can stimulate humoral and cellular immunity.
- Targeted delivery: Can be tailored to target specific cells or tissues.
- Better prognosis: no live virus and reduced risk of side effects.
- Simplicity: can be designed to carry different antigens, because there are many different diseases.
Research continues on nanovaccine virosomes, with applications to:
- Influenza
- HIV
- Cancer
- COVID-19 (42)
APPLICATIONS OF VIROSOMES (43-51)
Virosomes conjugated to antibodies to improve tissue specificity as the antibody binds to the specific receptor Several virosome-based products have been approved by the USFDA for human use. A key feature of virosome design is the interaction between antigenic proteins from the virus and cell receptors. The interaction of the virus receptor was investigated for the treatment of parasitism, viral disease, neurological disease and other cancers. Virosomes contain antibacterial, antimalarial, antifungal, invitro and in vivo properties that have been proven effective, release of virus tranquilizers-and it is fast, protective and sustainable compared to other related systems. In addition, viruses can deliver proteins and peptides. The A-eggelonin component of diphtheria toxin is delivered to target cells by virosomes, along with ovalbumin.
Cancer is one of the major health problems worldwide and is still a major challenge because the intensive use of NDDS in cancer treatment has led to the development of new therapeutic strategies. Virosomes are also used in oncology because they can carry peptides to target antigens (TAA) such as peptides derived from parathyroid hormone-related protein (PTH-rp) from the recombinant protein such as Her-2/neu, Fab combined the antiproliferative properties of monoclonal antibodies and the cytotoxic effect of doxorubicin in vivo.
- Hemagglutinin gene delivery:
The membrane-bound protein of influenza virus, is known to mediate a small pH-dependent fusion reaction between the viral envelope and the limiting membrane of the cleavage endosome, with after cellular uptake of viral particles by mediators endocytosis.
Virosomes show good tolerance and very specific immune responses. Two peptide structures have been identified that serve as antigens for malaria vaccines. The NPNA regions of the circumsporozoite protein (CSP) ring I domain III antigen-1 in the apical membrane of merozoites (AMA-1) lead to other structural antigens that have been identified.
Virosomes display a pathogen-associated molecular pattern (PAMP) that provides stimulatory signals to APCs.
The delivery of RNA/DNA genetic material in virosomes capable of synthesizing new proteins can be produced and overcomes the lack of a proper delivery mechanism or these molecules
REFERENCES:
- Singh N, Gautam SP, Kumari N, Kaur R, Kaur M. Virosomes as novel drug delivery system: an overview. PharmaTutor. 2017;5(9):47-55.
- Shaikh SN, Raza S, Ansari MA, Khan G, Athar SHM. Overview on virosomes as a novel carrier for drug delivery. Journal of drug delivery and therapeutics. 2018;8(6-s):429-34.
- Singh A, Kathuria T, Kalra S, Yashpal M. Virosomes: A Drug Delivery System. Microbial Products: CRC Press; 2022. p. 401-15.
- Mughees MM, Ansari MA, Mughees A, Farooque F, Wasi M. Virosomes as drug delivery system: An updated review. International Journal of Research in Pharmaceutical Sciences. 2021;12(3):2239-47.
- Rathor S, Soni P, Lal D. Unique drug delivery system: Virosomes. Research Journal of Pharmaceutical Dosage Forms and Technology. 2019;11(4):304-8.
- Alenzi AM, Albalawi SA, Alghamdi SG, Albalawi RF, Albalawi HS, Qushawy M. Review on different vesicular drug delivery systems (VDDSs) and their applications. Recent Patents on Nanotechnology. 2023;17(1):18-32.
- Kalra A, Sharma S. Virosomes: a viral envelope system having a promising application in vaccination and drug delivery system. Nanopharmaceutical Advanced Delivery Systems. 2021:145-60.
- Nalawade R, Nalawade A, Patwa H, Ghode S, Raut R, Chatur VM. Virosomes: A Drug Carrier System. J Hosp Pharm. 2021;16(1):1-9.
- Prabahar K, Alanazi Z, Qushawy M. Targeted drug delivery system: Advantages, carriers and strategies. Indian J Pharm Educ. 2021;55:346-53.
- Medarametla RT, Gopaiah V. A comprehensive study on the review of virosomes As a novel drug delivery system. UPI Journal of Pharmaceutical, Medical and Health Sciences. 2023:1-6.
- Myneni GS, Radha G, Soujanya G. Novel vesicular drug delivery systems: a review. J Pharm Res. 2021;11(4):1650-64.
- Ali H, Akbar M, Iqbal B, Ali F, Sharma NK, Kumar N, et al. Virosome: An engineered virus for vaccine delivery. Saudi Pharmaceutical Journal. 2023;31(5):752-64.
- Ansari MH. Virosome Delivery System: Concepts and Applications. Int J Med Phar Sci| Vol. 2021;11(05):1.
- Alavi M, Karimi N, Safaei M. Application of various types of liposomes in drug delivery systems. Advanced pharmaceutical bulletin. 2017;7(1):3.
- Wang Y, Li B, Luo Y, Yang T, Zhao X, Ding P. Virosome, a promising delivery vehicle for siRNA delivery and its novel preparation method. Journal of Drug Delivery Science and Technology. 2022;74:103490.
- Deshmukh PK, Jain SN, Patil PO, Pardeshi CV. Vesicular carriers for direct nose-to-brain drug delivery. Direct Nose-to-Brain Drug Delivery: Elsevier; 2021. p. 209-23.
- Karpe S, Gupta K, Vyas G, Rana P, Khan F, Kumar R, editors. Virosome: A vector in vaccine delivery. BIO Web of Conferences; 2024: EDP Sciences.
- Kumar V, Kumar R, Jain V, Nagpal S. Comparison of Virosome vs. Liposome as drug delivery vehicle using HepG2 and CaCo2 cell lines. Journal of Microencapsulation. 2021;38(5):263-75.
- Prathyusha H, Venkatesh P. Review on virosomes. Journal of Innovations in Applied Pharmaceutical Science (JIAPS). 2022:18-23.
- Pattnaik S, Swain K, Singh SP, Sirbaiya AK. Lipid vesicles: Potentials as drug delivery systems. Nanoengineered Biomaterials for Advanced Drug Delivery: Elsevier; 2020. p. 163-80.
- Satija S, Dhanjal DS, Sharma P, Hussain MS, Chan Y, Ng SW, et al. Vesicular Drug Delivery Systems in Respiratory Diseases. Advanced Drug Delivery Strategies for Targeting Chronic Inflammatory Lung Diseases: Springer; 2022. p. 125-41.
- Supraja B, Mulangi S. An updated review on pharmacosomes, a vesicular drug delivery system. Journal of drug delivery and therapeutics. 2019;9(1-s):393-402.
- Radwan Y, Karaly AH, El-Sherbiny IM. Nanovesicles for delivery of antiviral agents. Viral Infections and Antiviral Therapies: Elsevier; 2023. p. 493-518.
- Mbah CC, Attama AA. Vesicular carriers as innovative nanodrug delivery formulations. Organic Materials as Smart Nanocarriers for Drug Delivery: Elsevier; 2018. p. 519-59.
- Asadikaram G, Poustforoosh A, Pardakhty A, Torkzadeh-Mahani M, Nematollahi MH. Niosomal virosome derived by vesicular stomatitis virus glycoprotein as a new gene carrier. Biochemical and Biophysical Research Communications. 2021;534:980-7.
- Ahmadi D, Zargar M, Zolfaghari MR, Kazemimanesh M, Ghaemi A. Construction of Cationic Virosome Derived from Vesicular Stomatitis Virus as a Promising Candidate for Efficient Gene Delivery to the Central Nervous System. The Neuroscience Journal of Shefaye Khatam. 2020;8(2):72-81.
- Bagmar NA, Hatwar PR, Bakal R. A review on Targeted Drug Delivery System. WJPR. 2023;12(19):288-98.
- Chivte P, Pardhi V, Pillai A. Biological Methods for Drug Delivery. Advanced Drug Delivery: Methods and Applications: Springer; 2023. p. 1-20.
- Nayak AK, Hasnain MS, Aminabhavi TM, Torchilin VP. Nanovesicular systems in drug delivery. Systems of Nanovesicular Drug Delivery: Elsevier; 2022. p. 1-15.
- Witika BA, Makoni PA, Mweetwa LL, Ntemi PV, Chikukwa MT, Matafwali SK, et al. Nano-biomimetic drug delivery vehicles: potential approaches for COVID-19 treatment. Molecules. 2020;25(24):5952.
- Mohammad Z, Zeeshan A, Faisal S, Suhail A, Sahar I, Mohd S, et al. Vesicular drug delivery system used for liver diseases. World Journal of Pharmaceutical Sciences. 2017:28-35.
- Babar MM, Najam-us-Sahar Sadaf Zaidi A, Kazi G, Rehman A. Virosomes-Hybrid drug delivery systems. LIPOSOME & NANOTECHNOLOGY. 2013:415.
- Kalra N, Dhanya V, Saini V, Jeyabalan G. Virosomes: as a drug delivery carrier. American Journal of Advanced Drug Delivery. 2013;1(1):29-35.
- Sen R, Gupta R, Singh S, Mantry S, Das S. A Review on Cubosome and Virosome: the novel drug delivery system. UJPSR. 2017;3(1):24-33.
- Bhattacharya S, Mazumder B. Virosomes: A novel strategy for drug delivery and targeting. BioPharm International. 2011;2011(1).
- Kapoor D, Vyas R, Lad C, Patel M. A multipurpose and novel carrier for drug delivery and targeting-virosomes. Journal of drug delivery and therapeutics. 2013;3(5):143-7.
- Kamboj S, Saini V, Magon N, Bala S, Jhawat V. Vesicular drug delivery systems: a novel approach for drug targeting. brain. 2013;1(11).
- Rathore P, Swami G. Virosomes: a novel vaccination technology. International Journal of pharmaceutical sciences and research. 2012;3(10):3591.
- Shinde NG, Aloorkar NH, Kulkarni AS. Recent advances in vesicular drug delivery system. Research journal of pharmaceutical dosage forms and technology. 2014;6(2):110-20.
- Garg T, Rath G, Goyal A. Colloidal drug delivery systems: current status and future directions. Critical Reviews™ in Therapeutic Drug Carrier Systems. 2015;32(2).
- Asadi K, Gholami A. Virosome-based nanovaccines; a promising bioinspiration and biomimetic approach for preventing viral diseases: A review. International journal of biological macromolecules. 2021;182:648-58.
- Moser C, Müller M, Kaeser MD, Weydemann U, Amacker M. Influenza virosomes as vaccine adjuvant and carrier system. Expert review of vaccines. 2013;12(7):779-91.
- Chime SA, Onyishi IV. Lipid-based drug delivery systems (LDDS): Recent advances and applications of lipids in drug delivery. Afr J Pharm Pharmacol. 2013;7(48):3034-59.
- Kaneda Y. Virosome: a novel vector to enable multi-modal strategies for cancer therapy. Advanced drug delivery reviews. 2012;64(8):730-8.
- Jamali A, Holtrop M, de Haan A, Hashemi H, Shenagari M, Memarnejadian A, et al. Cationic influenza virosomes as an adjuvanted delivery system for CTL induction by DNA vaccination. Immunology letters. 2012;148(1):77-82.
- Liu Q, Jung J, Somiya M, Iijima M, Yoshimoto N, Niimi T, et al. Virosomes of hepatitis B virus envelope L proteins containing doxorubicin: synergistic enhancement of human liver-specific antitumor growth activity by radiotherapy. International journal of nanomedicine. 2015:4159-72.
- Lande C, Cecchettini A, Tedeschi L, Taranta M, Naldi I, Citti L, et al. Innovative Erytro-Magneto-HA Virosomes for gene-drug delivery in porcine model of VSMC activation: basis for local therapy to prevent restenosis. Cardiovasular & Hematological Disorders Drug Targets. 2012;12(1):68-75.
- Saga K, Kaneda Y. Virosome presents multimodel cancer therapy without viral replication. BioMed Research International. 2013;2013(1):764706.
- Kheiri MT, Jamali A, Shenagari M, Hashemi H, Sabahi F, Atyabi F, et al. Influenza virosome/DNA vaccine complex as a new formulation to induce intra-subtypic protection against influenza virus challenge. Antiviral research. 2012;95(3):229-36.
- Moser C, Amacker M. Influenza virosomes as antigen delivery system. Novel Immune Potentiators and Delivery Technologies for Next Generation Vaccines: Springer; 2012. p. 287-307.
- Liu H, de Vries-Idema J, Ter Veer W, Wilschut J, Huckriede A. Influenza virosomes supplemented with GPI-0100 adjuvant: a potent vaccine formulation for antigen dose sparing. Medical microbiology and immunology. 2014;203:47-55.


 Prajapati Rahul Vinitbhai *
Prajapati Rahul Vinitbhai *
 Soni Dhruvi Nileshkumar
Soni Dhruvi Nileshkumar
 Khatiya honey Girdharilal
Khatiya honey Girdharilal
 Rajput Shruti Rakeshbhai
Rajput Shruti Rakeshbhai
 Mahendrakumar R Dubey
Mahendrakumar R Dubey





 10.5281/zenodo.13955400
10.5281/zenodo.13955400