Abstract
Human immunodeficiency virus type 1 (HIV-1) remains a global health challenge despite advancements in antiretroviral therapies. Capsid inhibitors have emerged as promising agents due to their unique mechanism of action targeting the viral capsid, which plays a critical role in viral replication, assembly, and uncoating. Among these, lenacapavir—a first-in-class, long-acting capsid inhibitor—has shown substantial efficacy in preclinical and clinical trials, demonstrating prolonged antiviral activity, reduced dosing frequency, and a favorable safety profile. This review explores the pharmacology, clinical efficacy, and safety of novel capsid inhibitors, with a focus on lenacapavir’s potential to address issues of adherence and resistance. Additionally, the article discusses practical considerations, dosing strategies, and future directions in capsid inhibitor research, offering insights into the next generation of HIV therapeutics.
Keywords
Lenacapavir, HIV-1 Treatment, Capsid Inhibitors Drugs.
Introduction
Human immunodeficiency virus (HIV) infection remains a significant global public health challenge. There are two main types of HIV, HIV-1 and HIV-2, with HIV-1 accounting for around 95% of infections worldwide due to its higher global prevalence. An estimated 38.4 million people are living with HIV-1 globally, and approximately 28.7 million of them are receiving antiretroviral therapy (ART). HIV-1 infection progressively weakens the immune system and can lead to acquired immunodeficiency syndrome (AIDS), characterized by a CD4+ T-cell count below 200 cells per microliter or the presence of opportunistic infections, such as tuberculosis, cryptococcal meningitis, lymphomas, and Kaposi’s sarcoma. Key indicators of HIV-1 infection progression or treatment failure include an increased viral load and a declining CD4+ T-cell count. If left untreated, HIV-1 infection may eventually lead to death following a variable latency period. Although ART regimens can manage HIV-1 infection, they cannot cure it (Figure 1).
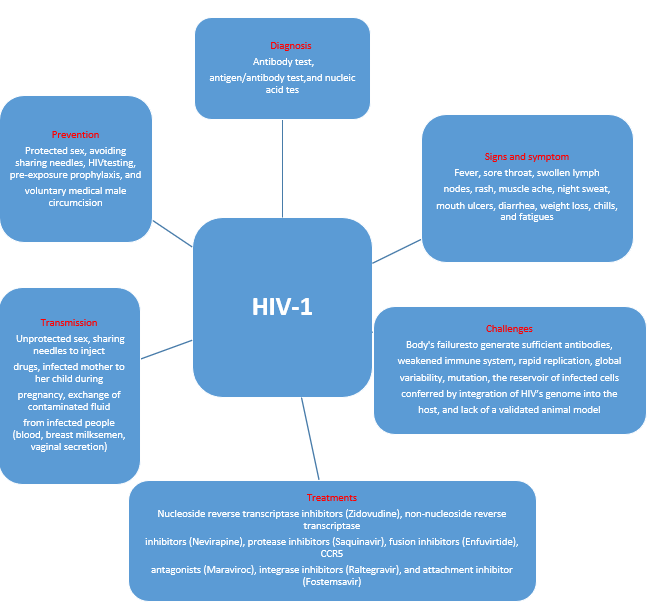
Figure 1. The mode of transmission, symptoms, diagnosis, treatment, prevention, and challenges of HIV-1 infection.
The rise in ART use has been accompanied by an increase in drug resistance, including multi-drug resistance (MDR). Unaddressed, this drug resistance poses a serious threat to ART effectiveness, potentially leading to higher HIV-1 transmission rates and increased morbidity and mortality. MDR HIV-1 infection, which remains an unmet medical need, is often resistant to current ART regimens, necessitating the development of novel treatment options . Recently, the United States Food and Drug Administration (USFDA), Health Canada, and the European Medicines Agency (EMA) approved Lenacapavir (LEN) as the first-in-class capsid inhibitor specifically designed to treat MDR HIV-1 infection. Although previous reviews on LEN exist, they often overlook pharmaceutical aspects, innovations, and patent literature related to LEN. This review aims to highlight LEN's pharmaceutical properties, development, Novel capsid approved and non approved drugs , and future potential. The literature sources used include PubMed, authoritative sites (USFDA, Health Canada, EMA, Gilead,
and NIH),with keywords such as GS-6207, GS-714207, Lenacapavir, Sunlenca, and J05-AX31.
2. Lenacapavir (LEN)
2.1. Description
Lenacapavir (LEN), marketed as Sunlenca and known by other synonyms including GS-6207, GS-714207, GS-CA1, and J05-AX31, is a novel antiretroviral agent targeting the HIV-1 capsid. With the molecular formula C??H??ClF??N?O?S? and a molecular weight of 968, LEN is a weakly acidic indazole derivative categorized under BCS class 4 due to its low water solubility and permeability, posing unique formulation challenges. Approved by regulatory authorities in the USA, Europe, and Canada (Table 1), LEN represents a groundbreaking advancement in HIV treatment options, particularly for individuals with multi-drug-resistant (MDR) HIV-1 who have limited treatment alternatives.
Table 1. Product Details of Sunlenca
|
Active Pharmaceutical Ingredient (Proprietary Name; Applicant)
|
Dosage Form (Route; Strength)
|
Approval Date (Marketing Status)
|
Indication
|
|
Lenacapavir sodium (Sunlenca;GileadSciences)
|
Solution
For
injection (Subcutaneous; EQ 463.5 mg base/1.5 mL and EQ 309 mg base/mL)
|
22 December 2022
(USFDA); 1 November 2022 (Health Canada); 17 August 2022 (EMA); Licensed for manufacturing and distribution in India by Dr. Reddy’s and Hetero, October 2024
|
In combination with other ARTs, for the treatment of heavily treatment-experienced patients with previous failed ART due to MDR HIV-1 infection, intolerance, or safety issues
|
|
Lenacapavir Sodium
|
Tablet (Oral; EQ 300 mg base)
|
|
|
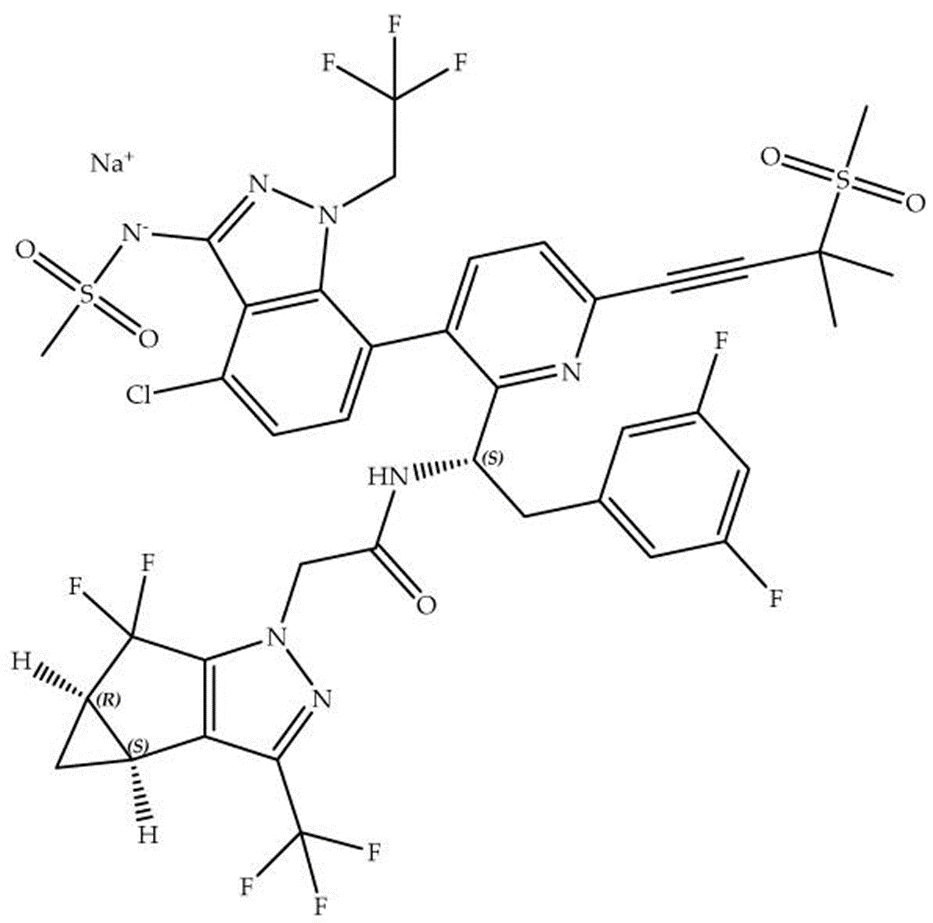
Figure 2. Chemical structure of Lenacapavir sodium.
Lenacapavir sodium (C??H??ClF??N?NaO?S?, molecular weight 990) features a unique structural composition, with specific stereochemistry defined by its (2S,3bS,4aR)-isomer as the principal active form. It exists in multiple polymorphic forms, including crystalline and amorphous states, with the crystalline form preferred in Sunlenca for enhanced stability, reproducibility, and consistent biopharmaceutical characteristics. This choice is crucial given LEN’s susceptibility to oxidative, hydrolytic, and photolytic degradation. The subcutaneous injection of LEN is supplied as a preservative-free, clear yellowish-brown solution containing polyethylene glycol 300 and water as non-medicinal components. The oral LEN tablet, a beige, capsule-shaped film-coated form, incorporates additional non-medicinal excipients to support stability and bioavailability.
2.2. Mechanism of Action of LEN
HIV-1 is an enveloped retrovirus, with its capsid—a protective protein shell—housing essential components like viral RNA, nucleocapsid, reverse transcriptase, and integrase (Figure 3). This capsid plays a crucial role across various stages of HIV-1 replication, performing functions like
safeguarding the viral genome, facilitating its transport, interacting with host cellular structures, and ultimately releasing the viral genetic material within the host cell (Figure 4). Disruption in the normal function of the capsid can significantly hinder different phases of the HIV-1 life cycle, such as the entry of viral DNA into the nucleus and its integration into the host genome. These unique characteristics of the HIV-1 capsid underscore its potential as a promising target for anti-HIV drug development.
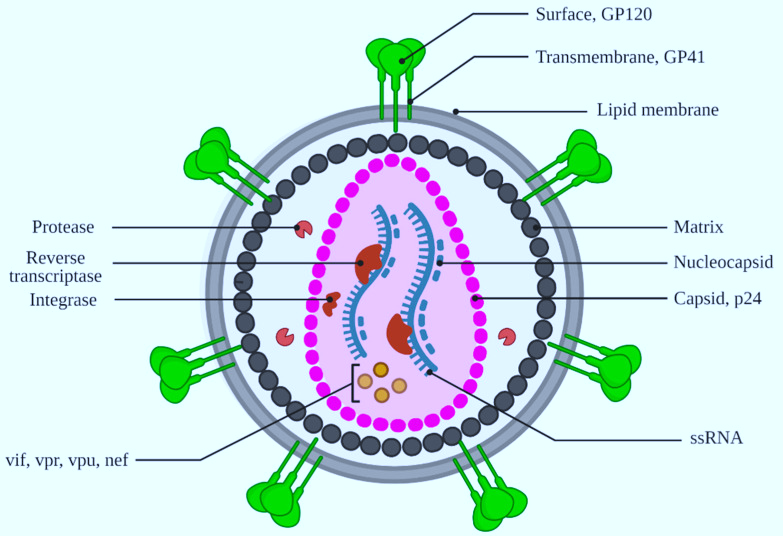
Figure 3. Representation of the HIV-1 virion structure. Illustration created using Biorender.com.
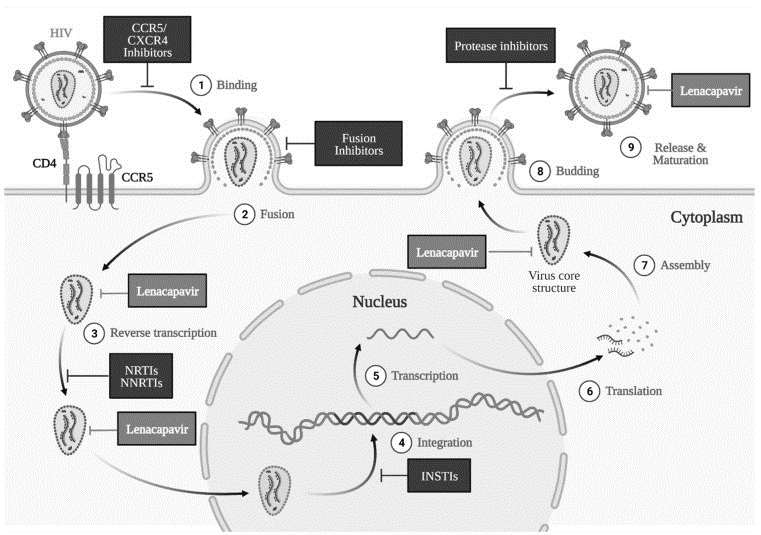
Figure 4. Mechanism of action of Lenacapavir (LEN) across various stages of the HIV-1 life cycle. Image generated with Biorender.com.
The HIV-1 capsid protein structure is composed of repeating subunits known as protomers (hexamers). Lenacapavir (LEN) binds specifically at the junction between two hexamer subunits, targeting the N74 residue on the N-terminal domain of one hexamer and two residues (N183 and K70) on the C-terminal domain of an adjacent hexamer. This binding interferes with capsid formation, leading to the development of immature or inhibited capsids, which ultimately affects multiple capsid-dependent functions throughout the HIV-1 replication cycle (Figure 4). As a result, LEN disrupts essential stages in the HIV-1 life cycle, including capsid-mediated nuclear entry, viral assembly and release, and the formation of the capsid core (Figure 4).
2.3. Preclinical Studies
Preclinical studies of Lenacapavir (LEN) have been well documented. Regulatory agencies, including the USFDA, Health Canada, and EMA, have reviewed and approved LEN based on comprehensive pharmacological data. Here, we summarize key preclinical findings.
LEN has shown strong antiviral activity against HIV-1, with an effective concentration (EC50) of 105 pM in MT-4 cells, 32 pM in human CD4+ T cells, 20–160 pM across various HIV-1 isolates, 56 pM in macrophages, and 885 pM for HIV-2 isolates. LEN's effectiveness against HIV-1 is about 8–10 times higher than for HIV-2.Tests for cytotoxicity in human cell lines (like MT4, Huh-7, and MRC-5) and primary cells (hepatocytes, PBMCs, CD4+ T-cells, and macrophages) showed a half-maximal cytotoxic concentration (CC50) of 24.7 µM to over 50 µM. This translates to a very high selectivity index (CC50/EC50), ranging from 140,000 to over 1.6 million, showing LEN’s safety margin in targeting HIV-1. Off-target tests against 87 receptors, enzymes, and ion channels revealed no significant interactions at a 10 µM concentration. This highlights LEN's potency against HIV-1 with minimal side effects. LEN also demonstrated efficacy against drug-resistant HIV-1 strains (Table 2).
Table 2 Effectiveness of LEN Against Drug-Resistant HIV-1 Strains [35].
|
Drug Class
|
HIV-1 Mutation
|
LEN Resistance (Mean ± SD)
|
Control Drug Resistance
|
|
Nucleoside RT Inhibitor
|
K65R
|
0.6 ± 0.2
|
Emtricitabine: 14.1 ± 2.6
|
| |
M184V
|
0.3 ± 0.3
|
Emtricitabine: >23.0
|
|
Non-nucleoside RT Inhibitor
|
Y188L
|
0.5 ± 0.1
|
Efavirenz: >22.5
|
|
Integrase Inhibitor
|
E138K + Q148K
|
0.6 ± 0.3
|
Elvitegravir: >53.8
|
|
Maturation Inhibitor
|
V230I in Capsid
|
0.7 ± 0.2
|
Bevirimat: >67.5
|
|
Protease Inhibitor
|
M46I + I50V
|
0.7 ± 0.2
|
Darunavir: 27.1 ± 23.1
|
LEN’s pharmacokinetics, studied in animal models (rats, dogs, and monkeys), are detailed in regulatory filings [20,24,25,36,37]. At a dose of 100 mg/kg, LEN showed no adverse effects on the cardiovascular system in dogs, nor on the central nervous or respiratory systems in rats. LEN was also shown to be non-carcinogenic, non-mutagenic, and had no impact on fertility.
This data highlights LEN’s high efficacy and selectivity against HIV-1, with minimal off-target effects, making it a promising candidate for further clinical development.
2.4. Clinical Studies on LEN
Here’s a simplified summary of the Gilead-sponsored clinical trials on Lenacapavir (LEN) for treating and preventing HIV infection, with focus on main outcomes, phases, and locations:
Table 3: Summary of Gilead-Sponsored and India-Based Clinical Studies on LEN for HIV Treatment
|
NCT Number
|
Objective/Interventions
|
Phase
|
Status / Location
|
|
NCT05502341
|
Comparing safety and efficacy of Bictegravir/LEN vs. standard ART
in HIV treatment (671 participants)
|
Phase
2/3
|
Recruiting, United States
|
|
NCT05052996
|
Examining safety and efficacy of LEN with islatravir in HIV treatment (136participants)
|
Phase
2
|
Active, United States
|
|
NCT04994509
|
Evaluating LEN with F/TAF for HIV prevention—PURPOSE 1 study
(5,010 participants)
|
Phase
3
|
Recruiting, South Africa, India
|
|
NCT04925752
|
Testing LEN's efficacy in HIV-1 prevention—PURPOSE 2 study (3,000 participants)
|
Phase
3
|
Recruiting, United States, India
|
|
NCT04811040
|
Studying safety of LEN combined with teropavimab and zinlirvimab
(32 participants)
|
Phase
1
|
Active, United States
|
|
NCT04150068
|
LEN added to failing HIV-1 therapy due to resistance—CAPELLA study (72 participants)
|
Phase 2/3
|
Active, United States; Results posted
|
|
NCT04143594
|
Assessing LEN + ART combinations for HIV treatment—CALIBRATE study
(183 participants)
|
Phase
2
|
Active, United States; Results posted
|
|
NCT03739866
|
Evaluating LEN’s safety, antiviral activity, and pharmacokinetics in HIV-1 patients
(53 participants)
|
Phase
1
|
Completed, United States; Results posted
|
Highlights from Clinical Study Findings
- Phase 1 (NCT03739866): Demonstrated LEN’s safety, effective antiviral action, and favorable pharmacokinetics
- Phase 2 (NCT04143594): LEN combined with ARTs (emtricitabine, tenofovir, bictegravir) showed 85–92% virologic suppression after 54 weeks. Common side effects were headache and nausea for oral LEN, and erythema and pain for subcutaneous (SC) administration.
- Phase 3 (NCT04150068): In patients with multidrug-resistant HIV-1, LEN led to >81% reduction in viral load compared to placebo.
- PURPOSE 2 Trial (NCT04925752): An ongoing study to assess LEN’s impact on HIV prevention, though conclusive outcomes are not yet available.
Additional studies have explored LEN’s effects on LEN-resistant mutations, with findings showing some mutation development (e.g., Q67H) in LEN monotherapy, but also a beneficial inverse relationship between viral replication capacity and resistance.
2.5. Pharmacological Properties of LEN
|
Parameter
|
Summary
|
|
Dose/Regimen
|
Option 1: Day 1: SC injection in the abdomen (2 injections of 463.5 mg/1.5 mL) + 2 tablets (300 mg each); Day 2: 2 tablets (300 mg each); Maintenance: SC injection every 6 months (2 injections of 463.5 mg/1.5 mL).
Option 2: Day 1: 2 tablets (300 mg each); Day 2: 2 tablets (300 mg each); Day 8: 2 tablets (300 mg each); Day 15: SC injection (2 injections of 463.5 mg/1.5 mL); Maintenance: SC injection every 6 months.
|
|
Absorption
|
Ora bioavailability: 6–10%; Subcutaneous (SC): 100%. Time to peak concentration (Tmax): 4 hours (oral), 77–84 days (SC).
|
|
Volume of Distribution
|
Oral: 19240 L; SC: 9500–11700 L; Total: 976 L.
|
|
Protein Binding
|
Over 98.5% protein-bound.
|
|
Metabolism
|
Primarily metabolized by CYP3A and UGT1A1; no induction of CYP3A4. Not significantly metabolized or inhibited by other major enzymes like CYP2D6, CYP2C19, CYP2C9, and others.
|
|
Elimination
|
Mostly excreted unchanged in feces; <1>
|
|
Half-life
|
Oral: 10–12 days; SC: 8–12 weeks.
|
|
Clearance
|
Oral: 55 days; SC: 4.2 weeks.
|
|
Adverse Effects
|
Common: Nausea, injection site reactions (swelling, pain, redness, nodule formation). Less common: immune reconstitution syndrome, proteinuria, hyperglycemia, glycosuria, elevated creatinine, and liver enzymes.
|
|
Drug Interactions
|
May interact with drugs like carbamazepine, phenobarbital, phenytoin, efavirenz, rifampin, and strong CYP3A inducers, reducing LEN’s effectiveness. Minimal interactions with drugs like darunavir, famotidine, and rosuvastatin.
|
|
Food Interactions
|
May be taken with or without food.
|
|
Contraindications
|
Avoid with strong CYP3A inducers or inhibitors, atazanavir/cobicistat, and herbal products like St. John's wort.
|
|
Warnings/Precautions
|
Risk of immune reconstitution syndrome; may trigger autoimmune conditions (e.g., Graves' disease, autoimmune hepatitis). Non-adherence can lead to resistance.
|
|
Toxicity/Overdose
|
Limited toxicity data; overdose treatment is supportive. Dialysis is likely ineffective due to high protein binding. No known cardiotoxicity.
|
|
Special Populations
|
Limited data for elderly, pediatric, and pregnant/breastfeeding populations.
|
3 Resistance Development
Resistance to Lenacapavir has been observed in both in vitro studies and clinical trials, particularly involving mutations in the capsid protein that reduce the drug’s binding affinity. Key mutations, such as M66I and Q67H, have been identified as contributing to reduced susceptibility to Lenacapavir. These mutations alter the capsid structure in a way that diminishes the inhibitory effect of the drug.
To address the potential for resistance, Lenacapavir is typically used in combination with other antiretrovirals as part of a highly active antiretroviral therapy (HAART) regimen. This combination approach helps to suppress viral replication more effectively and minimizes the risk of resistant strains emerging. Monitoring for resistance development and tailoring treatment regimens based on resistance profiles are essential components of clinical management when using Lenacapavir.
4 Safety and Tolerability
Safety and tolerability are critical considerations in the long-term management of HIV-1. Lenacapavir has been well-tolerated in clinical trials, with a safety profile comparable to other antiretroviral agents. The most common adverse events reported include injection site reactions, such as pain, erythema, and swelling, which are generally mild and transient.
Other reported side effects include headache, nausea, and diarrhea, which were mostly mild and resolved without intervention. Importantly, no serious drug-related adverse events were reported, and there was no evidence of significant drug-drug interactions, making Lenacapavir a suitable option for use alongside other antiretrovirals.
5 Novel Capsid Inhibitors:
Several novel capsid inhibitors have shown promise in preclinical and clinical studies:
1. GC376 : A broad-spectrum capsid inhibitor effective against picornaviruses, including rhinoviruses and enteroviruses.
2. VX-787 : A capsid inhibitor targeting influenza A and B viruses, demonstrating efficacy in phase II clinical trials.
3. PF-04868273 : A capsid inhibitor showing potent activity against respiratory syncytial virus (RSV).
4. Lenacapavir (LEN) : A long-acting capsid inhibitor targeting HIV-1, with a unique mechanism of action and potential for once-weekly dosing.
5. GS-6207 : A capsid inhibitor targeting HIV-1, with potent activity and potential for combination therapy.
6. BMS-986197 : A capsid inhibitor targeting influenza A and B viruses, demonstrating efficacy in phase I clinical trials.
Here are the approved and investigational capsid inhibitor drugs:
5.1 Approved Capsid Inhibitors:
1. Lenacapavir (LEN) - HIV-1
2. Glecaprevir (Mavyret) - Hepatitis C
4. Valomaciclovir (Valcyte) - CMV
5. Palivizumab (Synagis) - RSV
3. Pibrentasvir (Mavyret) - Hepatitis C
5.2 Investigational Capsid Inhibitors:
HIV-1:
1. GS-6207
2. BMS-986197
3. MK-8591
4. GSK3684934
Influenza:
1. VX-787
2. JNJ-63623872
3. S-033188
RSV:
1. PF-04868273
2. RSV604
Hepatitis B/C:
1. ABI-H0731
2. JNJ-47965567
3. GLS-0521
Herpesviruses:
1. Pritelivir (HSV)
2. Valomaciclovir (CMV)
Picornaviruses:
1. GC376 (Rhinovirus)
2. Vapendavir (Rhinovirus)
3. BTA-858
COVID-19:
1. PF-07304814
2. S-033188
Preclinical Capsid Inhibitors:(HIV-1, Influenza, RSV, 6Hepatitis B/C, Herpesviruses)
5.3 Potential Targets:
1. HIV-1 : Develop capsid inhibitors targeting HIV-1 capsid protein.
2. Influenza : Design capsid inhibitors effective against influenza A and B viruses.
3. RSV : Target RSV capsid protein for respiratory syncytial virus treatment.
4. HBV : Develop capsid inhibitors targeting hepatitis B virus.
5. COVID-19 : Investigate capsid inhibitors against SARS-CoV-2.
CONCLUSION
Lenacapavir and other emerging capsid inhibitors represent a promising frontier in HIV-1 treatment, offering new avenues to enhance therapy and address limitations associated with traditional antiretroviral treatments. Lenacapavir’s unique, long-acting mechanism of action, targeting the viral capsid, demonstrates substantial antiviral efficacy with reduced dosing frequency and an impressive safety profile, making it a potential game-changer in managing HIV-1 infection. Additionally, the broader scope of capsid inhibitor development highlights the adaptability of this approach in targeting other challenging viruses, such as influenza, RSV, hepatitis B/C, and COVID-19. These novel agents not only enhance treatment options but also have the potential to overcome challenges related to adherence and resistance. The continued exploration of capsid inhibitors, including their role in combination therapies and broader viral applications, underscores their potential in revolutionizing the landscape of antiviral therapy. Future research and clinical studies will be crucial in further evaluating lenacapavir’s long-term impact and the broader applicability of capsid inhibitors as a cornerstone of next-generation antiviral therapeutics.
REFERENCES
- Ceccarelli G., Giovanetti M., Sagnelli C., Ciccozzi A., d’Ettorre G., Angeletti S., Borsetti A., Ciccozzi M. Human Immunodeficiency Virus Type 2: The Neglected Threat. Pathogens. 2021;10:1377. doi: 10.3390/pathogens10111377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization HIV-AIDS. [(accessed on 23 February 2023)]. Available online: https://www.who.int/health-topics/hiv-aids#tab=tab_1.
- Chatzidaki I., Curteis T., Luedke H., Mezzio D.J., Rhee M.S., McArthur E., Eddowes L.A. Indirect Treatment Comparisons of Lenacapavir Plus Optimized Background Regimen Versus Other Treatments for Multidrug-Resistant Human Immunodeficiency Virus. Value Health. 2022 doi: 10.1016/j.jval.2022.12.011. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos G.K., Tsantes A.G. Recent HIV Infection: Diagnosis and Public Health Implications. Diagnostics. 2022;12:2657. doi: 10.3390/diagnostics12112657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg J., Keeshin S. Prevention and Initial Management of HIV Infection. Ann. Intern. Med. 2022;175:ITC81–ITC96. doi: 10.7326/AITC202206210. [DOI] [PubMed] [Google Scholar]
- Back D., Marzolini C. The challenge of HIV treatment in an era of polypharmacy. J. Int. AIDS Soc. 2020;23:e25449. doi: 10.1002/jia2.25449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas M., Moreno S. Global strategy in the treatment of HIV infection in 2022. Rev. Esp. Quimioter. 2022;35((Suppl. S3)):34–36. doi: 10.37201/req/s03.08.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Granich R., Williams B.G. Update on treatment as prevention of HIV illness, death, and transmission: Sub-Saharan Africa HIV financing and progress towards the 95-95-95 target. Curr. Opin. HIV AIDS. 2022;17:368–373. doi: 10.1097/COH.0000000000000761. [DOI] [PubMed] [Google Scholar]
- Ndashimye E., Reyes P.S., Arts E.J. New antiretroviral inhibitors and HIV-1 drug resistance: More focus on 90% HIV-1 isolates? FEMS Microbiol. Rev. 2023;47:fuac040. doi: 10.1093/femsre/fuac040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl A.C., Garzino Demo A., Makarov V.A., Ekins S. New targets for HIV drug discovery. Drug. Discov. Today. 2019;24:1139–1147. doi: 10.1016/j.drudis.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N.E., Sutherland R.K. Lenacapavir and the novel HIV-1 capsid inhibitors: An emerging therapy in the management of multidrug-resistant HIV-1 virus. Curr. Opin. Infect. Dis. 2023;36:15–19. doi: 10.1097/QCO.0000000000000896. [DOI] [PubMed] [Google Scholar]
- Paik J. Lenacapavir: First Approval. Drugs. 2022;82:1499–1504. doi: 10.1007/s40265-022-01786-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvory-Sobol H., Shaik N., Callebaut C., Rhee M.S. Lenacapavir: A first-in-class HIV-1 capsid inhibitor. Curr. Opin. HIV AIDS. 2022;17:15–21. doi: 10.1097/COH.0000000000000713. [DOI] [PubMed] [Google Scholar]
- Gilead Lenacapavir. [(accessed on 23 February 2023)]. Available online: https://www.gilead.com/utility/search?q=lenacapavir.
- Orkin C. Lenacapavir in first-line therapy. Lancet HIV. 2023;10:e2–e3. doi: 10.1016/S2352-3018(22)00375-7. [DOI] [PubMed] [Google Scholar]
- Gupta S.K., Berhe M., Crofoot G., Benson P., Ramgopal M., Sims J., McDonald C., Ruane P., Sanchez W.E., Scribner A., et al. Lenacapavir administered every 26 weeks or daily in combination with oral daily antiretroviral therapy for initial treatment of HIV: A randomised, open-label, active-controlled, phase 2 trial. Lancet HIV. 2023;10:e15–e23. doi: 10.1016/S2352-3018(22)00291-0. [DOI] [PubMed] [Google Scholar]
- Walter M. Lenacapavir—Der erste Kapsid-Inhibitor. MMW Fortschr. Med. 2022;164:64. doi: 10.1007/s15006-022-1912-x. [DOI] [PubMed] [Google Scholar]
- Marrazzo J. Lenacapavir for HIV-1—Potential Promise of a Long-Acting Antiretroviral Drug. N. Engl. J. Med. 2022;386:1848–1849. doi: 10.1056/NEJMe2204376. [DOI] [PubMed] [Google Scholar]
- Segal-Maurer S., DeJesus E., Stellbrink H.J., Castagna A., Richmond G.J., Sinclair G.I., Siripassorn K., Ruane P.J., Berhe M., Wang H., et al. CAPELLA Study Investigators. Capsid Inhibition with Lenacapavir in Multidrug-Resistant HIV-1 Infection. N. Engl. J. Med. 2022;386:1793–1803. doi: 10.1056/NEJMoa2115542. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency Summary of Product Characteristics. [(accessed on 23 February 2023)]. Available online: https://www.ema.europa.eu/en/documents/product-information/sunlenca-epar-product-information_en.pdf.
- Center For Drug Evaluation And Research Product Quality Review(s) [(accessed on 23 February 2023)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2023/215973,215974Orig1s000ChemR.pdf.
- United States Food and Drug Administration Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. [(accessed on 23 February 2023)]; Available online: https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm.
- European Medicines Agency Sunlenca. [(accessed on 23 February 2023)]. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/sunlenca#authorisation-details-section.
- Health Canada Product Monograph Including Patient Medication Information. [(accessed on 23 February 2023)]. Available online: https://pdf.hres.ca/dpd_pm/00068216.PDF.
- Rossi E., Meuser M.E., Cunanan C.J., Cocklin S. Structure, Function, and Interactions of the HIV-1 Capsid Protein. Life. 2021;11:100. doi: 10.3390/life11020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selyutina A., Hu P., Miller S., Simons L.M., Yu H.J., Hultquist J.F., Lee K., KewalRamani V.N., Diaz-Griffero F. GS-CA1 and lenacapavir stabilize the HIV-1 core and modulate the core interaction with cellular factors. iScience. 2021;25:103593. doi: 10.1016/j.isci.2021.103593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bester S.M., Wei G., Zhao H., Adu-Ampratwum D., Iqbal N., Courouble V.V., Francis A.C., Annamalai A.S., Singh P.K., Shkriabai N., et al. Structural and mechanistic bases for a potent HIV-1 capsid inhibitor. Science. 2020;370:360–364. doi: 10.1126/science.abb4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer L.E., Gorman E.M., Mulato A.S., Rhee M.S., Rowe C.W., Sellers S.P., Stefanidis D., Tse W., Yant S.R., Chiu A. Capsid Inhibitors for the Treatment of HIV. Application Publication Number WO2020018459A1. PCT Patent. 2020 January 23;
- Neogi U., Sonnerborg A., Singh K., Quinn T.P., Gallazzi F. Peptide Inhibitors for the Inhibition of HIV Capsid. Application Publication Number US2022306690A1. U.S. Patent. 2022 September 29;
- Zhang X., Xu S., Sun L., Ding D., Tao Y., Kang D., Liu X., Zhan P. HIV-1 capsid inhibitors: A sword to destroy the virus. Future Med. Chem. 2022;14:605–607. doi: 10.4155/fmc-2022-0008. [DOI] [PubMed] [Google Scholar]
- Troyano-Hernáez P., Reinosa R., Holguín Á. HIV Capsid Protein Genetic Diversity Across HIV-1 Variants and Impact on New Capsid-Inhibitor Lenacapavir. Front. Microbiol. 2022;13:854974. doi: 10.3389/fmicb.2022.854974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S., Torbett B.E. Interactions of HIV-1 Capsid with Host Factors and Their Implications for Developing Novel Therapeutics. Viruses. 2021;13:417. doi: 10.3390/v13030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirra R.T., Dos Santos N.F.B., Zadrozny K.K., Kucharska I., Ganser-Pornillos B.K., Pornillos O. A molecular switch modulates assembly and host factor binding of the HIV-1 capsid. Nat. Struct. Mol. Biol. 2023;30:383–390. doi: 10.1038/s41594-022-00913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link J.O., Rhee M.S., Tse W.C., Zheng J., Somoza J.R., Rowe W., Begley R., Chiu A., Mulato A., Hansen D., et al. Clinical targeting of HIV capsid protein with a long-acting small molecule. Nature. 2020;584:614–618. doi: 10.1038/s41586-020-2443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency Assessment Report. [(accessed on 23 February 2023)]. Available online: https://www.ema.europa.eu/en/documents/assessment-report/sunlenca-epar-public-assessment-report_en.pdf.
- Center For Drug Evaluation And Research Integrated Review. [(accessed on 23 February 2023)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2023/215973,215974Orig1s000IntegratedR.pdf.
- National Institutes of Health National Library of Medicine. [(accessed on 23 February 2023)]; Available online: https://www.clinicaltrials.gov/
- Margot N., Vanderveen L., Naik V., Ram R., Parvangada P.C., Martin R., Rhee M., Callebaut C. Phenotypic resistance to lenacapavir and monotherapy efficacy in a proof-of-concept clinical study. J. Antimicrob. Chemother. 2022;77:989–995. doi: 10.1093/jac/dkab503. [DOI] [PubMed] [Google Scholar].


 Ravula Tulasi Naga pavan kumar*
Ravula Tulasi Naga pavan kumar*
 Panja Aishwarya Sai
Panja Aishwarya Sai
 P. Suma Sri
P. Suma Sri
 Boddani Sunil
Boddani Sunil




 10.5281/zenodo.14208676
10.5281/zenodo.14208676