Abstract
Novel drug delivery System, like niosomes, extend the drug's shelf life and efficacy by shielding it from environmental influences and enzymatic destruction. By using different concentration of polymers, the formulation and evaluation of chlorpheniramine niosomes has been prepared by using ether injection method. A14 gauge needle was selected for preparation of formulation. Pre-formulation analysis uses FTIR to ensures the drug purity and its compatibility with excipients. The gel, however, exhibit some instability in accelerated conditions (40°C), suggesting that higher temperatures may have an impact on the formulation's long-term stability. The chlorpheniramine niosomal gel's pH was found to be approximately 5.9, which is within acceptable range for topical application. The maximum cumulative drug release (93.25%) in 8 hours and hence it is the best formulation.
Keywords
Chlorpheniramine, Niosomal gel, Novel drug delivery, FTIR, SEM.
Introduction
A technique that combines innovative development, formulations, new technology, and novel methodology for delivering pharmaceutical substances throughout the body as needed to safely achieve its targeted pharmacological effects is known as a novel drug delivery system (NDDS). An existing therapeutic molecule can be given new life in the form of a novel drug delivery system. When a novel drug delivery system is properly constructed, it can be a significant advancement in addressing issues with drug release at a specific place at an appropriate movement. A novel drug delivery system serve to improve therapeutic value by lowering toxicity, raising bioavailability and reducing the need for repeated administration to overcome non-compliance. (1) The effectiveness of a medication can be greatly impacted by how it is taken. For many drugs, which have an ideal concentration range within which optimum benefit is attained, concentrations above or below this range may be harmful or fail to produce any therapeutic benefit. However, the extremely slow development in the efficacy of treating major illnesses has pointed to the growing necessity of a multidisciplinary strategy for delivering medications to tissue targets. As a result, new ideas for controlling the pharmacokinetics, pharmacodynamics, immunogenicity, non-specific toxicity, and efficacy of drugs were created. This novel method referred as drug delivery system. (2) Major function of niosomes is that they hinder its removal from bloodstream thereby improving the APIs therapeutic efficacy. When niosomes are applied topically, with human skin it facilitate the desired interaction by modifying the properties of the stratum corneum, that help in reduction of transdermal water loss and eliminates the skin through skin lipid replenishment. (3)
MATERIAL AND METHODS
Pre-Formulation Study
Melting point and Fourier transform infrared (FTIR) spectroscopy have been employed in the Preformulation studies to identify Chlorpheniramine. The Odor, colour, and physical appearance of the drug samples were examined.
1.Preliminary drug solubility studies
50mg of chlorpheniramine drug was weighed accurately and solubility was checked in 50 ml of Distilled water, Methanol, Toluene and Ethanol. It was discovered that the drug was freely soluble in Distilled water, Methanol and Ethanol but practically insoluble in toluene. Therefore, distilled water was selected as diluent as they are freely available and experimental stability experiments revealed that the medication remained stable in methanol for 24 hours. (4)
2.Melting point study of drug chlorpheniramine
To determine melting point, a capillary tube method was used to recognize the melting point of chlorpheniramine. A thick-walled capillary tube sealed from one end and the other side of the tubes were filled with drug. By using a digital melting point apparatus, subsequently the capillary was positioned within the melting point apparatus and thermometer was set. Once the drug gets down to melting and achieved complete liquid condition, the temperature was recorded using a thermometer. This procedure was recorded for five times and then the obtained value were then compared with a reference value for verification. (5)
3.Preparation of standard graph for Chlorpheniramine using distilled water (6)
3.(i) Determination of absorption maxima (?max).
100mg drug of chlorpheniramine was precisely weighed and dissolved in 100ml of distilled water in 100ml volumetric flask and this result in preparation of stock Solution A. Now 10 ml was taken from stock solution A and transferred into 100 ml volumetric flask and diluted up to 100 mL with distilled water. The resulting solution was labelled as standard working Solution B. Then 2 ml of the working solution B was withdrawn and diluted up to 10 mL with distilled water in 10 ml volumetric flask. This solution spectrum was measured using a UV- visible spectrophotometer in the 200-400 nm range. It was discovered that the drug chlorpheniramine has a ? max of 261nm.
3. (ii) Preparation of standard graph
To get concentration of 1?g, 2?g, 3?g, 4?g, 5?g, 6?g, 7?g and 8?g respectively 1, 2, 3, 4, 5, 6, 7 and 8 ml of the chlorpheniramine standard working Solution B were taken out and diluted with 10 ml of distilled water in a 10 ml volumetric flask. Using the distilled water as a blank, the absorbance of each solution was measured at 261nm using a UV-visible spectrophotometer.
4.Preparation of calibration curve
From the above working standard stock solution B (100 ?g/ml), 1 ml was pipetted out and then transferred to a series of 10 ml volumetric flasks. The final volume was adjusted with diluent to provide solutions of 1 to 8 ?g/ml of Chlorpheniramine. Solutions were then scanned in the range of 200-400 nm against diluent as blank. The absorbance maxima (? max) was found to be 261nm for Chlorpheniramine and then calibration curve was plotted as absorbance vs concentration. (7)
5.Partition coefficient of drug
By shaking equal amounts of oil and the aqueous phase in a separating funnel, the drug's partition coefficient was ascertained. 50 ml of a drug solution containing 1 mg per ml was placed in a separating funnel, shaken for 20 minutes with an equivalent volume of n-octanol, and then left to stand for 8 hours while being shaken intermittently. Next, a UV spectrophotometer was used to measure the aqueous phase and oil phase after partitioning in order to determine the values of the partition coefficient. (8)
Formulation of chlorpheniramine niosomal gel
(i)Preparation of Chlorpheniramine niosomes
As indicated in table, various niosomal formulations were made by means of an ether injection technique with cholesterol at varying doses and a non-ionic surfactant (Span 60). In short, Chlorpheniramine (CPM) was dissolved in 6 ml of methanol, while Span 60 and cholesterol were dissolved in 18 ml of diethyl ether. The resultant solution was then gradually infused into 20 ml of PBS (pH 7.4) hydration solution at a rate of 1 mL/min using a micro syringe. A magnetic stirrer was used to continually agitate the solution while keeping the temperature between 55 and 60 °C. 14 gauze needle is used for slow injection of the lipid solution into the aqueous phase resulted in solvent evaporation and the subsequent temperature differential between the phases, which led to spontaneous vesiculation and niosome formation. For additional research, the resulting niosomes were refrigerated between 4 and 8° C.
(ii)Preparation of gel formulation
Batches with high entrapment efficiency were chosen for additional niosomal gel formulation based on the outcomes of drug-loaded niosome formulation. In order to create a viscous solution, 2g of carbopol-934 was first dissolved in 20 g of PG. Next, 1.5 g of PEG was dissolved in 15 ml of methanol to create a thin solution, and 50 g of F4 was added to it (10 mg/g of gel). To create a CPM-loaded niosomal gel of pH 7.4, a thin solution was added to a viscous solution all at once while being continuously stirred at 55 C. The pH was then adjusted by dropwise Triethanolamine (TEA) to create a homogenous gel, which was then kept in collapsible tubes for future research.
Evaluation of Gel
- Drug Entrapment Efficiency (9)
A sample of 200 mg of niosomal gel was dissolved in distilled water and heated at 40°C in order to determine the entrapment efficiency of niosomes using the centrifugation method. The niosomal dispersion was centrifuged for 40 minutes at 14,000 rpm. To calculate the amount of free drug, the supernatant layer 1was gathered and separated. The drug concentration in the sample was measured using spectrophotometry at the determined ?max (261 nm). The following formula was used to determine the percentage of drug encapsulation.
EE % = [(T-C)/T] ×100
Where, T = total amount of drug (calculated both in supernatant and sediment),
C = amount of drug found only in the supernatant.
2. pH (10)
Using pH indicator paper, the pH of the chlorpheniramine niosomal gel was assessed. To create a homogenous solution, around 1 g of the gel was dissolved in 10 mL of distilled water and gently agitated. After dipping a pH strip into the mixture, the colour shift was contrasted with the reference colour chart that came with the pH paper.
3.Viscosity (11)
A rotational digital viscometer with an N4 spindle operating at 12 rpm and room temperature was used to measure the viscosity of the optimized niosomal gel of Chlorpheniramine.
4.Spreadability (12)
A crucial factor in assessing the effectiveness of topical preparations, such as gels, is spreadability. It establishes how easily a formulation can be applied evenly to the skin. Better application, consistent drug distribution, and patient compliance are all guaranteed by good spreadability. The two-glass slide method was used to assess the chlorpheniramine maleate niosomal gel's spreadability. Two horizontal glass slides were enclosed by a 1 g gel sample. To give the gel time to spread, a consistent 2 kg weight was placed on the top slide for 30 minutes. A calibrated scale was used to measure the gel's spread diameter after the allotted amount of time.
The spreadability of the gel was calculated using the following formula:
S = M.L/T
Where:
S = Spreadability (g·cm/s)
M = Weight applied (g)
L = Length of spread (cm)
T = Time taken for spreading (s)
5.In Vitro Drug Release Study (13)
Specific ratios of chemicals such as cholesterol, surfactant, and drug (chlorpheniramine) were used
to create the formulation (F5). These components most likely improved the stability, niosome size, and drug entrapment effectiveness, which resulted in improved release behaviour.
The following in vitro diffusion techniques were used in the drug release study:
Dialysis Membrane Method: The niosomal gel is immersed in a dissolving liquid (such as phosphate buffer or simulated fluids) while enclosed in a dialysis membrane. To maintain sink conditions, a tiny portion of the dissolving medium was removed and replaced with fresh medium at predetermined intervals (e.g., 1 hour, 2 hours, etc). The amount of chlorpheniramine released into the medium was ascertained by analysing the extracted samples using UV-visible spectrophotometry at a wavelength of 261 nm.
6.Stability studies of Chlorpheniramine Niosomal Gel (14)
The physical, chemical, and rheological stability of the chlorpheniramine maleate niosomal gel was evaluated over a period of a month under various storage circumstances. To imitate normal storage circumstances and ascertain the gel samples' shelf-life, they were kept at different temperatures and humidity levels. The storage conditions listed below were used:
Room Temperature (25°C ± 2°C)
Refrigeration (4°C ± 2°C)
Accelerated Conditions (40°C ± 2°C)
At the conclusion of each week and after a month of storage, the gel samples were assessed for changes in appearance (such as phase separation, colour, and texture), pH, viscosity, and spreadability.
RESULT AND DISCUSSION
Preformulation Studies
The drug's organoleptic properties were found to be within the following ranges, as indicated in the table.
Table:1 Organoleptic Properties of Chlorpheniraie
|
S. No.
|
Properties
|
Inference
|
|
1
|
Colour
|
White to off white
|
|
2
|
State
|
Powder is Crystalline
|
|
3
|
Odour
|
Odourless
|
|
4
|
Taste
|
Astringent
|
Melting Point
The melting point of drug chlorpheniramine at1370C.
Solubility
It was discovered that the drug was freely soluble in Distilled water, Methanol and Ethanol but practically insoluble in toluene.

Fig: 1 Solubility study of Chlorpheniramine
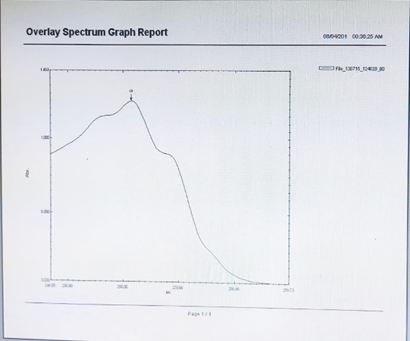
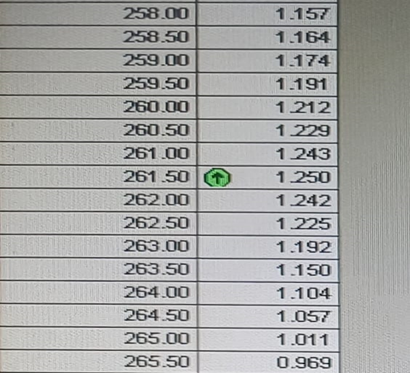
Fig:2 Peak Pick UV Spectrum Report of Chlorpheniramine
Table:2 Construction of Standard Curve
Calibration data of chlorpheniramine niosomal gel in distilled water at 261nm.
|
S. No.
|
Concentration in (?g/mL)
|
Absorbance at 261 nm
|
|
1
|
1 (?g/mL)
|
0.170
|
|
2
|
2 (?g/mL)
|
0.289
|
|
3
|
3 (?g/mL)
|
0.407
|
|
4
|
4 (?g/mL)
|
0.526
|
|
5
|
5 (?g/mL)
|
0.644
|
|
6
|
6 (?g/mL)
|
0.763
|
|
7
|
7 (?g/mL)
|
0.881
|
|
8
|
8 (?g/mL)
|
0.962
|
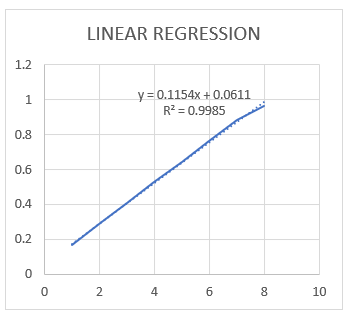
Fig:3 Standard graph of chlorpheniramine niosomal gel
Partition coefficient of drug
The hydrophilicity of chlorpheniramine was justified by the K value of chlorpheniramine which was found to be 1300 in n-octanol and 3.929 in distilled water.
FTIR Of Chlorpheniramine
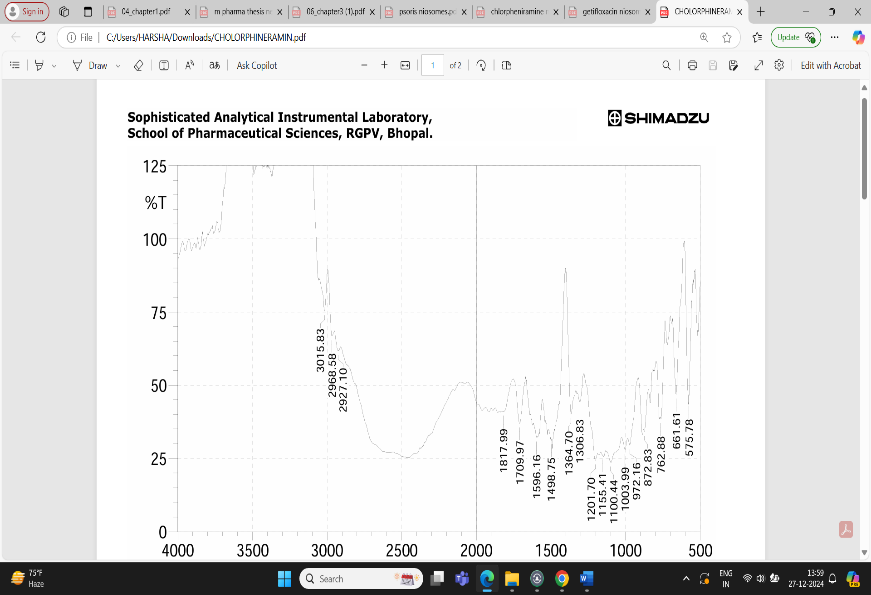
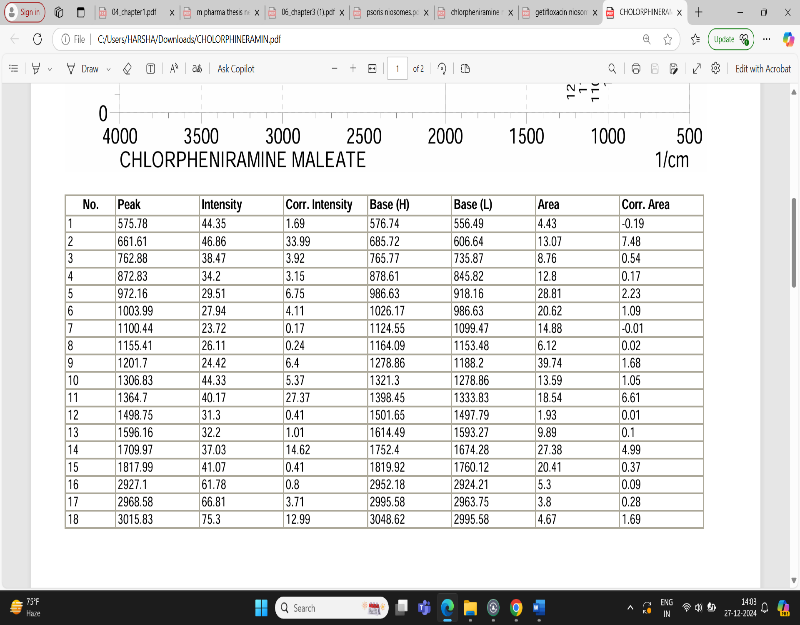
Fig: 4 FTIR Interpretation of data for Drug Chlorpheniramine
The result showed that a lot of number of peaks were detected, informing the complex structure material.
1.In single bond region (2500 – 4000cm-1)
a. No broad absorption found in the range of between 3650 and 3250 cm- indicating no hydrogen bonding.
b. Peak 3000-3200 cm- showing the presence of aromatic ring.
c. Peak at between 3000 cm-1 indicate presence of single bond of carbon.
2.Regarding the double bond region (1500-2000) several peaks were detected.
a. Between 1615 – 1495 cm-1 indicating the presence of aromatic ring.
3. Peak at 1596.10 shows the presence of aromatic ring C=C-C.
4. Peak at 3015.83 indicate aromatic C-H strech.
5. Peak at 872.83 indicating Aromatic phenyl ring having C-H 1,4 disubstitution.
6. Peak at 2927.1 shows methylene C-H absorbance strech, in fingerprinting region (600-1500cm-1).
7. Peak at 1201.7 shows presence of tertiary amines, C-N strech.
8. Strech at 872 shows presence of C-Cl group.
9. Strech at 1306.8 shows presence of aromatic secondary amine C-N strech.
Based on above analysis it showed that the material has aromatic ring, 1,4 di substituted phenyl ring, methylene group, tertiary amine group, chloro group and aromatic secondary amine. This is good acquirement that this chemical compound is chlorpheniramine.
FTIR of Drug + Excipients
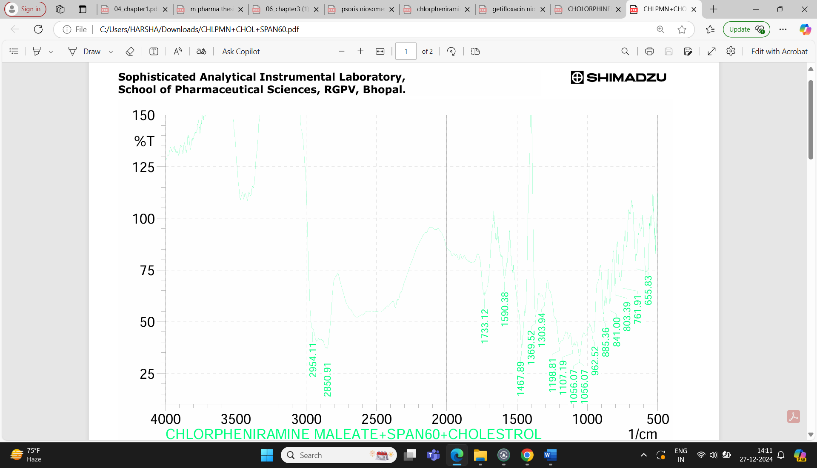
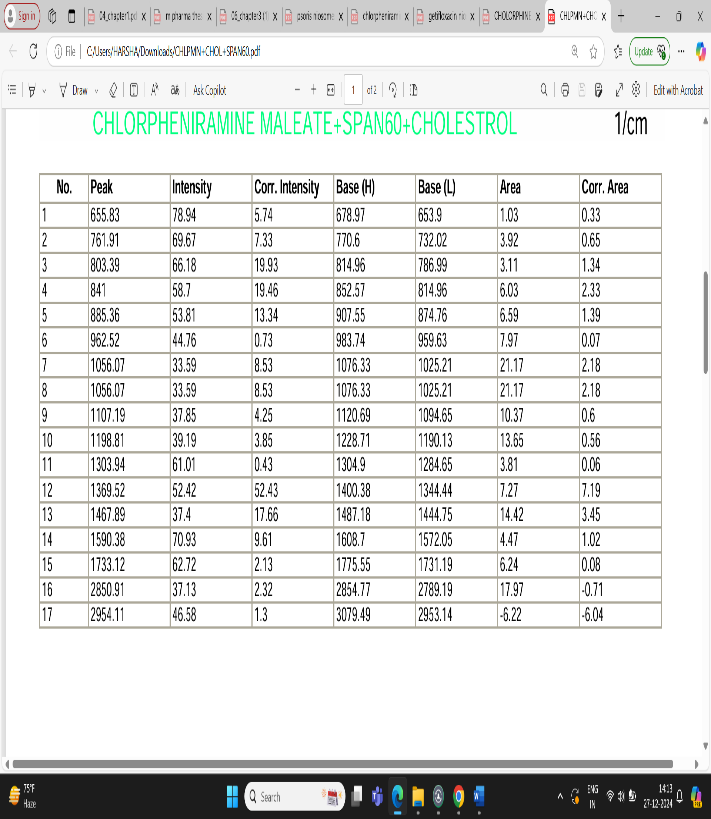
Fig: 5 FTIR Interpretation of data for Drug and Excipients (CPM + Span60 + Cholesterol)
The result showed that a lot of number of peaks were detected, informing the complex structure material.
1.In single bond region (2500 – 4000cm-1)
a. No broad absorption found in the range of between 3650 and 3250 cm- indicating no hydrogen bonding.
b. Peak at between 3000 cm-1 indicate presence of single bond of carbon.
2.Regarding the double bond region (1500-2000) several peaks were detected.
a. Range between 1750 – 1700 cm-1 describing the presence carbonyl (ketone) group (aryl ketone).
b. Between 1615 – 1495 cm-1 indicating the presence of aromatic ring.
3. Peak at 1596.10 shows the presence of aromatic ring C=C-C.
4. Peak at 885.36 shows aromatic phenyl ring having C-H 1,4 disubstitution.
5. Peak at 2954.11 shows methylene C-H absorbance strech, in fingerprinting region (600-1500cm-1).
6. Peak at 1198.81 shows presence of tertiary amines, C-N strech.
7. Peak at 885.36 shows presence of C-Cl group.
8. Peak at 1303.94 shows presence of aromatic secondary amine C-N strech.
Based on above analysis it showed that the material has aromatic ring, 1,4 disubstituted phenyl ring, methylene group, tertiary amine group, chloro group and aromatic secondary amine. This is good acquirement that this chemical compound is chlorpheniramine and it is compatible with span 60 and cholesterol.
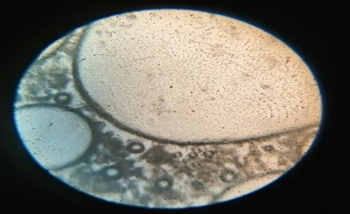
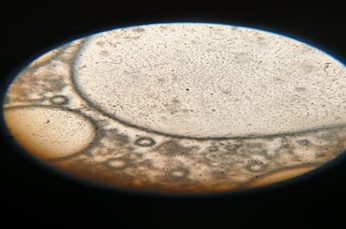
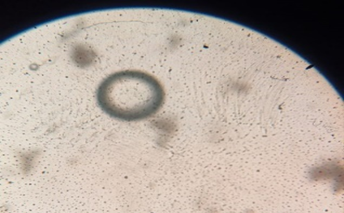
Fig: 6 Photomicrographs of Chlorpheniramine Niosomes
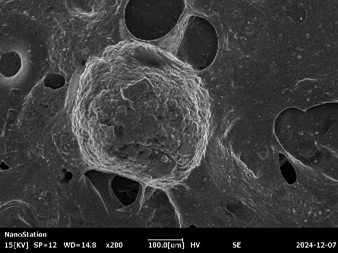
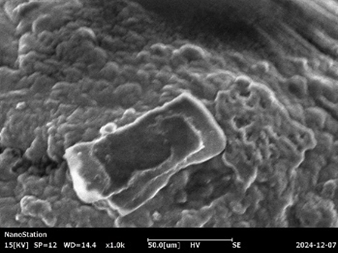
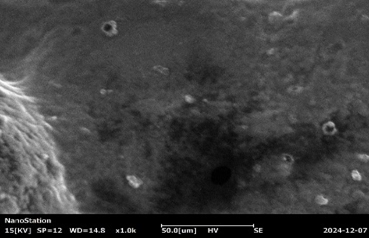
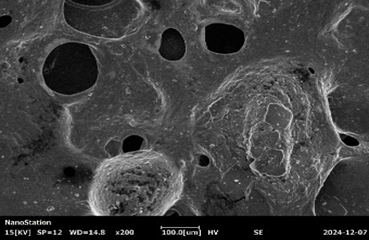
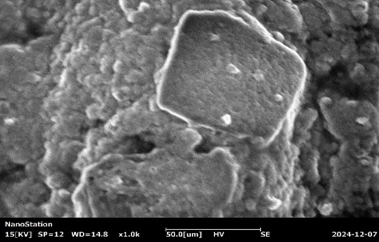
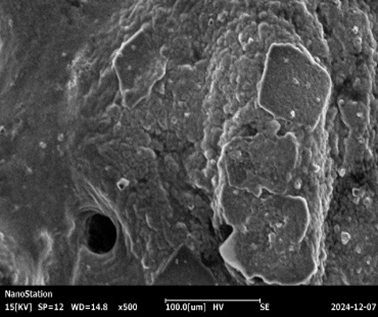
Fig :7 SEM Images of Chlorpheniramine Niosomal Gel
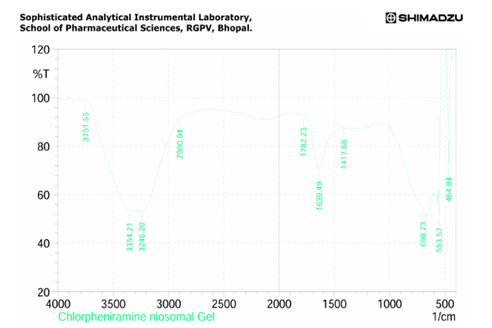
Fig: 8 FTIR Report of Chlorpheniramine Niosomal Gel
Table: 3 Drug Entrapment Efficiency
|
S. No.
|
Formulation Code
|
Entrapment Efficiency
|
|
1
|
F1
|
68.4
|
|
2
|
F2
|
72.1
|
|
3
|
F3
|
76.5
|
|
4
|
F4
|
78.9
|
|
5
|
F5
|
80.2
|
|
6
|
F6
|
81.5
|
|
7
|
F7
|
82.0
|
|
8
|
F8
|
82.5
|
|
9
|
F9
|
83.0
|
|
10
|
F10
|
82.8
|
Formulation F9, with 500mg of drug shows 83.0?, which is the highest among all formulations. However, Formulation F3 (with 200mg of drug) yeilds good entrapment efficiency of 76.5%, making it a strong formulation with improved efficiency. As the drug concentration increases, the entrapment efficiency generally improves, but excessive drug quantities (500mg) slightly reduced efficiency due to limitations in vesicle formulation and drug entapment. The best entrapment efficiency (EE%) was achieved with Formulation F9 (500mg drug), but Formulation F3 (200mg drug) showed an acceptable and reliable entrapment efficiency of 76.5%. This formulation may be ideal for further clinical development due to its balanced drug loading and entrapment efficiency.
Viscocity Of Chlorpheniramine Niosomal gel
A Brookfield digital viscometer, a device frequently used to assess the rheological characteristics of semi-solid formulations, was used to test the viscosity of the chlorpheniramine maleate niosomal gel. The viscosity of the gel is an important factor since it has a direct impact on patient compliance, stability, and spreadability. In order to guarantee accurate findings, the measurement was carried out at room temperature using the N4 spindle at an appropriate rotational speed of 12rpm and 100rpm. The spindle's resistance to flow, measured in centipoise (cP), can be accurately estimated from its interaction with the gel. This technique helps to optimize the gel's rheological characteristics for topical application and guarantees consistency in the formulation. In order to assess the gel's performance and make sure it is appropriate for the intended therapeutic usage, the determined viscosity values are crucial.


Fig: 9 Brookfield Viscometer
The observed viscosity of the gel was found to be 1880 cP at 100 rpm and 14800 cP at 12 rpm. This number indicates the gel's ideal consistency, which guarantees suitable spreadability and topical application simplicity. The spindle speed was chosen to guarantee precise gel-instrument contact and to provide trustworthy viscosity measurements, which are crucial for assessing the stability and performance of the formulation. Speadibility of Chlorpheniramine Niosomal Gel
The gel's ideal consistency and ease of application for topical use were demonstrated by its 5 cm spreadability. This test makes sure the formulation has enough spreadability, which is essential for consistent skin application and patient compliance. The technique aids in the evaluation of the gel's quality by offering a consistent and accurate measurement of its physical characteristics.
Spreadability Study of Chlorpheniramine Niosomal Gel
The spreadability of the gel was calculated using the following formula:
S = M.L/T
Where:
M = 2000 g (2 kg weight)
L = 5 cm (spread diameter)
T = 1800 s (30 minutes converted to seconds)
The spreadability of the chlorpheniramine maleate niosomal gel was calculated to be 5.56 g·cm/s, indicating adequate consistency and ease of application. This ensures the gel is suitable for uniform spreading during topical use.
pH of Chlorpheniramine Niosomal Gel
The chlorpheniramine niosomal gel's pH was found to be approximately 5.9, which is within acceptable range for topical application (4.5-6.5). This ensures that the gel is non-irritating to the skin and suitable for use. And the colour found was light yellow-green in colour. This colou indicates a slightly acidic pH which is close to the neutral range suitable for topical application.
Table: 4 Stability Study Result of Chlorpheniramine Niosomal Gel
|
Storage Condition
|
Appearance
|
pH
|
Viscosity
|
Spreadibility
|
|
Room Temperature (25°C ± 2°C)
|
No phase seperation, no discolouration,
Smooth texture
|
5.8
|
Slight increase
|
5.0
|
|
Refrigeration (4°C ± 2°C)
|
No phase seperation, no discolouration,
Smooth texture
|
5.5
|
Increased viscosity
|
4.8
|
|
Accelerated Conditions (40°C ± 2°C)
|
Slight phase seperation, slight discolouration.
|
5.5
|
Incresed viscosity
|
4.8
|
Stability Studies of Chlorpheniramine Niosomal Gel
At Room Temperature (25°C ± 2°C)
The gel showed no visible signs of phase separation, discolouration, or texture changes after a month. The viscosity very slightly increased and the pH stayed at 5.8 (original pH: 5.9), suggesting that the gel's consistency had not changed much. The spreadability was still 5 cm. At room temperature, the formulation demonstrated high stability.
At Refrigeration (4°C ± 2°C)
Additionally, there were no indications of phase separation or discoloration in the gel that was refrigerated. The viscosity was somewhat higher than in the original formulation, while the pH was constant at 5.9. The spreadability did not significantly change (5 cm). When kept at fridge temperature, the gel remained stable overall.
At Accelerated Conditions (40°C ± 2°C)
After a month, the gel kept under accelerated conditions displayed a small rise in viscosity and indications of phase separation. Spreadability dropped to 4.8 cm and the pH dropped to 5.5. This implies some instability at higher temperatures, which might have an impact on the formulation's long-term shelf life. Hence the stability study of chlorpheniramine niosomal gel under varying storage conditions over a period of one month showed that, under refrigeration (4°C) and at room temperature (25°C), the chlorpheniramine niosomal gel showed outstanding stability. The gel, however, exhibit some instability in accelerated conditions (40°C), suggesting that higher temperatures may have an impact on the formulation's long-term stability. According to the findings, the gel is most stable when kept either at normal room temperature or in a refrigerator, and exposure to high temperatures should be avoided.
Table: 5 Cumulative Percentage Drug Release of Formulations
|
Time (hrs)
|
F1
|
F2
|
F3
|
F4
|
F5
|
F6
|
F7
|
F8
|
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
1
|
24.18
|
29.05
|
31.22
|
30.15
|
40.35
|
34.12
|
30.09
|
31.01
|
|
2
|
33.89
|
39.54
|
44.21
|
41.25
|
54.75
|
50.07
|
39.75
|
43.21
|
|
3
|
39.85
|
43.89
|
50.13
|
46.32
|
59.65
|
54.72
|
43.52
|
49.25
|
|
4
|
51.23
|
56.98
|
63.21
|
62.14
|
72.21
|
67.85
|
54.32
|
64.10
|
|
5
|
56.41
|
58.12
|
70.04
|
68.01
|
78.35
|
73.62
|
59.42
|
70.11
|
|
6
|
65.72
|
69.23
|
81.15
|
75.22
|
86.01
|
84.14
|
71.12
|
79.42
|
|
7
|
67.85
|
73.15
|
83.25
|
79.41
|
90.15
|
87.05
|
76.51
|
82.35
|
|
8
|
71.14
|
77.89
|
85.62
|
81.23
|
93.25
|
90.03
|
80.25
|
84.89
|
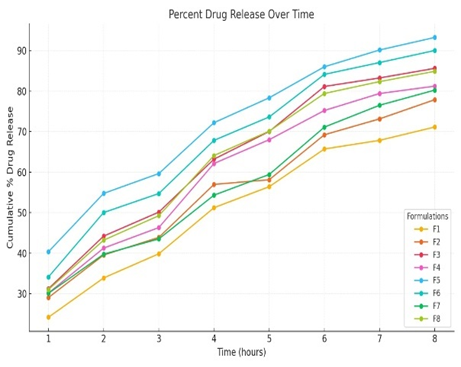
Fig: 10 In Vitro Drug Release Study
The graph illustrates the cumulative percentage of drug released of several formulations (F1 to F8) over time (in hours), as explained. The way the drug is released from the niosomal gel is depicted by each line, which is the drug release profile of a particular formulation.
The time (hours) X-axis shows how long the drug release was measured.
At every time point, the cumulative percentage of medication release is displayed on the Y-axis.
Observations:
1. The medication release from all formulations increases gradually over time.
2. All formulas exhibit the same release pattern, with some achieving greater release percentage sooner than others.
3. Formulations like F5 and F6 have a higher cumulative release and a faster pace than the others, approaching 90% and 93% at 8 hours, respectively.
4. The release rates for F1, F2, and F7 are slower and remain below 75?ter 8 hours.
Since F5 produced the maximum cumulative drug release (93.25%) in 8 hours, it is the best formulation according to the data. This implies that F5 might have a perfect composition (for example, the ratio of cholesterol to
surfactant) to improve the release efficiency.
With a release rate of 90.03%, F6 is the second-best formulation; it has similar advantageous qualities to F5, although it is little less effective.
The optimal formulation is chosen based on how well it releases the medication over an extended period of time. This balance can be achieved by F5, which makes it perfect for therapeutic use.
CONCLUSION
By using ether injection method chlorpheniramine niosomal gel was successfully prepared. Through FTIR reports, the Preformulation study of chlorpheniramine confirms the purity of drug and compatibility of drug with excipients. The best entrapment efficiency (EE%) was achieved with Formulation F9 (500mg drug), but Formulation F3 (200mg drug) showed an acceptable and reliable entrapment efficiency of 76.5%. This formulation may be ideal for further clinical development due to its balanced drug loading and entrapment efficiency. According to reports from stability study of gel is most stable when kept either at normal room temperature or in a refrigerator, and exposure to high temperatures should be avoided. Research has to be carried out extensively to have commercially available niosomal formulations. The finding of this investigation have conclusively demonstrated that encapsulation of chlorpheniramine into niosomal gel formulation improves skin retention on which significantly improved therapeutic response and considerably reduced adverse symptoms.
REFERENCES
- Gavhane, S. A. et al., “The Novel Drug Delivery System”, International Journal of Creative Research Thoughts (IJCRT), Volume 9, Issue 9 September 2021, 2320-2882.
- Jain Sameeksha et.al., “Novel Drug Delivery Systems: An Overview” Asian Journal of Dental and Health Sciences, 2022; Vol:2, Issue:1, 33-39.
- Kumari Mamta et.al., Formulation and Characterization of Novel Non-Ionic Surfactant Based Aceclofenac Gel as a Potential Drug Delivery System, Journal of Pharmaceutical Research International, Vol:33, Issue:36A, 176-187.
- Suknuntha K et.al., “Solubility and Physical Stability Enhancement of Loratadine by Preparation of Co-Amorphous Solid Dispersion with Chlorpheniramine and Polyvinylpyrrolidone”, Pharmaceutics. 2023, Vol:15, Issue:11, 2558.
- Amlan Bisha et.al., “Development and Evaluation of Chlorpheniramine Maleate Orally Disintegrating Tablets”, Journal of Chemical Health Risks, 2023, Vol:13, Issue:4, 468-481.
- Raad Anfal et.al., UV-Spectral Studies on Chlorpheniramine Maleate in Pure Form and Pharmaceutical Preparations, 2021, Egyptian Journal of Chemistry, Vol:64, Issue:8, 4151-4156.
- Anfal R. Mahmoud et.al., UV- Spectral Studies on Chlorpheniramine Maleate in Pure Form and Pharmaceutical Preparations, Egyptian Journal of Chemistry, Vol:64, Issue:8, 2021, 4151 – 4156.
- Urooj Afreen et.al., “Formulation and evaluation of niosomes-based chlorpheniramine gel for the treatment of mild to moderate skin allergy”, Journal of Experimental Nanoscience, 2022, Vol:17, Issue:1, 467-495.
- Hayder K. Abbas, et al. “Preparation and evaluation of proniosomal gel containing diphenhydramine HCl”, Drug Invention Today, 2020, Vol:13, Issue 2, 313-322.
- Shivhare U et.al., “Formulation, development and evaluation of diclofenac sodium gel using water soluble polyacrylamide polymer. Digest. J. of Nanomaterials and Biostructures, 2009, Vol:4, 285-290.
- Swami H, et al. Formulation and Evaluation of Liposomal Gel of Lornoxicam. WJPR. 2015, Vol:4, Issue:9, 2312–2338.
- Rukmani K and Sankar V, “Formulation and Optimization of Zidovudine Niosomes”. AAPS Pharm Sci Tech 2010; Vol:11, Issue:3, 1119–1127.
- Prem Kumar et.al., “Formulation and Evaluation of Econazole Niosomes”, Sch. Acad. J. Pharm., 2013, Vol:2, Issue:4, 315-318.
- Jain Vivek et.al., “Formulation and In-vitro Evaluation of Niosomal Gel of Gatifloxacin”, Journal of Medical Pharmaceutical & Allied Sciences, Vol:10, Issue:2, 1050, 2021, 2681-2687..


 Harsha Sahu *
Harsha Sahu *
 Dr. Kamlesh Dashora
Dr. Kamlesh Dashora
 Dr. Darshan Dubey
Dr. Darshan Dubey





















 10.5281/zenodo.14822854
10.5281/zenodo.14822854