Abstract
In recent advances in drug delivery, the oral route continues to be the most convenient and preferred route for drug administration to attain maximal therapeutic benefits-leading to patient compliance. Nowadays, jelly candies are readily accepted by children with a full set of teeth as they enjoy the taste and chewability of the jelly candies due to the frequent addition of fruit juice and fruit extracts. Most patients with dysphagia would choke on water while administering highly viscous liquid formulations that should be avoided, thus the enhancement of pharmaceutical preparations of this sort. Modern development of oral medicated jelly is, in fact, one of the novel approaches that target the improvement of safety and efficacy. The formulations are more patient compliant when it comes to administering, especially for dysphagic patients, who constitute a significant proportion of the population in this modern world. This review aims to briefly address its advantages and disadvantages, gelling agents, excipients, methods of preparation, evaluation parameters, and other aspects in its preference over the conventional dosage forms of drugs. Oral medicated jelly with cumin, fennel, and carom primarily exhibits antacid properties and essentially treats GIT disorders, which is very much significant.
Keywords
Oral medicated jelly, oral route, patient compliant, gelling agent, dysphagia.
Introduction
In simplest words, jelly consists of semisolid preparations that may be transparent or translucent without greasiness, meant for topical and internal uses. Jellies refer to water-soluble bases derived from natural materials like tragacanth, pectin, boroglycerin, and alginates or the synthetic derivatives of those natural substances by means of cellulose, sodium carboxymethylcellulose, methylcellulose, and sodium carboxymethyl cellulose. An ideal dosage regimen for any drug treatment of a disease is usually one that achieves the desired therapeutic concentration of the drug in plasma (or at the site of action) as quickly as possible and keeps it generally constant for the entire duration of the treatment. Drugs are very often administered orally, which is somewhat contradictory to the idea. Drug administration is for the most part a very natural, simple, convenient, and safe option, allowing considerations of great flexibility in design of dosage form, ease of manufacture, and small cost, with user-appropriateness, aggregation, and ease of production. The most apparent disadvantage of the traditional oral dosage form, which would be tablets, is that they are difficult to swallow, causing noncompliance in patients, especially in pediatric and geriatric patients. But this can also apply to people who are sick in bed and those active working patients who tend to be busy or traveling, particularly those who have no access to water. To cater to these medical needs, Pharmaceutical Tech in a broad sense developed a new oral dosage form called Oral Medicated Jellies (OMJs), which dissolve quickly in saliva in a matter of seconds without the merchant use of water. OMJs indeed act as a means to dissolve the drug and absorb it into the system faster than the onset of clinical effects and bioavailability of the drug as compared to conventional dosage forms. Demand for developing these products has approximately quadrupled in over a decade, mainly because of their significant impact on patient compliance. The marketing and development of such oral medicated jellies are, therefore, quite significant. Medicated jellies intended for oral administration are favoured by a considerable segment of the people, especially those who typically have difficulty swallowing, which, contrary to popular opinion, is an uncommon situation. Basically, it can be reported that dysphasia refers to the difficulty in swallowing, and the condition is very common among all age-groups-but is more specific with paediatric, geriatric, and a few select patient groups, such as institutionalized, psychiatric patients and those who have complications from nausea, vomiting, and motion sickness. Dysphasia is common across almost all age groups, with an incidence of about 35% of the population; approximately 60% is seen amongst the geriatric, especially those that are institutionalized, and even a substantial 18 to 22% of all patients in long-term care can be practically assessed. The oral medicated jellies with good taste and flavour increase the acceptability of bitter drugs by various groups of the population and that holds quite an importance.
Oral Mucosa [2,5]
The term is used to refer to the soft tissue lining of the oral cavity, which comprises the - mucosa, epithelium, lamina propria, and submucosa. The total area of the oral cavity comes to about 100 cm2, and the oral mucosal surfaces are constantly bathed by saliva (0.5-2 L per day turnover). The pH of saliva ranges from 5.6 to 7.9 based on the flow rate.

Figure No. 1 Oral Mucosa
Types Of Jelly [2]
Medicated Jelly
These mainly act in the mucous membranes and skin due to their spermicidal, local anaesthetic, and antiseptic properties. These jellies contain adequate water content. Upon evaporation of the water, the jellies provide a local cooling effect and protect through a residual film. For example, ephedrine sulphate jelly acts as a vasoconstrictor in stopping nosebleeds.

Figure No. 2 Medicated Jelly
- Lubricating Jelly
The jellies are intended to act as lubricants for diagnostic equipment, such as cystoscopes, catheters, surgical gloves.

Figure No. 3 Lubricating Jelly
- Miscellaneous jelly
The miscellaneous jelly are used for various applications like patch testing, electrocardiogram.

Figure No. 4 Miscellaneous Jelly
Ideal Characteristics Of Jells [5]
Advantages Of Jelly [4]
- Can give anywhere and anytime without water.
- It can prevent synthesis difficulty due to short drug action and variation in drug release and retention time through the oral mucosa.
- Less frequent dose when compared to other drug administering systems.
- Termination of treatment is comparatively easy at will.
- Drugs should either dissolve or suspend in saliva and be in a freely bioavailable form, so that after the initial jelly release, they are swallowed into the gastro-tract.
- Design flexibility.
- Improved patient compliance.
- Medicated jellies are practical for localized treatment against the diseases affecting systemic conditions or the oral cavity.
- High acceptance by children, geriatrics and patients with dysphagia.
- Good for immediate or emergency treatment.
Disadvantages Of Jelly [5]
- Because it is an aqueous formulation, proper packaging is necessary to preserve the stability of the medications in a variety of environments.
- If not properly prepared, it could result in a disagreeable flavour.
- It also exhibits the quality of effervescent, fragile granules.
- Standard blister packets lack physical resistance.
- Because oral medicated jelly is hygroscopic, it needs to be stored in a dry location.
- Special packaging is necessary for the stable product to be appropriately stabilized and safe.
Limitations Of Jelly [2]
- An expensive production method
- Standard blister packets lack physical resistance.
- For the stable product to be adequately stabilized and safe, OMJ needs appropriate packaging.
- It also exhibits the quality of fragile, effervescent granules.
- Limited capacity to add more active medication concentrations.
- ODT must be stored in a dry location due to its hygroscopic nature.
Key Ingredients Used in Preparation of Jelly [6]
Table No. 1 Key Ingredients
|
Sr no
|
Ingredients
|
Uses
|
|
1)
|
Gelling agent
|
Gellan gum, Gelatin, xanthan gum, sodium alginate, pectin, carrageneen, mcc and dvts etc
|
|
2)
|
Stabilizers
|
Propylene glycol, sorbitol
|
|
3)
|
Preservatives
|
Methyl paraben, propyl paraben, sodium benzoate
|
Gelling Agents Used in Formulation [4]
Table No. 2 Gelling Agents
|
Sr no
|
Gelling agents
|
Description
|
|
1
|
Sodium alginate
|
In a variety of topical and oral medicinal formulations, including pastes, creams, and gels, as well as in cosmetics and food items, it is frequently utilized as a thickening and suspending agent.
|
|
2
|
Gelatin
|
In an implanted delivery system, it serves as a biodegradable matrix material. Additionally, gelatin is frequently used in culinary items and emulsions for photography.
|
|
3
|
Pectin
|
It has been experimentally employed in gel formulation for oral sustained drug administration and is utilized as an adsorbent and bulk forming agent.
|
|
4
|
Tragacanth
|
It serves as an emulsifying and suspending ingredient in a number of medicinal compositions. Creams, gels, and emulsion formulas all employ it.
|
|
5
|
Xanthan gum
|
It is primarily utilized as a thickening, emulsifying, stabilizing, and suspending ingredient in food, cosmetics, and topical and medicinal formulations. Additionally, it is utilized as a hydrocolloid in the food sector and as a thickening ingredient in shampoo in cosmetics.
|
|
6
|
Cellulose derivatives
|
It is employed in the production of thermoplastic polymers and functions as a hydrophilic bulking agent. For instance, sodium carboxymethyl methyl cellulose.
|
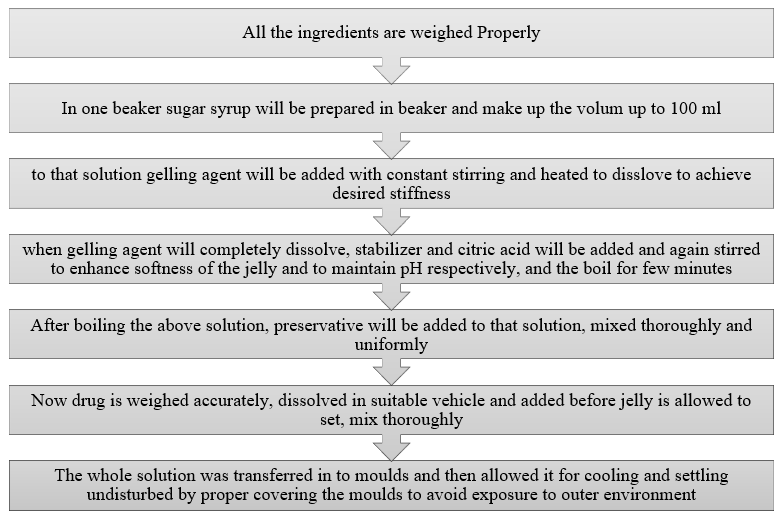

Figure No. 5 Prepared Jelly
Evaluation Of Jelly
- Physical Appearance
- pH Test
- Stickiness and grittiness
- Pourability of the mixture
- Taste evaluation
- Viscosity study
- Texture analysis
- Content uniformity
- In-vitro dissolution study
- Spreadability test
- Syneresis
- Stability Studies
- Physical Appearance [4]
For the most part, the medicated jelly may be physically inspected for appearance, including clarity, texture, transparency, and consistency, in an essentially significant manner.
- pH test [16]
At room temperature, the jellies pH was measured with a digital pH meter. To do this, 50 milliliters of distilled water should be combined with 0.5 grams of jelly to create a 1% solution, and the pH should be recorded. Both stability and flavour are impacted by the finished jelly's pH level.
- Stickiness and grittiness [14,15]
By gently rubbing the jelly sample between two fingers, one may visually check the formulations to determine their stickiness and grittiness.
- Pourability of the mixture [16]
It should be simple to pour the jelly formulation mixture into the moulds. Trisodium citrate and other buffer salts, also known as retarders, are crucial in this process because they interfere sterically with the pectin molecules' approach during the hot phase and elevate the pH level prior to the addition of acid, preventing pre-gelation. The longer the setting period, which gives enough time for the jelly to set and pour, and the lower the setting temperature, the greater the buffer salt, or retarder, concentration.
- Taste evaluation [15]
The volunteers evaluated the tastes. Experts on the taste panel should be given five grams of the improved formulation, and they should be instructed to put the gel in their mouths for five seconds. They were questioned about the flavour.
- Viscosity study [12]
The Fungi lab viscometer, which uses a non-Newtonian spindle number four, was used to measure the viscosity of the jelly. It was measured at 250 C ± 50 C for a set duration of 2 minutes at 1.5 rpm.
- Texture analysis [9]
This method involves using two fingers to push the gel surface. The geometry of a finger pushed into the material was replicated using a hemispherical probe with a 12 mm diameter. A load cell that analyses sample response as a function of probe penetration is directly attached to the traveling probe.
- Content uniformity [13]
Initially, each formulation's jelly was removed, crushed, and combined. The mixture's drug equivalent was extracted using the appropriate medium. A UV-visible spectrophotometer set at the appropriate wavelength should measure each solution's absorbance, or an appropriate analytical technique should be used to determine how much medicine was present in each extract. This test verifies that there is an equivalent quantity of medicinal compounds, or active pharmaceutical ingredients, in each dosage form throughout the batch.
- In-vitro dissolution study [14]
The USP paddle type apparatus was maintained at 370C ± 0.50C and 50 rpm for the in-vitro dissolution investigation using 900ml of dissolving liquid. After 10, 20, 30, 40, 50, 60, 90, and 120 minutes, 5 ml of the sample should be removed and diluted with 10 ml in a volumetric flask. The sink condition is then maintained by replacing the sample with new medium. Using a UV spectrophotometer or an appropriate analytical technique, the drug content of the sample was ascertained. After taking absorbance, the percentage of drug release was then computed.
- Spreadability test [11]
For the purpose of determining spreadability, 2.5 g of jelly was sandwiched between two glass slides and crushed to the appropriate thickness while maintaining a 1000 g weight for five minutes. Spredability was measured by measuring the amount of time in seconds required to separate two slides. Better Spredability was demonstrated with a shorter time interval to cross the 7.5 cm distance.
S = W × L/T
Where, S = Spreadability,
W = weight tide to upper slide,
L= length of glass slide (7.5cm),
T= time required to separate 2 slides.
- Syneresis [8]
It is the contraction of the gel during storage and the removal of water from the gel. It is more noticeable in the gels if a low dose of gelling ingredient is used. Every jelly was checked for syneresis at room temperature (25°C ± 5°C) and at 8°C ± 1°C. Syneresis-indicating formulations were rejected and not chosen for additional research.
- Stability Studies [10]
According to ICH rules, this investigation should be published. The samples should be kept at room temperature for three months as well as at different temperatures (0–8 °C). Every month, the jelly sample should be examined for viscosity, appearance, and pH. The samples were allowed to equilibrate at 250C for two hours before all measurements were taken.
25°C /60%RH (±2°C/±5%RH)
30°C /65%RH (±2°C/±5%RH)
CONCLUSION
This review leads to the conclusion that patient-compliance dose forms are superior to traditional ones since the new oral medicated jelly formulations are easily absorbed by paediatric and elderly patients as well as those with dysphagia. One of the innovative approaches, the formulation seeks to increase efficacy and safety. Several gelling agents and excipients must be employed in the formulation manufacturing process, and sugar syrup should be added for sweetness or to enhance the acceptable flavour, which is primarily accepted by children nowadays as jelly candies.
REFERENCES
- More MM, Jaiswal RS, Gawale DS, Chaudhari SA, Chavhan RP, Khairnar VT. A review on formulation and evaluation herbal oral medicated jellies of Glycyrrhiza and Ajwain.
- Sarojini S, Anusha K, Maneesha C, Mufaquam MA, Deepika B, Krishna Y. Oral Medicated Jellies–a Review. World J Pharm Res. 2018 Jan 29;7(6):352-65.
- Doolaanea AA, Bahari AZ. Advantages of jelly over liquid formulations for pediatrics. Journal of Formulation Science & Bioavailability. 2017;1:102-3.
- Yadav C, Tangri S, Yadav R. A review recent advancement in formulation of oral medicated jelly. World J Pharmacy Pharm Sci. 2018 May 5;7(7):417-26.
- Darade AD, Mundada AS. Oral medicated jellies as a emerging platform for oral drug delivery in pediatrics. World J. Pharm. Res. 2021 Apr 17;10:1628-47.
- Raja Manali M, Dhiren P. Oral medicated jelly: a recent advancement in formulation. An international journal of pharmaceutical sciences. 2016 Apr 1;7(2):13-20.
- Mondhe DS, SS A, AV B, MS T. Development & Quality Evaluation of Jelly Prepared from Guava Blended with Pomegranate. IRE J. 2018 Dec;2:44-50.
- Shirse P. Formulation and Evaluation of Oral Medicated Gelly Containing Cyclodextrin Inclusion Complexed Water Insoluble Drug–Glimipiride. IJPRD. 2011;4(4):142-53.
- Raja Manali M, Dhiren P. Oral medicated jelly: a recent advancement in formulation. An international journal of pharmaceutical sciences. 2016 Apr 1;7(2):13-20.
- Prakash K. Formulation development and evaluation of novel oral jellies of carbamazepine using pectin, guar gum, and gellan gum. Asian Journal of Pharmaceutics (AJP). 2014 Oct 18;8(4):241-9.
- Dosani MA, Sakarkar DM, Kosalge SB, Sheikh Shafiq SS. Formulation development and evaluation of unit moulded herbal semisolid jelly useful in treatment of mouth ulcer.
- Evangeline DD, Shankar RB, Kumar AB, Reddy YR. Formulation and evaluation of antimicrobial activity of medicated jelly with Ajowan extract.
- Prabhu C. Formulation Development and Evaluation of Tadalafil Oral Jelly Comparative with Marketed Product (Doctoral dissertation, Swamy Vivekanandha College of Pharmacy, Tiruchengode, Tamilnadu, India).
- Salunke T, Mayee R. Formulation and evaluation of medicated jelly of bitter drugs. Int J Pharm Innov. 2013;3(5):1-4.
- Godhwani T, Chhajed M, Chajed A, Tiwari D. Formulation development and evaluation of unit moulded semisolid jelly for oral administration as a calcium supplement. World journal of pharmaceutical research. 2012 May 6;1(3):629.
- Kapre S, Raskar G. Formulation Development Of Curcumin Loaded Solid Lipid Nanoparticulate Oral Jellies. International Journal Of Institutional Pharmacy and Life Science. 2014;4(5):77-99.


 Mansee Bhoir*
Mansee Bhoir*
 Aniket Gudur
Aniket Gudur








 10.5281/zenodo.14878511
10.5281/zenodo.14878511