Abstract
Transdermal drug delivery systems, or TDDSs, are adhesive patches applied to the skin with a precise dosage that are then absorbed into the bloodstream. It is important to take into account the entire morphological, biophysical, and physicochemical aspects of the skin when delivering medicinal substances through the human skin for systemic effects. A transdermal patch offers a regulated release and continuous drug administration, which is advantageous over other forms of pharmaceutical delivery. It also avoids pulsed entry into the systemic circulation, which frequently results in undesirable side effects. Transdermal patches are among the most significant new drug delivery systems; they are a painless method for. For a research scientist interested in TDDS, the TDDS review article offers a convenient source of information about TDDS and its evaluation method. Transdermal drug delivery systems (TDDS), sometimes referred to as "patches," are dosage forms intended to distribute a medication dosage that is therapeutically efficacious through a patient's skin. To administer therapeutic drugs for systemic effects via the human skin, it is necessary to take into account the skin's complete morphological, biophysical, and physicochemical features. Transdermal administration offers a significant advantage over oral and injectable methods due to its ability to prevent first pass metabolism and increase patient compliance, respectively. In addition to enabling continuous distribution of medications with brief biological half-lives, transdermal delivery also prevents pulsed entrance into systemic circulation, which frequently results in undesired side effects. The transdermal drug delivery systems and its evaluation process specifics are detailed in the TDDS review articles, which are a useful resource for research scientists working on TDDS.
Keywords
transdermal drug delivery systems, Transdermal Patch, Thermal ablation, microneedle, JET injector.
Introduction
A "drug delivery system" (DDS) is any of a number of sophisticated physicochemical techniques intended to optimize the therapeutic potential of pharmacologically active substances by controlling their release and distribution inside cells, tissues, and organs. DDS focuses on the formulation and administration techniques that effectively transport drugs in order to maximize treatment effectiveness and reduce side effects. There are numerous methods of administration, each with a distinct delivery mechanism, including mucosal, intravenous, transdermal, lung inhalation, and oral. The transdermal drug delivery system (TDDS) is one of these that has drawn a lot of interest as a very promising tactic. Contrary to traditional direct administration techniques, which usually entail injections using needles, transdermal drug delivery via skin (TDDS) has become a well-researched non-invasive alternative. Significant effects of TDDS have been seen in the distribution of many medicinal drugs, including those used to treat pain management, hormone therapy, and illnesses of the central nervous system and heart. By avoiding the digestive tract, TDDS guarantees that first-pass metabolism does not result in loss and that medications can be delivered without interference from intestinal flora, pH, or enzymes. The ability of TDDS to regulate drug release in accordance with usage restrictions and its non-invasive design are other factors contributing to its appeal. Scopolamine was the medication used in the first transdermal drug delivery (TDD) system, Transderm-Scop, which was created in 1980 to treat motion sickness. The transdermal device is a system that is membrane-moderated. In this technology, a microporous polypropylene sheet serves as the membrane. The medication is dissolved in a blend of mineral oil and polyisobutylene to form the drug reservoir. This research release is sustained for a duration of three days. The innovative drug delivery method for currently available pharmacological compounds has attracted increasing attention recently. In addition to increasing a medicine's safety and effectiveness, the innovative drug delivery method also boosts patient compliance. Transdermal drug delivery systems (TDDS) are discrete dose forms that are self-contained. Another benefit of the TDDS is that the dosage is lower than with the oral medication delivery method.
Advantages of transdermal drug delivery system: -
- One can lower the dose frequency.
- Because of increased bioavailability, drug concentration can be decreased.
- It is possible to avoid the liver's first pass metabolism.
- They can avoid problems with gastrointestinal medicine absorption brought on by enzymatic activity, stomach pH, and drug interactions with food, beverages, and other oral medications.
- Lower drug side effects and decreased plasma concentration levels of the medications.
- Because they are non-invasive, they eliminate the inconvenience of parenteral therapy.
- Because they provided prolonged therapy with a single application, they increased compliance in comparison to earlier dosage forms that necessitated more frequent dose administration.
- Drug therapy can be quickly stopped by removing the application from the skin's surface.
- With these systems, self-administration is feasible.
- It lessens drug interactions across the board.
- It provides a longer action duration.
Disadvantages of transdermal drug delivery system: -
- The medicine needs to have certain desirable physicochemical qualities in order to penetrate the stratum cornea. Transdermal delivery will be extremely challenging if the drug dose needed for therapeutic value exceeds 10 mg/day.
- Due to the skin's natural barriers to drug entrance, only somewhat powerful medications are appropriate candidates for TDDS.
- Some patients require stopping treatment because they get contact dermatitis at the application site for one or more system components.
- Another aspect that must be thoroughly considered before deciding to manufacture a transdermal medicine is clinical need.
- The skin's barrier function varies with age, from person to person, and from one place to another on the same individual.
Limitation of transdermal drug delivery system: -
- Low permeability of the skin
- Limited to strong medications
- Unsuitable for molecules larger than 500 Daltons
- The patch's adhesion to the skin 5. The drug's potential for skin degradation
- Because highly melting drugs are little soluble in fat and water, they cannot be administered via this method.
- If they irritate the skin, they are completely inappropriate.
- Ionic medications cannot be delivered by this technology.
An ideal drug candidate for transdermal drug delivery should require the following characteristics.
1. The molecular weight ought to be under 500 Da.
2. A log partition coefficient that is ideally between one and three
3. Strong chemical; fewer than ten milligrams.
4. The solubility in water should be more than 100 ug/ml.
Types of TDDS:
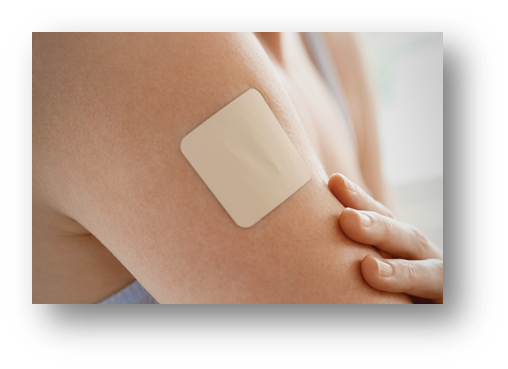
Transdermal Patch:
The application of a medicated adhesive patch to the skin allows for the controlled delivery of a prescribed dosage of medication via the skin and into the bloodstream, resulting in a therapeutic effect.
Advantages of Transdermal drug delivery System
- Reducing the frequency of doses.
- Increased bioavailability can lead to a decrease in medication concentration.
- The liver's first pass metabolism can be circumvented.
- They can avoid problems with gastrointestinal medicine absorption brought on by enzyme activity, stomach pH, and drug interactions with food, beverages, and other oral medications.
- Lower drug side effects and decreased plasma concentration levels of the medications.
- Because they are non-invasive, they spare users from the inconvenience of parenteral therapy.
- Because they provided prolonged therapy with a single application, they increased compliance in comparison to earlier dosage forms that necessitated more frequent dose administration.
- These methods allow for self-administration;
- Drug therapy can be stopped quickly by removing the application from the skin's surface.
- It lessens drug interactions across the board.
- It provides a longer action duration.
Disadvantage of Transdermal Drug Delivery System:
- Transdermal administration is appropriate only for strong medications.
- This technique is not cost-effective; some patients may experience skin irritation at the application location.
- Dosage dumping may occur if the medication binds to the skin.
- Only chronic illnesses, such as diabetes, hypertension, angina, and so on, should be treated with it; acute conditions should not be treated with it.
- Cutaneous metabolism may have an impact on the medication's therapeutic effectiveness.
- Transdermal treatment is not appropriate for ionic medications.
- Fit for medications with smaller molecular weights, meaning fewer than 500 Daltons
Anatomy and physiology of Skin:
With a total size of almost 20 square feet, the skin is the biggest organ in the body. In addition to helping to regulate body temperature and shielding us from germs and the environment, skin also allows us to feel touch, heat, and cold.
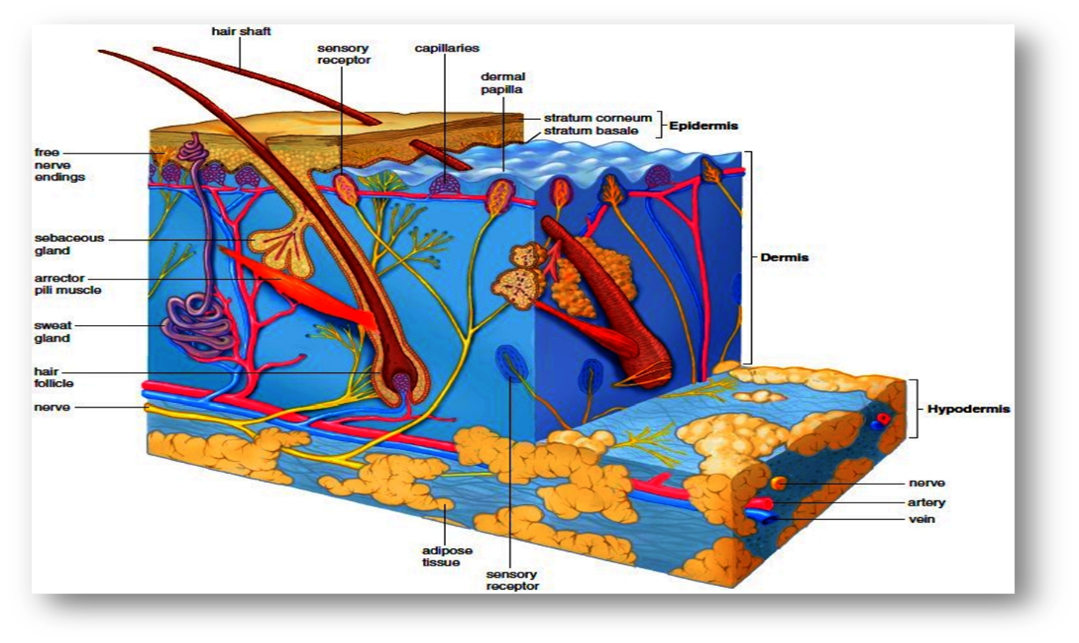
Fig-Anatomy and physiology of Skin
The skin is composed of three primary layers:
Epidermis: Keratinocytes, which create the protein keratin, make up the majority of the epidermis, the skin's outermost layer. This layer establishes our skin tone and acts as a waterproof barrier. The stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum, and stratum basale are among the sub-layers that make up the epidermis.
Dermis: Underneath the epidermis, the dermis is the middle layer of skin. It is made up of nerve endings, sweat glands, hair follicles, blood vessels, and connective tissue. The skin's dermis gives it flexibility, nutrients, and structural stability. The dermis is composed of two sub-layers: the reticular dermis and the papillary dermis..
Adipose tissue, or fat, and connective tissue make up the hypodermis, or subcutaneous layer, which is the innermost layer. This layer gives the body comfort, insulation, and energy storage. Additionally, it fixes the skin to the underlying bones and muscles. The skin also contains a variety of specialized cells and structures in addition to these major layers, including melanocytes (which are in charge of skin color), Langerhans cells (which control the immune system), and Merkel cells (which control feeling). The skin's intricate structure allows it to protect the body in an efficient manner while still performing vital tasks.
Types of Transdermal Patches
Single layer drug in adhesive:
This kind has the medication embedded in the sticky layer. The medicine is released into the skin via the adhesive layer, which also acts as a glue to hold the other layers together. There is a backing and a temporary liner surrounding the adhesive layer.

Multi-layer drug in adhesive:
This kind is comparable to a single-layer patch, but in addition to the adhesive layer, it also has two layers: one for instant medication release and another for regulated release. The medication is released because of the sticky layer. Both a permanent backing and a temporary liner are included with this patch.

Figure 2 Multilayer patch
Drug Reservoir-in-Adhesive:
It is distinguished by the presence of a liquid compartment with a medication solution or suspension that is kept apart from the release liner by an adhesive and semi-permeable membrane. The adhesive component of the product that is in charge of skin adhesion can be included in either a concentric design surrounding the membrane or a continuous layer between the membrane and the release liner.
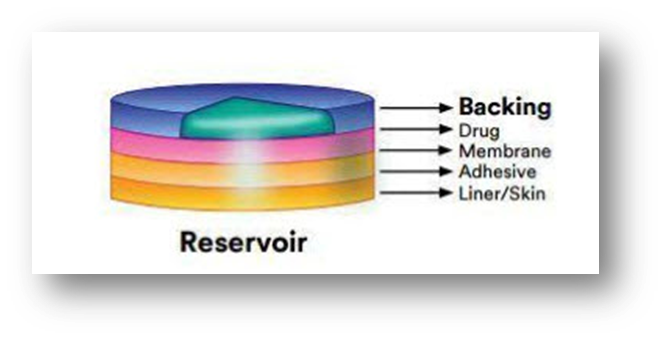
Drug Matrix-in-Adhesive:
It is distinguished by the presence of a semisolid matrix that is in direct contact with the release liner and contains a medication solution or suspension. The skin-adhering component is integrated into an overlay and surrounds the semisolid matrix in a circular pattern.

Vapour Patch:
This kind of patch uses an adhesive layer that releases vapour in addition to holding the different layers together. The newest products on the market are vapor patches, which release essential oils for as long as six hours. Vapour patches, which release essential oils for up to 6 hours, are just now beginning to appear on the market. The vapor patches release essential oils and are mostly used to treat cases of congestion. As an alternative, controller vapour patches are available that improve the quality of sleep. Additionally, there are vapour patches available that help reduce the number of cigarettes a person smokes each month. Month can also be found in the market.
Different Transdermal Drug Delivery Technologies:
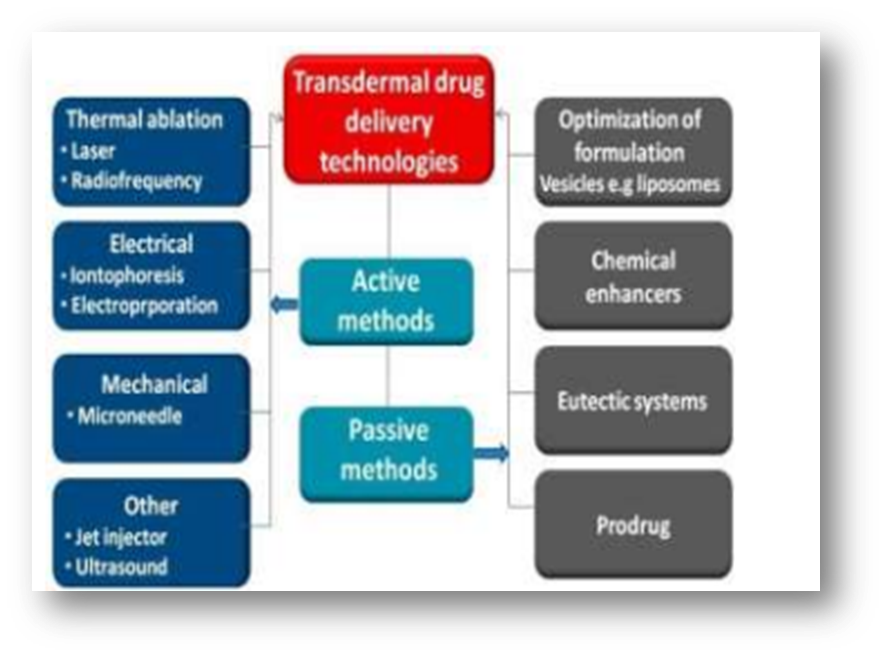
Fig- Transdermal Drug Delivery Technologies
Thermal ablation:
By using targeted heat to deliberately disturb the stratum corneum structure, thermal ablation, sometimes referred to as thermophoresis, is a promising technique that improves medication administration by creating microchannels in the skin. In order to thermally ablate the stratum corneum, a temperature well above 100 °C is necessary, which causes keratin to heat up and vaporize. Furthermore, the degree of the stratum corneum structure alteration is directly related to the locally elevated temperature, suggesting that this technique is perfect for accurate drug delivery management. Depending on the various sources of thermal energy, laser and radiofrequency techniques can typically generate thermal ablation.
Thermal ablation techniques use a laser to create the skin's micropore structure and raise the skin's temperature, which raises the diffusivity of the skin. Water excitation and explosive evaporation from the epidermis result from laser light energy being absorbed by skin pigments and water, which then turns into heat energy. Many parameters, including wavelength, pulse length, energy, number and repetition rate, tissue thickness, absorption coefficient, and laser exposure duration, can be carefully adjusted to determine the degree of ablated skin depth.
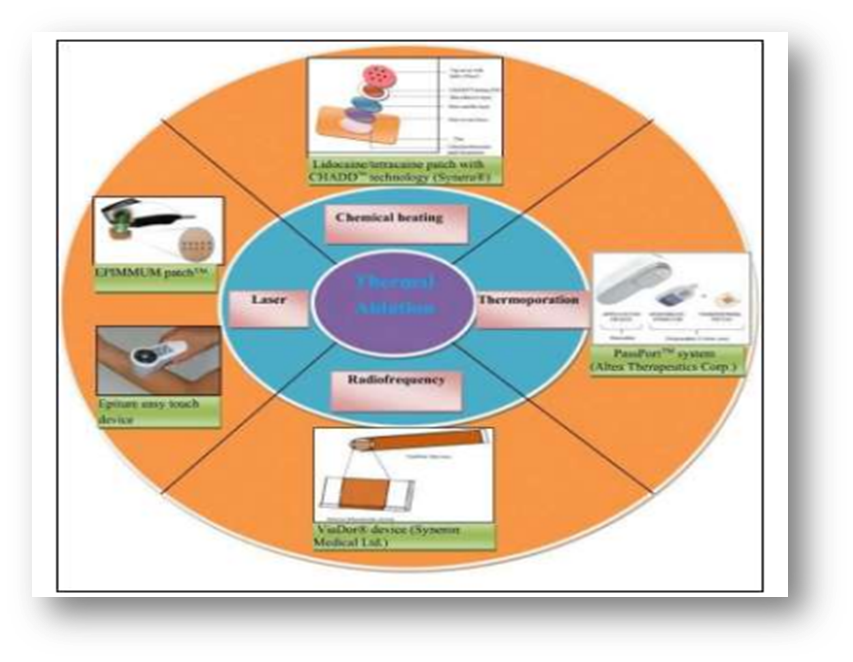
Fig- Thermal ablation
Microneedle:
A needle is used in the microneedle drug delivery system, a revolutionary drug delivery method, to administer medication to the circulatory system. This is a popular approach of transdermal drug delivery and a topic of ongoing research at the moment. This approach includes puncturing the skin's superficial layer with needles the size of microns, which diffuses the medicine throughout the epidermal layer. These short, thin microneedles carry medications directly to the blood capillary area for active absorption, reducing the risk of pain.
Fig- microneedle drug delivery
Researchers have endeavored to employ several methodologies to achieve the suitable optimization and geometric measurements necessary for the successful insertion of microneedles into human skin, which also signifies the overarching goal of microneedle research. Usually arranged on a single side of a supporting base or patch, MN are numerous tiny projections with dimensions typically between 25 and 2000 ?m in height, 50 to 250 ?m in base width, and 1 to 25 ?m in tip diameter. When implanted into layers of skin, the needles should have the proper length, breadth, and form to prevent contact with nerves. Typically, they are arranged in arrays to enhance the skin's surface contact and promote the skin's absorption of medicinal chemicals.
JET injector
The liquid is propelled by liquid-jet injectors through nozzles with orifice diameters varying from 50 to 360 ?m. This is significantly less than the usual hypodermic needle's outer diameter of 810 ?m for a 21G needle.
By adjusting the jet velocity and orifice diameter, the jet can transport a medicine into the intradermal (i.d.), subcutaneous (s.c.), or intramuscular (i.m.) layers of skin.The primary benefit of utilizing needle-free gadgets pertains to worries about secure needle disposal and preventing unintentional injuries from needle sticks. Cross-contamination is still possible, though, as the nozzle could get contaminated by interstitial liquid that splashes back from the skin. Consequently, the usage of multi-use nozzle jet injectors has been discontinued, and certain devices—like the Tjet® device, which distributes somatropin (human growth hormone (hGH))—are now limited to administering multiple doses of medication to the same patient.

Fig -T-jet
Compressed gas is used as the power source in solid jet injectors, which also have a drug-loaded chamber with a solid drug formulation and a nozzle to direct the particle flow towards the skin. Compressed gas expands when the actuation mechanism is activated, pushing medication powder via a nozzle and into the skin. Particles strike the skin, producing microscopic holes that settle in the viable epidermis or stratum corneum. Particle qualities (size, density, and impact velocity) are the most crucial factors that control particle distribution through the stratum corneum. For example, the particle size range for DNA vaccine should be between 0.5 and 3 µ.
Future prospective
Transdermal devices are expected to have a $2 billion US market. With a robust yearly growth rate of 25%, transdermal drug delivery has surpassed both the inhalation market and oral drug delivery systems, which both increase at a rate of 2%. As new devices are developed and the number of transdermal medications that are marketed grows, this number is anticipated to rise in the future. Comparing the gadgets in development to traditional transdermal patch therapy, they are more expensive and intricate. Data supporting the device's safety while worn on the skin for either short- or long-term use will also be needed for regulatory organizations. Therefore, in order for any of these innovative drug delivery systems to be successful and rival those that are now available on the market, they must exhibit cost-effectiveness, mobility, safety, and efficacy.
wide range of use. Addressing these issues will be essential to the creation and implementation of creative, patient-centered solutions that could transform medication administration and enhance patient outcomes as the area of transdermal drug delivery develops. Transdermal drug delivery systems' (TDDS) ongoing progress depends on the creation of novel technologies that can get over the drawbacks of current systems, like subpar medication
permeability and restricted suitability for a few class of medicinal compounds. New prospects for TDDS are emerging as research and development activities in this field advance, especially in the context of biologics, personalized medicine, and the delivery of complicated medications. The incorporation of cutting-edge materials, such nanotechnology and smart polymers, to improve medication delivery effectiveness and patient compliance, is one possible area of expansion for TDDS. For instance, more exact and regulated medication release patterns can be achieved with responsive polymers, which react to variations in environmental conditions like pH, temperature, or light. It is also possible to customize nanotechnology-based carriers, like liposomes and polymeric nanoparticles, to enhance medication encapsulation, stability, and permeability through the skin barrier. The integration of biosensors and digital health technologies into drug delivery platforms is a viable avenue for the advancement of TDDS. By offering real-time monitoring of physiological indicators, illness biomarkers, or drug levels, these devices can improve therapeutic outcomes and allow for customized dosage schedules. Additionally, the combination of TDDS and digital health tools can improve patient adherence and engagement, two important aspects of treatment plan efficacy. The need for TDDS that can efficiently deliver these treatments over the skin barrier is increasing along with the demand for large-molecule medicines and biologics.
Innovative approaches like sonophoresis, electroporation, and microneedle arrays have demonstrated efficacy in increasing macromolecule delivery and bioavailability, thus they have a lot of promise to address this difficulty. Lastly, TDDS's future also depends on how widely it is applied outside of established therapeutic domains. In the context of microbiome-based therapeutics, such as topical probiotics or prebiotics, new potential for transdermal drug administration may present themselves as research into the skin microbiota progresses. Moreover, focused, effective, and non-invasive delivery methods are needed for gene therapies and CRISPR-based treatments, both of which TDDS may help deliver.
CONCLUSION:
Because TDDS technology circumvents firstpass metabolism and other sensitivities associated with alternative drug administration routes, it has become the preferred mode of drug administration for transdermal delivery across a range of skin types due to its widespread acceptance as a mainstream delivery method. The controlled-release technique of TDDS makes it possible to provide medication consistently in a non-invasive and allergenic way. A number of industry and academic research institutes' research papers, patents, and commercially accessible products attest to the significant growth of TDDS in the local and global drug delivery system market. Improvements in vaccination and patient preferences for self-administering long-term treatment drugs could be spurred by the development of TDDS. It might also serve as a catalyst for lowering the prevalence.
REFERENCE
- Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008 Nov;26(11):1261-8
- Ita K. Transdermal drug delivery: progress and challenges. J Drug DelivSci Technol. 2014 Aug;24(4):245- 50.
- Chowdary K.P.R and Naidu R.A.S, Transdermal Drug Delivery, A Review of Current Status, Indian Drugs, 1995, 32(9), 414- 422.
- Alkilani AZ, McCrudden MTC and Donnelly RF: Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the stratum corneum. Pharmaceutics 2015; 7: 438-70.
- Department of Pharmaceutics, Centre for Pharmaceutical Sciences, JNTUH UCEST, JNTUH, Hyderabad, Telangana-500085, India. GSC Biological and Pharmaceutical Sciences, 2023, 22(02), 245–255 Publication history: Received on 01 January 2023; revised on 15 February 2023; accepted on 17 February 2023 Article DOI:.
- Abdul Hafeez* Doon College of Pharmacy, Sunderpur, Saharanpur, U.P., India247001 Dr. Upendra Jain Chandigarh College of Pharmacy, Mohali, Punjab, India- 140110 Jagpal Singh Doon College of Pharmacy, Sunderpur, Saharanpur, U.P., India247001 ArunMaurya, LakhanRanaDoon College of Pharmacy, Sunderpur, Saharanpur, U.P., India247001.
- Correspondence to Author: Dr. Avinash R. Tekade Professor & Head, Department of Pharmaceutics, MarathwadaMitraMandal’s College of Pharmacy, Thergaon, Pune - 411033, Maharashtra, India
- https://www.crxmag.com/issues/2020/summer/beyond-the-smoke.shtml
- Dragicevic N, Maibach HI, editors. Percutaneous penetration enhancers: chemical methods in penetration enhancement. Berlin: Springer; 2015.
- Mitragotri S, Kost J. Low-frequency sonophoresis: a review. Adv Drug Deliv Rev. 2004 Feb;56(5):589- 601.
- https://www.google.com/search?q=structure+of+anatomy+of+skin&sca_esv=579562946&tbm=isch&source=lnms&sa=X&ved=2ahUKEwj0-oLaoKyCAxVWTmwGHYECCXIQ_AUoAXoECAIQAw&biw=1517&bih=674&dpr=0.9#imgrc=WiTR4itXlm43_M
- Jain.N.K, Controlled and novel drug delivery, first edition, CBS publishers and distributors, New Delhi.1997.
- Mathiowitz.Z.E, Chickering, Lehr.C.M, Bio adhesive drug delivery systems; fundamentals, novel approaches and development, Marcel Dekker, Inc. New York. Basel
- Sharma N, Parashar B, Sharma S, Mahajan U. Blooming Pharma Industry with Transdermal Drug Delivery System. Indo Global J Pharm. Sci. 2012; 2(3): 262-278.
- Expert Opin Drug Deliv. 2014;11(4):599–614. https://doi. org/10.1517/17425247.2014.885501
- Escobar-Chávez JJ, Díaz-Torres R, Domínguez-Delgado CL, Rodríguez-Cruz IM, LópezArellano R, Hipólito EAM. Therapeutic applications of sonophoresis and sonophoretic devices. In: Percutaneous Penetration Enhancers Physical Methods in Penetration Enhancement. SpringerVerlag Berlin Heidelberg; 2017. p. 31–58.
- Leppert W, Malec–Milewska M, Zajaczkowska R, Wordliczek J. Transdermal and Topical Drug Administration in the Treatment of Pain. Molecules. 2018; 23(3):681
- Ruby PK, Pathak SM, Aggarwal D. Critical attributes of transdermal drug delivery system (TDDS) – a generic product development review. Dru
- 0. Bala I, Hariharan S, Kumar MN. PLGA nanoparticles in drug delivery: the state of the art. Crit Rev Ther Drug Carrier Syst. 2004;21(5):387-422


 Sayali shankhapal*
Sayali shankhapal*
 Vaishnavi pandav
Vaishnavi pandav










 10.5281/zenodo.13354827
10.5281/zenodo.13354827