Abstract
The oral mucosa is an intriguing site for drug delivery, offering promising opportunities for achieving high drug bioavailability, both locally and systemically. However, the formulation of oral dosage forms must consider the relative permeability of the oral mucosa and the potential influence of oral fluids and mechanical stresses. Sustained drug release necessitates prolonged contact between dosage forms and the oral site of absorption/application, underscoring the importance of developing mucoadhesive dosage forms. Mucoadhesion is a multifaceted process involving interactions between Mucoadhesive polymers and the mucous substrate. In addition to factors related to the oral mucosa and oral environment, the physical and chemical properties of mucoadhesive polymers significantly impact mucoadhesive bonds. While modifying mucosal features may be challenging, understanding the properties of polymers influencing mucoadhesion allows for the development of more effective mucoadhesive systems. This review aims to elucidate the various mechanisms and factors underlying Mucoadhesion, with particular emphasis on the features of the oral environment, oral mucosa, and polymeric compounds influencing the mucoadhesion process. Finally, the review briefly discusses the primary mucoadhesive dosage forms designed for oral transmucosal drug delivery.
Keywords
Transmucosal drug delivery, Anatomy and Physiology of the mouth cavity, Mucoadhesion, dosage form, drug controlled-release, mucoadhesive polymers, oral mucosa, mucosal permeability
Introduction
that develops on the oral cavity's mucous membrane is known as a mouth ulcer. "A break within the mucosal surface of the oral cavity" is its definition. An ulcer is an open skin or mucous membrane sore that is defined by the removal of dead, inflamed tissue. Although they can occur at almost any location, ulcers are most frequently found on the skin of the lower extremities and inside the digestive system. There are numerous forms of ulcer such mouth ulcer, esophagus ulcer, ulcer, and genital ulcer They are prevalent and can be related to local reasons such stress from dentures or fractured tooth or a limit smaller number of systemic disorders can present as ulcerations within the oral cavity. These are uncomfortable oval or circular sores that develop inside the mouth, usually on the inside of the lips or cheeks. Due to their prevalence and the variety of diseases they can accompany, as well as their various processes, they typically lack a significant underlying cause. One of the most frequent causes of recurring mouth ulcers is oral trauma. The mucosa becomes irritated mechanically, chemically, or thermally as a result. Usually, these are abrupt, severe episodes that result in painful ulcers that go away on their own in a few weeks. If the triggering stimuli is not eliminated, the ulcers may potentially become recurring. A mouth ulcer hurts when you eat, drink, and clean your teeth. Mouth ulcers are frequent and typically result from trauma, such as shattered teeth, poorly fitted dentures, or fillings. However, in order to rule out cancer (see previous article) or other dangerous illnesses such persistent infections, people with ulcers lasting more than three weeks should be referred for a biopsy or further examinations. After the cause is removed and benzydamine hydrochloride 0.15% mouthwash or spray (Difflam) is used to relieve symptoms, trauma-related ulcers often fade away in approximately a week. Chlorhexidine 0.2% aqueous mouthwash is also recommended to maintain good dental hygiene.Mouth ulcers are frequent and typically result from trauma, such as shattered teeth, poorly fitted dentures, or fillings. Nonetheless, individuals who have had an ulcer for longer than three weeks ought to be submitted for a biopsy. Further tests to rule out cancer (see to the preceding article) or other dangerous illnesses such persistent infections. After the cause is removed and benzydamine hydrochloride 0.15% mouthwash or spray (Difflam) is used to relieve symptoms, trauma-related ulcers often fade away in approximately a week. Chlorhexidine 0.2% aqueous mouthwash is also recommended to maintain good dental hygiene. (1)
Definition and Significance transmucosal delivery
Transmucosal delivery of therapeutic agents is a popular method because mucous membranes are relatively permeable, allowing for rapid uptake of a drug into the systemic circulation and avoiding the first pass metabolism. The mucoadhesion of the device is an important factor in the development of these drug delivery systems. Materials that attach themselves to the mucin layer of a biological membrane are frequently referred to as "mucoadhesive." Mucoadhesive polymers have been used in a variety of dosage formulations to try and accomplish systemic medication administration across the various mucosae. Tablets, patches, cassettes, films, semisolids, and powders are some of these dose forms. The polymers should have certain general physiochemical characteristics in order to function as mucoadhesive polymers. These characteristics include being primarily anionic hydrophilic with lots of hydrogen bond-forming groups, having a surface property that is appropriate for wetting mucus/mucosal tissue surfaces, and having enough flexibility to get into tissue crevices or the mucus network. (2)
NEED OF TRANSMUCOSAL DRUG DELIVERY
Transmucosal drug delivery is required for a number of reasons, including controlled release, targeted and localized drug delivery, bypassing first pass metabolism, preventing drug degradation, prolonging the effect, achieving high drug flux through the absorbing tissue, and minimizing fluctuations in the steady state plasma level. (3)
Advantage of TRANSMUCOSAL DRUG DELIVERY
The main advantages of Transmucosal Drug Delivery include: - (3)
- Localization of Drug Action: Targeting specific areas within the body for optimized therapeutic outcomes.
- Prolonged Residence Time: Enhanced duration of drug effectiveness or absorption at the intended site.
- Ease of Administration: Simplified methods for delivering medication, making it more accessible and feasible for patients.
- Convenient Termination of Therapy: Facilitating the conclusion of treatment when desired, except when administered through the gastrointestinal route, which might complicate termination due to continued absorption.
LIMITATIONS OF TRANSMUCOSAL DRUG DELIVERY.
This method cannot be used to give medications that irritate the oral mucosa, taste bitter or unpleasant, or have a disagreeable odor. This method cannot be used to give medications that are unstable at buccal pH. Only medications that require modest doses can be given. Saliva can be swallowed with drugs, negating the benefits of the buccal route. By this technique, only medications that are absorbed through passive diffusion can be delivered. Drinking and eating may become restricted, although the patient may still be able to swallow the formulation. Over-hydration can cause a slick surface to form, and the swelling and hydration of the bioadhesive polymers can compromise the formulation's structural integrity. (4)
ORAL ANATOMY AND Physiology:
Anatomy of the mouth ulcer:-
Mouth ulcers are frequent and typically result from trauma, including fillings, broken teeth, or ill-fitting dentures. However, in order to rule out cancer (see previous article) or other dangerous illnesses such persistent infections, people with ulcers lasting more than three weeks should be further referred for a biopsy or examinations. Recurrent canker sores, or aphthous stomatitis recurrent tiny, round, or ovoid ulcers with constricted edges, erythematous haloes, and yellow or grey floors are the typical early signs and symptoms of recurrent aphthous stomatitis in children or adolescents. It typically goes through an eventual remission and affects at least 20% of the population. Three primary types of clinical cases exist:
- Minor aphthous ulcers (80% of all aphthae) are less than 5 mm in diameter and heal in 7-14 days x
- Major aphthous ulcers are large ulcers that heal slowly over weeks or months with scarring x
- Herpetiform ulcers are multiple pinpoint ulcers that heal within about a month. (2)

Fig.1. Oral Ulceration
The oral mucosa is recognized as a distinctive route for delivering drugs due to its function as the primary barrier separating the oral cavity from underlying tissues. Its surface is lined with a layer of tightly adhering stratified squamous epithelium, predominantly made up of keratinocytes and a small number of nonkeratinocytes. Histologically, this epithelium can be classified as either keratinized or non-keratinized based on its characteristics, location, and role. In keratinized epithelium, keratinocytes are arranged into four layers: stratum basale, stratum spinosum, stratum granulosum, and stratum corneum. Examples of keratinized epithelium include the gingiva, hard palate, and dorsal surface of the tongue. In contrast, non-keratinized epithelium consists of stratum basale, stratum spinosum, stratum intermedium, and a superficial layer, covering areas like the buccal mucosa, lips, ventral surface of the tongue, and sublingual mucosa. (5)
Permeability:-
The oral mucosa's epithelium, consisting of around 40–50 layers of cells, exhibits varying thicknesses across different sites. Thickness ranges from 100 to 200 ?m in areas like the hard and soft palate mucosa, ventral tongue, and gingiva, while the buccal mucosa is thicker, measuring 500–600 ?m.
Tight junctions primarily facilitate cell interaction. The oral epithelium displays a unique trait with its proliferative stratum basale, housing progenitor cells such as stem cells and transiently amplifying cells. These cells, found in the basal layer and one to two layers above it, collectively form the stratum germinativum. Stem cells, albeit in limited quantities, either persist or transition into transiently amplifying cells upon division. Subsequently, these cells mature through multiple divisions and migrate toward the epithelial surface. (5) (6)

Fig.2 The anatomy and histology structure of oral cavity
Keratinized epithelium:
In keratinized epithelium, cells undergo differentiation and lipid accumulation as they enlarge during migration. Within the stratum spinosum, small organelles called membrane-coating granules or lamellar granules, which contain specific lipids, can be observed. Transitioning from the junction between the stratum granulosum and the stratum corneum, these membrane-coated granules migrate toward the upper region of the keratin-forming cell. Subsequently, organelle membranes start to fuse with the cytoplasmic membrane, resulting in the release of lipid sheets into the extracellular space through a process known as cytosolic vomiting. Upon enzymatic treatment, these lipid sheets undergo transformation into a blend of nonpolar lipids, including ceramides, cholesterol, and fatty acids. This blend occupies about half of the intercellular space between stratum corneum cells, forming broad lipid sheets, while the remaining half is filled with desmosomes. (5)
Non-keratinized epithelium
In non-keratinized epithelium, lipids predominantly consist of polar molecules such as cholesterol esters, cholesterol, and glycosphingolipids. Moreover, the presence of membrane-coated granules is less prominent in non-keratinized epithelium compared to the stratum spinosum of keratinized epithelium. A minority of granules contain small lipid sheets, which, upon extrusion into the extracellular space, are more inclined to adopt an amorphous lipid structure rather than forming distinct sheets. Research indicates the existence of electron-lucent material in non-lamellar phase lipids within non-keratinized epithelium, characterized by sporadic short layers of lipids. This unique and irregular lipid structure may contribute to the relatively higher permeability of non-keratinized epithelium in comparison to keratinized mucosa. Permeability can be influenced by both the thickness of the epithelium and its histological structures. Further details regarding the permeability of various regions are provided in the accompanying context. (5)

TABLE 1 Oral structure features
Mucoadhesion, dosage form:-
Mucoadhesion, a specialized form of adhesion, is derived from the Latin term "adhaerere," meaning to stick to. In general, adhesion refers to the joining of two material surfaces using an adhesive substance, forming a joint resistant to separation. This joint's integrity relies on both adhesion and cohesion forces. Adhesion occurs as the adhesive molecules establish close contact with the substrate surface, a process often described as wetting. Conversely, cohesion arises from interactions among the adhesive molecules themselves, representing the energy required to rupture the adhesive and create two distinct surfaces. Adhesives are typically applied in liquid form to promote wetting before undergoing processes like chemical reactions or solvent evaporation to achieve strong cohesion. (6) (7)
Methods to Evaluate Mucoadhesion Dosage Form:-
Testing plays a crucial role in the development, characterization, and practical application of mucoadhesive systems. However, it can be challenging to accurately predict the behavior of a mucoadhesive system in vivo based solely on in vitro testing. In vitro tests are typically conducted under controlled conditions, which differ from the dynamic and variable conditions encountered in vivo. Consequently, various approaches have been explored to devise accurate,

straightforward, and practical test setups for measuring mucoadhesive ability. (11) Three common types of adhesion assays are utilized to assess adhesion properties: tensile, shear, and peeling tests. In a tensile test, a machine measures the force required to detach the substrates in the axial dimension. In contrast, a shear test measures the force needed to detach the substrates in a tangential axis. In a peeling test, at least one substrate is made of a flexible material, allowing for plastic deformation during measurements. Typically, a flexible tape bonded with the adhesive material is peeled off a rigid substrate, with the peeling force assumed to maintain a steady rate of peeling.

While most adhesion tests for mucoadhesive systems fall into the categories of tensile or shear assays, various new methods have been developed to evaluate mucoadhesive ability at both macroscopic and microscopic levels. These include spectroscopic methods, contact angle measurements, dielectric measurements, and others. These methods contribute to a

comprehensive understanding of mucoadhesion and its practical implications. (10) (9)
Mucoadhesive Materials:-
Mucoadhesive polymers boast a plethora of hydrophilic entities, ranging from the ubiquitous hydroxyl to the versatile carboxyl, amide, and sulfate groups. These molecular constituents possess an inherent affinity for mucus or cellular membranes, forging bonds through a diverse array of interactions, including the delicate interplay of hydrogen bonding, hydrophobic attractions, and electrostatic forces. Consequently, these hydrophilic moieties imbue the polymers with a propensity to swell upon contact with aqueous environments, thereby unfurling a myriad of adhesive sites, poised to engage in intricate molecular dialogues with their surroundings.(12)
An ideal polymer for a bioadhesive drug delivery system should have the following characteristics
- Biocompatibility:
The polymer and its breakdown products should be nontoxic and not absorbed by the body, ensuring safety for the patient.
- Nonirritating:
It should not cause irritation or adverse reactions when in contact with biological tissues.
- Strong Adhesion:
Ideally, the polymer should form robust noncovalent bonds with mucosal or epithelial surfaces, ensuring long-lasting adhesion.
- Rapid Adhesion:
The polymer should adhere quickly to moist tissues and ideally exhibit some level of specificity for targeted sites, enhancing drug delivery efficiency.
- Drug Compatibility:
It should allow for easy incorporation of the drug and facilitate its release without hindrance, ensuring efficient delivery of therapeutic agents.
- Stability:
The polymer should remain stable during storage and throughout the shelf life of the dosage form, preventing premature degradation and ensuring efficacy.
- Cost-effectiveness:
The cost of the polymer should be reasonable to maintain the competitiveness of the final dosage form in the market.
Factors Affecting Mucoadhesion:-
"The interpenetration of polymer molecules is more likely with low molecular weight polymers, while entanglements become predominant as molecular weight increases. The ideal molecular weight for achieving maximum mucoadhesion varies depending on the polymer type. Bioadhesive forces increasing with the molecular weight of the polymer up to 100,000. Beyond

this level, there is no further gain.
Cross-linking and Swelling:
Cross-link density is inversely proportional to the degree of swelling.[The lower the cross-link density, the higher the flexibility and hydration rate; the larger the surface area of the polymer, the better the mucoadhesion. To achieve a high degree of swelling, a lightly cross-linked polymer is favored.
Spatial Conformation:
Besides molecular weight or chain length, spatial conformation of a polymer is also important. Despite a high molecular weight of 19,500,000 for dextrans, they have adhesive strength similar to that of polyethylene glycol (PEG), with a molecular weight of 200,000. The helical conformation of dextran may shield many adhesively active groups, primarily responsible for adhesion, unlike PEG polymers, which have a linear conformation.
pH:
The pH at the bioadhesive to substrate interface can influence the adhesion of bioadhesives possessing ionizable groups. Many bioadhesives used in drug delivery are polyanions possessing carboxylic acid functionalities. If the local pH is above the pK of the polymer, it will be largely ionized; if the pH is below the pK of the polymer, it will be largely unionized (13)
Drug controlled-release:-
Incorporating mucoadhesive properties into drug delivery systems offers several advantages, including prolonged contact time at the site of application, enhanced drug absorption, and improved therapeutic outcomes. Here's a brief overview of drug controlled release utilizing mucoadhesive technology:
Extended Residence Time:
Mucoadhesive polymers have the ability to adhere to mucosal surfaces, such as those in the gastrointestinal tract or nasal cavity. This prolonged residence time allows for sustained drug release and absorption, leading to a more controlled and predictable therapeutic effect.
Improved Bioavailability:
By increasing the contact time between the drug and the mucosal membrane, mucoadhesive drug delivery systems enhance drug absorption. This can result in higher bioavailability compared to conventional drug formulations, reducing the required dosage and potential side effects.
Site-Specific Delivery:
Mucoadhesive formulations can be designed to target specific sites within the body, such as the gastrointestinal tract or the nasal mucosa. This targeted delivery minimizes systemic exposure and maximizes the therapeutic effect at the desired site.
Enhanced Patient Compliance:
Controlled release mucoadhesive formulations often require less frequent dosing due to their sustained release profile. This can improve patient compliance and convenience, particularly for medications that need to be administered multiple times a day.
Versatility:
Mucoadhesive drug delivery systems can be tailored to accommodate a wide range of drugs, including small molecules, peptides, and proteins. This versatility makes them suitable for various therapeutic applications, including local and systemic drug delivery.
Customization:
Formulation scientists can adjust the properties of mucoadhesive polymers, such as molecular weight, cross-linking density, and composition, to optimize drug release kinetics and mucoadhesive strength for specific applications.

Fig.3. Mucoadhesion determination (14)
Mucoadhesive/ Bioadhesive Systems:
The Mucoadhesive/Bio adhesive system for the stomach mucous membrane has increased the gastric retention period. The adherence of the delivery system to the stomach wall extends residence time or duration, thereby enhancing bioavailability. Chemicals like polycarbophil, Carbopol, lecithin, chitosan, carboxyl methylcellulose, gliadin, etc. are used to promote mucoadhesion. Novel adhesive materials produced from bacteria fimbrae or synthetic counterparts have also been tested for gut attachment. However, the gastric Mucoadhesive force is not strong enough to resist the propulsion force of the stomach wall. Another problem of such a system is the continuous formation of mucus and dilution of the stomach content. Many researchers have experimented with combining the floating and Bio adhesion systems in a synergistic manner. (Fig.3 : Mucoadhesive drug deliverysystem). (2, 17)
Binding of polymers to the mucin/epithelial surface can be divided into three broad categories:-
- Hydration-mediated adhesion
- Bonding-mediated adhesion
- Receptor-mediated adhesion
Mucoadhesive polymers:-
Polymers play an essential role in MFDDS design by boosting the GRT of the drug at the site of action. The structure and functional groups of the polymer being used can have a direct impact on the capacity for mucoadhesion and the strength of interactions. There are essentially two types of Mucoadhesive polymers. These polymers have swellable networks and are both water-soluble and insoluble in water. An ideal Mucoadhesive polymer should be inert, nonirritating, and nontoxic; it should stick fast to most tissues; and it should be site specific. Another desirable property of a Mucoadhesive polymer is the ability to build a strong, non-covalent connection with the mucin epithelial cell surface. Mucoadhesive polymers are classified according to their characteristics. (16, 15)

Table no 2 Mucoadhesive polymers. [Adapted from Lohani et al., 2012].
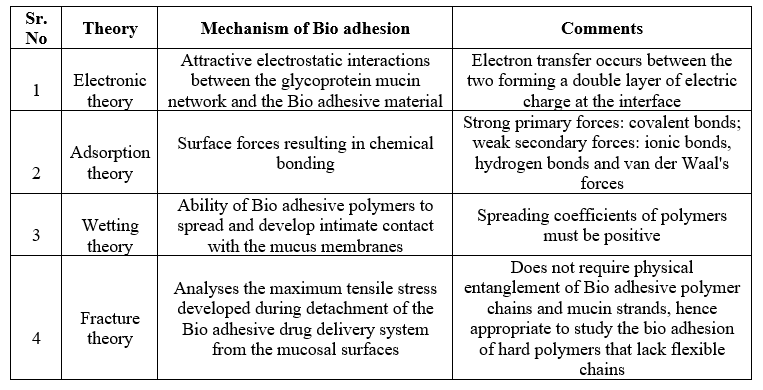
Table no 3Mechanisms of Mucoadhesion [Adapted from Vasir et al., 2003].
CONCLUSION: -
In summary, the oral mucosa stands as a promising terrain for drug delivery, offering avenues for both local and systemic bioavailability enhancement. However, the formulation of oral dosage forms necessitates meticulous consideration of factors such as mucosal permeability, oral fluid dynamics, and mechanical stresses. The quest for sustained drug release underscores the imperative of developing mucoadhesive formulations, ensuring prolonged contact with the absorption site. Mucoadhesion is a nuanced process dictated by interactions between mucoadhesive polymers and mucosal substrates, with polymer properties exerting significant influence. While modifying mucosal characteristics may pose challenges, a deep understanding of polymer attributes affecting mucoadhesion holds the key to crafting effective mucoadhesive systems. This review endeavors to elucidate the multifaceted mechanisms and variables governing mucoadhesion, with a spotlight on oral environmental factors, mucosal nuances, and polymer characteristics. Ultimately, through a comprehensive exploration of mucoadhesive technology, this review aims to shed light on its potential in revolutionizing oral drug delivery, fostering enhanced therapeutic outcomes and patient welfare.
REFERENCES:-
- Scully C, Shotts R. Mouth ulcers and other causes of orofacial soreness and pain. Bmj. 2000 Jul 15;321(7254):162-5.
- Abhang P, Momin M, Inamdar M, Kar S. Transmucosal drug delivery-an overview. Drug Delivery Letters. 2014 Apr 1;4(1):26-37.
- Flaia, C.; Marcos, L.B.; Raul, C. Mucoadhesive drug delivery syste ms. Brazilian J. Pharm. Sci., 2010, 46(1), 224-230.
- Vyas S.P.; Khar R.K. Controlled drug delivery-concepts and advances. Vallabh Prakashan, first edition, New Delhi, 2002
- Pan Z, Zhang X, Xie W, Cui J, Wang Y, Zhang B, Du L, Zhai W, Sun H, Li Y, Li D. Revisited and innovative perspectives of oral ulcer: from biological specificity to local treatment. Frontiers in Bioengineering and Biotechnology. 2024 Feb 22;12:1335377.
- Campisi, G., Paderni, C., Saccone, R., Di Fede, O., Wolff, A., and Giannola, L. I. (2010). Human buccal mucosa as an innovative site of drug delivery. Curr. Pharm. Des. 16, 641–652
- Packham DE, editor. Handbook of adhesion. Longman Scientific and Technical; 1992.
- Davidovich?Pinhas M, Bianco?Peled H. Methods to study mucoadhesive dosage forms. Mucoadhesive materials and drug delivery systems. 2014 May 2:175-96.
- Pocius AV. Adhesion and adhesives technology: an introduction. Carl Hanser Verlag GmbH Co KG; 2021 Mar 8.
- Mortazavi, S.A. and Smart, J.D. (1995) An investigation of some factors influencing the in vitro assessment of mucoadhesion. Int. J. Pharm., 116, 223–230.
- Davidovich?Pinhas M, Bianco?Peled H. Methods to study mucoadhesive dosage forms. Mucoadhesive materials and drug delivery systems. 2014 May 2:175-96.
- Yang X, Robinson JR. Bioadhesion in mucosal drug delivery. Biorelated polymers and gels. 1998:135-92.
- Shaikh R, Singh TR, Garland MJ, Woolfson AD, Donnelly RF. Mucoadhesive drug delivery systems. Journal of pharmacy and Bioallied Sciences. 2011 Jan;3(1):89.
- Andrews GP, Laverty TP, Jones DS. Mucoadhesive polymeric platforms for controlled drug delivery. European journal of pharmaceutics and biopharmaceutics. 2009 Mar 1;71(3):505-18.
- Singh BN, Kim KH. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. Journal of Controlled release. 2000 Feb 3;63(3):235-59.
- Sai D, Naveen Kumar K, Preethi K. Gastro retentive drug delivery systems: A review. INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY 2020, 10(1)96-104. Available from: www.ijrpc.com
- Patil H, Tiwari RV, Repka MA. Recent advancements in mucoadhesive floating drug delivery systems: A mini-review. Journal of Drug Delivery Science and Technology. 2016 Feb 1;31:65-71.


 Sayyed ahamad Sayyed Kaleem *
Sayyed ahamad Sayyed Kaleem *
 Quazi Majaz
Quazi Majaz










 10.5281/zenodo.10913108
10.5281/zenodo.10913108