Abstract
Topical drug delivery is one of the most effective ways to provide a wide range of therapeutic candidates, and emulgels are developing as a novel formulation that combines the properties of emulsions and gels. Emulgel has numerous promising features for dermatological usage, including being greaseless, readily removable, easily spreadable, emollient, non-staining, having a longer shelf life, being transparent, having an elegant appearance, and having a low risk of major adverse effects. Several investigations have shown that emulgel has enhanced penetrability and is capable of delivering both hydrophilic and hydrophobic medicines, overcoming the constraints of commonly accessible topical formulations. Emulgel is made using the inclusion method. Emulgels are commonly used to administer analgesics, anti-inflammatory drugs, antifungal medications, acne treatments, and a variety of cosmetic formulations.
Keywords
Emulgel, Pharmaceutical, Industry, skin, gelling, API, Uses
Introduction
Topical medicine. The transport system has a few advantages, including the avoidance of gastrointestinal incompatibility, metabolic degradation associated with oral administration, and the capacity to deliver the medicine more selectively to a specific location. Furthermore, topical treatments improve bioavailability by avoiding hepatic first-pass metabolism and administering over extended periods of time 1,2. Gel is a handy and popular dosage form for delivering active chemicals to their intended site of action. Because of its cross-linked and three-dimensional structure, gel absorbs tiny drug particles and promotes controlled release. The three-dimensional network is made up of macromolecules and can entrap a huge number of solvent molecules. Because of their muco-adhesive properties, gels increase the drug's contact time with the skin. The majority of pharmaceutical gels are created by dispersing hydrophilic polymers in a sufficient aqueous phase. When hydrophilic polymers dissolve in an aqueous phase, they transform into lyophilic colloids, which are self-associating and have two types of self-association (reversible and irreversible). The resulting lyophilic gels are classified as either type 1 or type 2 gels. Emulgels have recently emerged as promising delivery vehicles for both hydrophilic and lipophilic functional components and nutraceuticals, including carotenoids, vitamins, probiotics, and unsaturated fatty acids. Emulgels are used to overcome low chemical stability, low solubility, and poor absorption of food ingredients, as well as to improve the sensory textures of formulations, bioavailability, bioaccessibility, and release control. Emulsions are thermodynamically unstable biphasic dose forms made up of two immiscible liquids, with one uniformly spread as globules (internal phase) throughout the second phase (external phase). Oil-in-water (O/W) emulsions are created when hydrophobic therapeutic ingredients are included into the oil phase, facilitating the dispersion of oil globules in the aqueous phase. Moreover, emulsions can function as regulated medication delivery systems in which the drug of choice is kept in reserve inside the oil phase. An emulsion's interior oil phase will serve as a drug reservoir, releasing the drug into the skin in a regulated manner. First, there are two types of gels: hydrogels, which are hydrophobic and based on organic solvents. The first is made of a basis liquid paraffin with polyethylene or fatty oils gelled with colloidal silica, soaps made of aluminium or zinc, and the second is made of a base of water, glycerol, or propylene glycol. The drug particles in the emulsion are absorbed into the skin through the exterior phase and internal phases. According to the BCS classification systems, emulgel is a better option for class 2 drugs, which have poor solubility and are thixotropic, grease-less, easily spreadable, easily removable, emollient, non-staining, water soluble, have a long shelf life, and have a pleasing appearance that increases patient acceptability 3.
With the help of recent developments in emulgel research studies, this review seeks to provide a comprehensive overview of emulgels as a contemporary method of delivering active chemicals. This includes formulation consideration, preparation, and characterization of emulgels.
Rationale of Emulgel as a Topical Drug delivery system:
Numerous topical medications, including creams, lotions, and ointments, have a host of drawbacks. When applied, they are very sticky, which makes the patient uncomfortable. Numerous topical medications, including creams, lotions, and ointments, have a host of drawbacks. When applied, they are very sticky, which makes the patient uncomfortable. They also need to be applied vigorously and have a reduced spreading coefficient. They struggle with consistency as well
Within the principal group of semisolid preparations, all of these considerations have led to an increase in the usage of translucent gels in pharmaceutical and cosmetic preparations. Usually composed of a little amount of a gelating material, a gel is a colloid that is 99 percent liquid and is held in place by surface tension between the gel and a macromolecular network of fibres. Despite the many benefits of gels, one significant drawback is their inability to effectively transport hydrophobic pharmaceuticals. To get around this issue, an emulsion-based method is being employed, enabling the successful incorporation and distribution of hydrophobic therapeutic component through gel 3,4. Thixotropic, greaseless, readily spreadable, easily removable, emollient, non-staining, water-soluble, longer-lasting, transparent, biocompatible, and aesthetically pleasant are among the qualities of emulgels intended for dermatological usage. Dual drug release control from the formulation is possible with Emulgel 5.

Figure No.1 Structure of Emulgel
3. Drug Delivery Across The Skin:
Skin is one of the major sense organs in the human body, performing a variety of critical tasks and acting as a first line barrier. It accounts for around 10% of total body mass and covers an average area of 1.7m2. Skin is also capable of absorbing topically applied chemicals, therefore its acceptability as a suitable route for delivering a wide range of pharmacological compounds is growing. The structure of the skin allows topically applied substances to reach the various layers of skin as well as the systemic circulation. Most of the chemicals permeate the skin through three principal pathways: stratum corneum, sweat ducts, and sebaceous follicle. The epidermis is the skin's most superficial layer, made up of stratified keratinized squamous epithelium with varying thicknesses throughout the body. It is thickest on the elastic fibres. The skin creates a somewhat waterproof barrier to protect the deeper and more delicate structures. Blood vessels are widely spread beneath the skin. A continuous venous plexus that receives blood from skin capillaries is especially significant. Blood is also given to the plexus directly from the tiny arteries via highly muscular arteriovenous anastomoses in the body's most exposed parts, such as the hands, feet, and ears 6 Dermatological pharmacology is distinguished by its direct access to the skin as a target organ for diagnostic and treatment. The skin functions as a two-way barrier, preventing neither absorption nor loss of water and electrolytes. The three main methods of topical medication absorption are transcellular, intercellular, and follicular. Most medications travel through the tortuous path of corneocytes and the lipid bilayer to reach viable skin layers 7.

Figure No.2 Structure of Skin
The pilosebaceous route is the next most common (and possibly underappreciated in the clinical setting) mode of distribution. The barrier is located in the epidermis's outermost layer, the stratum corneum, as indicated by chemical penetration rates that are roughly identical across isolated stratum corneum and whole skin. For years, creams and gels rubbed into the skin have been used to give pain relief and infection-fighting medications to an affected area of the body 6. These include, among other things, gels and creams for vaginal yeast infections, topical creams for skin diseases, and creams for arthritic relief. New technologies now enable various medications to be absorbed through the skin (transdermal). These can be used to treat not only the damaged parts (for example, the skin), but the entire body (systemic).
Factors to be considered when choosing a Topical preparation:
- The effect of the vehicle is to be assessed; for example, an occlusive vehicle promotes active component penetration and efficacy. The vehicle itself may have cooling, drying, emollient, or protecting properties.
- The type of preparation should be appropriate for the type of lesions. For acute weepy dermatitis, avoid using oily ointments.
- Match the type of preparation to the place (for example, gel or lotion for hairy areas).
- Potential for irritation or hypersensitivity. Should be alerted 3.
Formulation of Emulgel:
Emulgel features such as non-toxicity, non-irritation, non-sensitization, and non- comedogenicity are mostly determined by the emulgel composition. The basic prerequisite for emulgel formulation is the proper combination of oil phase, emulsifier, and gelling ingredient. The right selection of emulgel excipients would result in optimal active ingredient release, penetration into/through biological barriers (skin or intestinal mucosa), and hence biological or pharmacological effects. The presence of an emulsion and a gel system allows for controlled release and targeted medication administration. Aside from the physiochemical properties of the active substances (molecular weight, solubility, partition coefficient, etc.) and physiological factors (biological barrier properties such as membrane thickness, pH, blood flow, etc.), formulation factors have been shown to have a significant impact on active substance release and membrane transport The particle size of a colloidal system (for example, an emulsion in an emulgel) plays an important role in determining the release profile of compounds included within the particles, with smaller sizes increasing the amount of active substances released and permeating the skin. Surfactants, which are employed to disperse and emulsify one phase into another, influence medication release not just through their effects on oil droplet size but also through the structure of the lipid particles themselves. Polymers employed as gelling agents in emulgels improve the physical stability of an emulsion by raising the viscosity of the continuous phase, resulting in a slower release of active ingredients. Furthermore, it should be mentioned that increasing drug loading in a formulation accelerates release rates, as well as the presence of penetration enhancers in emulgels 8.
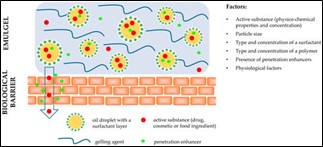
Figure No. 3 Factors affecting the release of active substances from emulgel formulation and their permeation
Formulation Considerations:
Oil Phase Selection –
The oil phase serves as a carrier for hydrophobic medicines while also determining the final product's physical qualities, such as occlusive and sensory properties. In addition, the oil phase can alter the emulsion's viscosity, permeability, and stability, as well as drug release. Oils produced from several types of plants contain therapeutic properties; these parts can be used to make an effective treatment using the newly invented Emulgel transport method. Geranium is a medicinally important oil. Geranium is used to stop bleeding, cure wounds, ulcers, and skin problems, as well to treat diarrhoea, dysentery, and colic. The oil contains insecticidal and antibacterial effects. Other medicinal plants that have been studied for their antibacterial action include Arnelia nobilis, Vitex nigundu, Buniun persicum, Acacia concinna, Albizia lebbeck, and Colla millenii. Oil Source Charactristic Preparation Uses Castor Oil Ricinus Communis Vegetable Oil Microemulsions, Ointment Antimicrobial Olive Oil Olive Seeds Vegetable Oil Emulsion, Microemulsion Anti Inflammatory Anti-Viral Birch Oil Betula Alba vegetable Oil Cream, Ointment, Gel Analgesic, Antiseptic Thyme Oil Thyme Plant Vegetable Oil Nanoemulsions, Cream Anti-Rheumati Shahin et al. (2011) conducted one such study with jojoba oil as the oil phase for Emulgel. They created an antifungal Emulgel from clotrimazole, using jojoba oil as the oil section. The objective of using jojoba oil in the oil phase was to help lessen the inflammation that is usually linked with yeast infections. Shrikhande et al. (2013) conducted a study utilising tea tree oil, lemon grass oil, ginger, and Capsicum oleoresin and concluded that formulations containing cow ghee as an excipient are appropriate products with improved pharmacokinetic and pharmacodynamic profiles.
X. W. Chen et al. (2016) Zein-based completely oil-in-glycerol emulgels supplemented with b-carotene as margarine substitutes. The study found that azein stabilised high (/ = 0.6) oil-in-glycerol (O/G) emulgels with b-carotene can be easily homogenised in a single step 9,10.
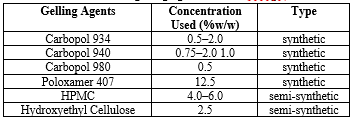
Table no. 1. List of Gelling Agent

Selection of Gelling agents:
Gelling agents used in emulgel formulation are classified as natural, synthetic, or semi-synthetic according to their origin. Natural gelling agents are extremely biocompatible and biodegradable; nonetheless, their main disadvantage is microbial destruction. The Swedish National Encyclopaedia (1989-1996) defines thixotropy as "the property of being viscous (viscid) or gel - like a product that becomes more liquid and vigorous the longer it lasts, which deforms (e.g., by stirring)." Thixotropy is usually considered as a fluid phenomenon with a reversible structure. Natural gelling agents include bio-polysaccharides and their derivatives, as well as proteins. Bio-polysaccharides include pectin, carrageenan, alginic acid, and gelatin, as well as derivatives such as xanthan gum, starch, and dextran 11. However, a variety of semi-synthetic and synthetic gelling agents are frequently utilised in emulgel formulation. Semi-synthetic gelling agents outperform natural ones in terms of stability and environmental tolerance. Methylcellulose, carboxymethylcellulose, and hydroxypropylmethylcellulose (HPMC) are examples of cellulose derivatives that are widely used in topical formulations. HPMC-based Emulgel is preferable to emulgel-based Carbopol since it demonstrated a higher drug release rate 2.
Table no. 2. List of various gelling agents used in emulgel formulations.
Table no. 2. List of various gelling agents used in emulgel formulations.
Viscosity is a crucial physical parameter for topical drug delivery systems, since it determines the consistency of any semisolid formulation. Gelling agents have a significant impact on the formulation's spreadability and viscosity, which in turn affects drug release. Daood et al. demonstrated that the viscosity of emulgels increases as the concentration of gelling agents increases.
Aqueous Phase:
Water and alcohol are the most typical aqueous ingredients used to prepare the aqueous phase 8.
Emulsifiers:
The emulsifiers are used to promote emulsification throughout the preparation process and to ensure the emulsion's physical stability during storage. Emulsifying agents are chosen based on their emulsifying ability, toxicity, and mode of delivery. Nonionic surfactants are widely utilised in biomedical formulations due to their low toxicity and compatibility with most active compounds. The most common emulsifiers used in emulgels are Tween 20 (polysorbate 20) in the aqueous phase and Span 20 (sorbitan monolaurate 20) in the oil phase. Other emulsifiers utilised in emulgel formulations include sorbitan monooleate, polyethylene glycol stearate, stearic acid, and sodium stearate 12. Proteins have amphiphilic, film-forming, and biocompatibility qualities, hence they are commonly utilised as emulsifiers in the food sector. Proteins stabilise emulsions by adsorbing on the oil-water interface, resulting in lower interfacial tension, viscoelastic interface layer, and the previously stated surfactants.
When globular proteins like ?-lactoglobulin adsorb at an interface, their hydrophilic groups remain in the aqueous phase and their hydrophobic groups face the oil phase. Thus, the interfacial layer generated by globular proteins is more compact and resistant to deformation, resulting in high viscoelasticity 13.

Figure 4. Configuration of conventional and polymeric emulsifiers at oil/water interfaces and the ‘train-loop-tail’ model of adsorption of flexible proteins
Penetration Enhancers:
Penetration enhancers are substances that improve medication penetration through the skin. These compounds promote drug absorption by a variety of ways, including briefly breaking the skin barrier, shifting drug partitioning into skin structures, fluidizing lipid channels between corneocytes, and so on. Oleic acid, clove oil, and menthol are popular penetration enhancers used in emulgels used for topical drug administration.
- Penetration enhancer properties include non-toxicity, non-irritation, and non-allergy, as well as pharmacological inactivity. Therefore, they should not bind to receptor .
- Drugs should have predictable activity and duration, prevent loss of endogenous material, be compatible with excipients and active ingredients, and be cosmetically acceptable and suitable for the skin 1,14.
Table No. 3 List of penetration Enhancers

pH Adjustment :
pH adjustment The pH of the emulgel formulation, like all topical formulations, should be compatible with skin pH to avoid skin irritation during application. The pH of adult skin ranges from 4.1 to 5.8, and topical skin care products are adjusted to pH 5.4 or 5.5 to preserve the "physiological" skin pH, however this might vary depending on their purpose. 15 TEA (triethanolamine) is typically utilised to regulate pH in emulgel compositions since carbopol is widely employed as a thickening, among other reasons. TEA is required to uncoil the polymer chain of Carbopol and generate a gel structure 16.
Preservatives :
These are chemicals that prevent the formulation from spoiling by inhibiting or slowing microbial growth. The ideal preservative should be active against the majority of germs even at low concentrations, although this is not always true. Parabens are more efficient against fungi than bacteria, and antibacterial activity is lower against Gram-negative bacteria than against Gram-positive pathogens 17. The most commonly used preservatives include methylparaben, propylparaben, benzalkonium chloride, benzoic acid, and benzyl alcohol. Some gelling agents are incompatible with specific preservatives; for example, methylcellulose reacts with methylparaben, propylparaben, butylparaben, and cetylpyridinium chloride. As a result, we must carefully select appropriate preservatives.
Humectants:
Humectants are commonly employed in emulgel formulations to reduce moisture loss while also improving formulation properties such as ease of application and consistency. Glycerol and propylene glycol are the most often utilised humectants in topical formulations 15.
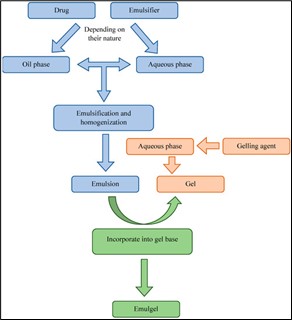
Figure no. 5 Steps in the process of emulgel preparation.
Preparation Processes of Emulgels:
STEP 1: Emulsion formulation (o/w or w/o)
STEP 2: Formulation of Gel Base
STEP 3: Incorporating emulsion into gel
Base with continuous stirring 9 .
Types of Emulgel :
Emulgels are classified into two categories based on the emulsion in their composition: oil-in-water (O/W) and water-in-oil (W/O) emulgels. Both varieties are frequently employed in the pharmaceutical business as vehicles for administering medications into the skin.
Emulgel can be classified as a macroemulsion gel, nanoemulgel, or microemulsion gel based on the particle size and dispersion of the emulsion droplets
Macroemulsion Gel:
The most prevalent emulgels are macroemulsion gels. The particle size of droplets is often greater than 400 nm, resulting in emulgel opacity and the ability to observe individual droplets under an optical microscope. Macroemulsions, like other emulsions, are thermodynamically unstable systems that can be stabilised by surface-active chemicals
Nanoemulgel:
When a nanoemulsion is mixed with a gel, nanoemulgels form. In comparison to other nanolipidal delivery techniques, nanoemulsion is one of the most effective for lipophilic and low bioavailable medicines. Nanoemulsions are optically transparent or translucent, isotropic structures. Nanoemulsions have various advantages, including improved physical stability, a high drug-loading capacity for both lipophilic and hydrophilic medicines, and greater solubility enhancement capacity 18. Nano emulsion formulations have superior transdermal and dermal transport capabilities in vitro and in vivo as compared to traditional topical formulations.
Nanoemulgel demonstrated higher drug penetration and greater transdermal flux as compared to both traditional emulgel and gel 19.
Microemulsion Gel:
Microemulsions are typically defined as thermodynamically stable, transparent, and isotropic systems with droplet sizes ranging from 10-100 nm and composed of water, oil, and a surfactant, usually in conjunction with a cosurfactant. Microemulsions feature physical properties such as transparency, low viscosity, and small particle size, and unlike ordinary emulsions, they can develop spontaneously. Furthermore, micro emulsions do not exhibit phase separation over a broad temperature range.
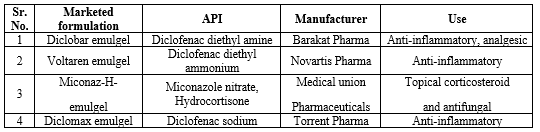
Table No. 4 Marketed formulation of emulgel
Advantages of Emulgel Formulations :
- The first and most basic advantage of emulgels is the ability to incorporate hydrophobic medicines into the emulsion's oil phase. The produced emulsion is simply combined with the gel basis. This way, the problem of low solubility of hydrophobic medications can be overcome, and their administration and penetration through the skin can be accomplished.
- Ajazuddin et al. have emphasised the advantage of emulgels in drug release over commonly used topical preparations such as ointments, creams, lotions, and pastes, which contain a high number of oil excipients that give emollient characteristics but slow drug release.
- Emulgel provides controlled release and targeted drug administration via dual controlled release mechanisms due to the inclusion of an emulsion and gel system. As a result, emulgel can prolong the effect of medications by regulating their release from the formulation, allowing for a longer period of action.
- Emulgels offer features such as good spreadability, greaselessness, thixotropy, and a long shelf life, and they are more comfortable to use than ointments and creams, which have poorer spreadability, stickiness, and require rubbing to apply.
- Emulgels, when properly prepared, often have good physical stability and low interfacial tension, which is obtained by adding an appropriate emulsifier, resulting in a prolonged shelf life.
- Emulgels have a higher loading capacity than other innovative drug delivery methods such as niosomes and liposomes, which may result in leakage and reduced trapping efficiency due to their vesicular structure.
- The final and equally essential feature of emulgels is their production feasibility and low preparation cost, which include simple and short processing processes. Emulgel manufacture does not require the use of highly specialised instruments 20–22.
Disadvantages:
- Poor macromolecule absorption.
- Bubbles are trapped during formulation
- Hydrophobic medicines are the optimal choice for these delivery systems.
- Emulgel might irritate contact dermatitis and trigger allergic reactions.
- Topical drug delivery systems have poor permeability due to the skin's thick and complicated structure, resulting in limited absorption of big particles 21.
CONCLUSIONS:
Emulgels are unique, modern drug delivery systems that combine the benefits of emulsions with gels, and as a result, they have piqued scientists' interest during the last decade. Emulsion gels, with their customisable appealing features and distinctive characteristics, have been shown to be effective and convenient delivery vehicles for a variety of bioactive compounds. The benefits of emulgels make them intriguing formulations for targeted medication administration with controlled release, as they can encapsulate and protect diverse pharmacological, cosmetic, and food constituents when administered topically or orally. The use of proteins and other natural elements in emulgel formulations is currently on the rise due to public health concerns about the increased substitution of synthetic substances with natural ones. The usage of emulgels is constantly rising in the pharmaceutical, cosmetic, and food industries, with considerable potential for future advancement.
REFERENCES :
- Devi Suman, Sangeeta, & Kumari Beena. Emugel for topical drug delivery: A novel approach. GSC Biol. Pharm. Sci. 11, 104–114 (2020).
- Lilhare, K. T., Borkar, S. S. & R. Baheti, J. Recent Update on Topical Drug Delivery Systems: Emulgel. Asian J. Pharm. Res. Dev. 11, 133–138 (2023).
- Netam, R. & Mishra, M. A. Recent Advancement in Emulgel: An Updated Review. 7, (2022).
- Jones, D., Woolfson, D. & Brown, A. F. Textural, viscoelastic and mucoadhesive properties of pharmaceutical gels composed of cellulose polymers. Int. J. Pharm. 151, 223–233 (1997).
- Bhaskar, D. R., Ola, D. M., Suroshe, Y. & Gaware, S. Review on: - Emulgel: A Emerging Trend in Topical Drug Delivery System. 7, (2022).
- Ahmed, S., Verma, S., Khan, S. & Sharma, A. Emulgel: A revolution in topical drug delivery system. Int. J. Health Sci. (2022) doi:10.53730/ijhs.v6nS4.10902.
- Arora, R., Khan, R., Ojha, A., Upadhyaya, K. & Chopra, H. EMULGEL: A NOVEL APPROACH FOR HYDROPHOBIC DRUGS. Int. J. Pharm. Biol. Sci.
- Milutinov, J., Krstonoši?, V., ?irin, D. & Pavlovi?, N. Emulgels: Promising Carrier Systems for Food Ingredients and Drugs. Polymers 15, 2302 (2023).
- Jain, A., Gautam, S., Gupta, Y., Khambete, H. & Jain, S. Development and characterization of ketoconazole emulgel for topical drug delivery. Pharm. Sin. 1, 221–231 (2010).
- Sriaandhal Sabalingam & Malitha Aravinda Siriwardhene. A review on emerging applications of emulgel as topical drug delivery system. World J. Adv. Res. Rev. 13, 452–463 (2022).
- Donthi, M. R. et al. Nanoemulgel: A Novel Nano Carrier as a Tool for Topical Drug Delivery. Pharmaceutics 15, 164 (2023).
- Research scholar, Amity Institute of Pharmacy, Amity University, Noida (U.P), India 201303, Singh, S., Singh, I., & Assistant Professor, Amity Institute of Pharmacy, Amity University, Noida (U.P), India 201303. EVOLVING IMPLEMENTATION OF EMULGEL AS A TOPICAL DRUG DELIVERY SYSTEM: A SYSTEMATIC REVIEW. Curr. Res. Pharm. Sci. 12, 121–131 (2022).
- Biviano, M. D., Böni, L. J., Berry, J. D., Fischer, P. & Dagastine, R. R. Viscoelastic characterization of the crosslinking of ?-lactoglobulin on emulsion drops via microcapsule compression and interfacial dilational and shear rheology. J. Colloid Interface Sci. 583, 404–413 (2021).
- Siddhant Yadav, S. W. Topical Emulgel Of Tolnaftate With Penetration Enhancer: Development, Characterisation And Antifungal Activity. (2017) doi:10.5281/ZENODO.1038500.
- Emulgel: A Boon for Enhanced Topical Drug Delivery | Journal of Young Pharmacists. https://jyoungpharm.org/article/1531 (2021).
- Muskan Kankane, Vijay Nigam, Shailendra Modi, Sanjay Jain, & Pooja Adhikari. Emulgel: A dual release system for hydrophobic drug delivery. World J. Biol. Pharm. Health Sci. 12, 335–347 (2022).
- Sasseville, D. Hypersensitivity to preservatives. Dermatol. Ther. 17, 251–263 (2004).
- Ali, A., Ansari, V. A., Ahmad, U., Akhtar, J. & Jahan, A. Nanoemulsion: An Advanced Vehicle For Efficient Drug Delivery. Drug Res. 67, 617–631 (2017).
- Anand, K. et al. Nano-emulgel: Emerging as a Smarter Topical Lipidic Emulsion-based Nanocarrier for Skin Healthcare Applications. Recent Patents Anti-Infect. Drug Disc. 14, 16–35 (2019).
- FORMULATION, DEVELOPMENT AND EVALUATION OF TOPICAL EMULGEL OF GRISEOFULVIN Citefactor.org-Journal|Research Paper|Indexing|Impact factor. https://www.citefactor.org/article/index/29562/formulation-development-and-evaluation-of-topical-emulgel-of-griseofulvin.
- Navneet Kumar Verma. Emulgel: A Recent Technique For Topical Drug Delivery- A Review. (2023) doi:10.5281/ZENODO.7593756.
- Prabhakara, P. et al. Preparation and evaluation of Transdermal patches of Papaverine hydrochloride. Int. J. Res. Pharm. Sci. 1, 259–266 (2010)


 Saleha Firoz Tamboli *
Saleha Firoz Tamboli *









 10.5281/zenodo.11550736
10.5281/zenodo.11550736