Abstract
Transdermal drug delivery system (TDDS) is a novel strategy in pharmaceutical research that provides a patient-friendly, non-invasive way to deliver therapeutic drugs. This abstract offers a summary of the most important developments in transdermal drug delivery technology, emphasizing their possible effects on healthcare. Nanotechnology has played a pivotal role, enabling the development of novel drug carriers that enhance permeation through the skin barrier. These nanocarriers, such as liposomes and polymeric nanoparticles, facilitate the controlled release of drugs, improving therapeutic efficacy while minimizing side effects. Moreover, the integration of smart materials and microfabrication techniques has led to the creation of responsive transdermal patches. These patches can actively modulate drug release in response to physiological cues, ensuring optimal therapeutic levels and personalized treatment regimens.The abstract also explores the challenges associated with TDDS, including skin permeability variability among individuals and the need for standardized testing methods. Strategies to address these challenges, such as predictive modeling and personalized medicine approaches, are discussed and recent strides in transdermal drug delivery systems, showcase the potential of these innovations to revolutionize drug administration, improve patient compliance, and enhance therapeutic outcomes. The ongoing research in this field holds promise for the development of next-generation TDDS with broader clinical applications.
Keywords
TDDS, Novel Drug Delivery System, Skin permeation
Introduction
Anatomy of skin
Epidermis:
squamous, stratified epithelium that keratinizes. The majority of cells (>90%) are made up of keratinocytes, which are also in charge of the development of barrier function. As keratinocytes migrate to the outer layer of the skin, they undergo physical features such as form and size changes. Under a microscope, the epidermis is further separated into five anatomical layers, each measuring between 100 and 150 micrometers thick. The outermost layer of the epidermis is called the stratum corneum (SC), moreover, it is exposed to the outside world. The nature of this layer makes it the most crucial for transdermal distribution since it keeps water in the body and foreign things out. Large, flat, polyhedral, plate-like envelopes that are packed with keratin and derived from dead cells that have moved up from the stratum granulosum are known as stratum granulosum (SC).The SC is made up of 10–15 layers of corneocytes, and when it's dry, its thickness is between 10–15 ?m and when it's wet, 40 ?m.
Dermis:
The large microvasculature network structures found in the dermis include hair follicles, sweat glands, and smaller blood vessels. Consequently, for a medicine to be delivered through the skin, it must cross the epidermis and enter the dermis, where it can be absorbed by capillaries and enter the circulatory system. Connective tissue constitutes the bulk of the inner and bigger (90%) skin layer, which supports the skin's epidermal layer. The dermal-epidermal junction, which separates the dermis from the epidermis layer, acts as a physical barrier to keep big drug molecules and cells out. It includes nerve terminals, blood, and lymphatic vesicles. The two anatomical regions of the dermis are the reticular dermis and the papillary dermis. The thinner outermost layer of the dermis is called the papillary. In the papillary region, collagen and elastin fibers are primarily vertically orientated and attached to the dermal-epidermal junction.
Hypodermis:
The third layer below the dermis is called the subcutaneous layer, or hypodermis in histology. Huge amounts of fat cells make up the elastic subcutaneous layer, which acts as a shock absorber for blood arteries and nerve endings. This layer ranges in thickness from 4 to 9 mm on average. The exact thickness, however, varies from one individual to another and is dependent upon the specific body area. A molecule comes into contact with sebum, natural microbial flora, cellular detritus, and other things when it reaches undamaged skin.[1-4]

Figure 1: Anatomy Of The Skin
Routes of skin penetration
Transcellular transport is the primary mode of transport for compounds soluble in water. It involves the organization of lipids in the stratum corneum and transit through the cytoplasm of corneocytes. Lipid soluble compounds are transported through an intercellular channel that appears to involve the endogenous lipid in the stratum corneum. The trans-epidermal route, as illustrated below, refers to the combination of the transcellular and intercellular pathways.
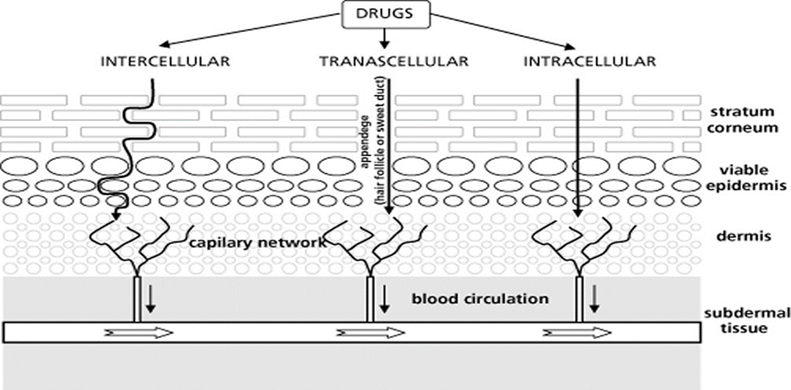
Figure 2: Skin Penetration Via Different Route
Solute molecules can enter the skin by the sweat duct, sebaceous glands, or hair follicles. The terms "shunt" and "appendageal route" refer to these passageways collectively. It is widely acknowledged that the appendages of the skin account for roughly 0.1% of the fractional area available for drug absorption. Therefore, rather than concentrating on the appendages, the key goal is to create penetration strategies through the stratum corneum. The dead cells of the SC are the primary obstacles to absorption because they limit the passage of pharmacological molecules both inward and outward and have a high electrical resistance. The SC is made up of flattened keratinized cells and is a heterogeneous tissue. Compared to the cells next to the underlying granular layer, these cells' outer layers are less densely packed. As a result, the epidermal barrier becomes increasingly impermeable in the bottom half. This finding has given rise to theories that a further barrier, known as the SC, exists at this level. Therefore, the SC serves as the rate-limiting barrier, or the tissue that offers the greatest resistance to the passage of molecules, as they enter the skin from the environment. Transdermal permeation or percutaneous absorption can be seen as a combination of several processes after the dose form is administered topically.
- Adsorption of a penetrating chemical onto the SC surface layers.
- Diffusion via the viable epidermis and SC.
Percutaneous Absorption
It can be broken down into three parts: the gradual process by which chemicals permeate different layers of skin and then cross the skin to enter systemic circulations.
- A substance's entry into a certain layer is known as penetration.
- Permeation:
The process of moving from one layer into another; this layer differs from the first layer in terms of both structure and function.
- Absorption:
The process by which a material enters the bloodstream.[5-7]
TDDS
Human societies have been applying substances to the skin as cosmetic and medical agents for thousands of years. However, the utilization of the skin as a medicine delivery system did not begin until the 20th century. A transdermal patch or skin patch is another name About transdermal medication delivery system, which is a medical device that delivers a prescribed dosage of medication into the bloodstream. This sticky patch is medicated. When delivering therapeutic substances through the human skin for systemic effects, it is important to take into account the morphological, biophysical, and physicochemical features of the skin. The first transdermal patch was scopolamine, which received FDA approval in 1981. Scopolamine (TransdermScop ALZA Corp) and nitroglycerine (Transderm Nitro) are transdermal delivery mechanisms that are employed to reduce motion sickness and angina pectoris related to coronary artery disease, respectively. Patients benefit therapeutically from products that deliver drugs transdermally. More than 35 transdermal medication delivery solutions and about 16 active components have received approval for usage internationally and for sale in the United States, respectively. According to statistical study, the market was valued at $12.7 billion in 2005 and $21.5 billion in 2015. It is expected to reach $31.5 billion by the year 2015.
The use of patches to the skin replaces the necessity of using pumps or syringes to gain vascular access. A variety of patches are currently available for medications, including clonidine, fentanyl, lidocaine, nicotine, nitroglycerin, oestradiol, oxybutynin, scopolamine, and testosterone. In addition, combination patches for hormone replacement therapy and contraception are available. The duration of the patches varies from one to seven days, contingent on the medication. Transdermal drug delivery systems, or "patches," are topically applied medications that are intended to distribute a therapeutically effective dose of a medication at a regulated pace across the patient's skin for a systemic effect. The main barrier to topical medication distribution is the limited rate at which medications diffuse through the stratum corneum, the skin's outermost, somewhat impervious layer.Furthermore, the primary route for lipophilic medicines, the interstitial lipid area, has a diffusion path length of around 500 mm, which is significantly greater than the stratum corneum's (20 mm) thickness.
Table.01 : Below Table Describes Approved Drug In History With The Therapeutic Use, Manufacturing Name And Industry Production.
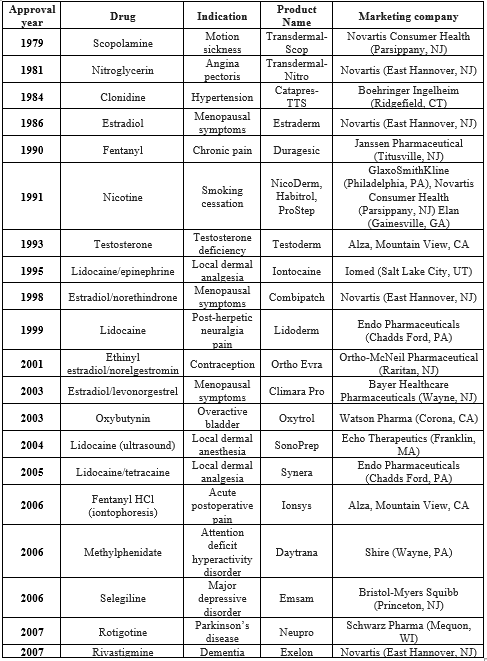
* The FDA-approved transdermal patches and delivery methods are included in this list. The first authorized product using a certain delivery mechanism to administer a specific medicine or drug combination is the only one displayed. Not included are topical creams, ointments, gels, or sprays. Very few medication candidates have been licensed for transdermal distribution, despite the benefits and interests in this drug delivery method. The primary hurdle to this mode of delivery, aside from the skin toxicity of the medication or drug excipients, is the skin's barrier properties, which restrict the amount of molecules that can penetrate it to just those that satisfy specific requirements. These compounds must to have certain physicochemical characteristics, like a low melting point (less than 10 mg). Few medications fit these requirements.
As a result, numerous methods have been developed in an effort to get beyond the barrier qualities and administer the majority of medications via the skin. They consist of both the physical and chemical methods of augmentation. Supersaturated systems, pro-drugs, colloidal formulations, and penetration enhancers are examples of the former tactic's chemical techniques. The latter tactic uses physical techniques like electroporation and phonophoresis, among others. Therefore, more studies have been conducted recently to examine the impact of various chemical, physical, or combination of both enhancers on the skin permeability of most commonly used medications, particularly those that already have issues with their current route of administration. There are various methods for doing percutaneous research, such as in vitro and in vivo penetration experiments.[8-12]
THE TRANSDERMAL DRUG DELIVERY SYSTEM’S (TDDS) ADVANTAGES
The subsequent items are the benefits of transdermal distribution versus alternative delivery methods:
- Steer clear of medications that undergo first pass metabolism.
- Lower medication plasma concentration levels with fewer adverse effects.
- Utilizing medication candidates with short half-lives and low therapeutic indices to lessen variations in drug plasma levels.
- Simple drug delivery removal in the event of toxicity.
- Lowering the frequency of doses and improving patient adherence.
- Over an extended period of time, transdermal drugs provide a consistent infusion of a substance. It is also possible to prevent side effects or treatment failure, which are often linked to sporadic dosing.
- Transdermal administration can improve many medications' therapeutic usefulness by preventing certain side effects. For instance, the "hepatic first pass" impact might cause GI discomfort, decreased absorption, and breakdown.
- Because of the aforementioned benefit, transdermal drug input may be able to elicit a comparable therapeutic effect with a lower daily dose of the drug than would be required, for example, if the drug were administered orally.
- Less variation between and within patients and increased patient compliance are the results of the streamlined drug schedule.[13-15]
DISADVANTAGES OF TDDS
The following are transdermal delivery techniques' drawbacks:
- Medication that needs high blood levels cannot be given; only strong molecules, those needing a daily dosage of 10 mg or less, can be used.
- Transdermal administration is often intended to provide steady, sustained drug delivery; it is not a method for achieving quick bolus-type drug input.
- Sufficient solubility of the medication in aqueous and lipophilic conditions to penetrate cutaneous microcirculation and make their way into the circulatory system..
- The drug's molecular size should be appropriate for transdermal absorption. When it comes to this route of administration, tolerance-inducing drugs are not a wise choice unless a suitable wash-out period is scheduled in between the dose schedule.
- The medication finds it difficult to get through human skin because of the skin's barrier function.
- Another significant drawback is dermatitis or skin irritation brought on by the enhancers and excipients of the medication administration mechanism employed to boost percutaneous absorption.
- Not all skin types will adhere to adhesive properly.
- Inconvenient to wear.
- Might not be cost-effective.[16-18]
FACTORS AFFECTING TRANSDERMAL DRUG DELIEVERY SYSTEM
Skin condition
The skin's barrier function is maintained by the intact layer, however numerous substances, like acids and alkali, can pass through the skin's barrier cells. Many solvents break down the lipid fraction and create artificial shunts that allow drug molecules to flow through, opening up the intricate, dense structure of the horny layer. Examples of these solvents are methanol and chloroform.
Skin age
It is observed that younger and adult skin is more porous than older skin. However, there isn't a noticeable change. Children's larger surface area per unit body weight has harmful effects. As a result, strong steroids, hexachlorophene, and boric acid have caused serious side effects. The intact skin itself acts as a barrier, but many agents like acids and alkali cross the barrier cells and penetrate through the skin. Many solvents open the complex dense structure of the horny layer: solvents like methanol and chloroform remove the lipid fraction, forming artificial shunts through which drug molecules can pass easily.
PHYSICOCHEMICAL FACTOR
Hydration of skin
Water saturation of the skin generally causes tissues to swell, wrinkles to soften, and an increase in The skin's permeability to allow medication molecules to pass through.
Temperature and PH of the skin
Adequate clothing on the body minimizes wide changes in temperature and penetration rates. The diffusion coefficient lowers as the temperature drops and the penetration rate fluctuates with temperature. Only unionized molecules are capable of penetrating the lipid membrane with ease, and weak acids and bases exhibit varying degrees of dissociation based on their pKa and pH or pKb values. The effective membrane gradient, which is directly correlated with pH, will therefore depend on the focus of unionized prescription drugs in the applied phase.
ENVIRONMENTAL FACTOR
Sunlight
Sunlight causes blood vessel walls to thin, which results in bruises with just slight stress in the sunexposed areas. Additionally, a sun lentigo or freckle is pigmentation, the most obvious pigment alteration brought on by the sun.
Cold season
Dry, itchy skin is a common side effect of the winter season. In response, the skin produces more oil to counteract the drying effects of the weather. Using a quality moisturizer can help reduce dry skin complaints. Additionally, consuming a lot of water helps maintain moisturized, glowing skin.
Air pollution
Dust can alter the way drugs are delivered via the skin by clogging pores and increasing germs on the countenance and skin's surface, which both can result in spots or acne. The natural oils the skin naturally produces to retain moisture and maintain its suppleness can be degraded by imperceptible chemical contaminants in the air, interfering with the skin's defense mechanism.[19-20]
IDEAL REQUIREMENTS FOR TDDS:
- The shelf life is two years.
- The patch is small in size (less than 40 cm2) and has a convenient dosing frequency (one dose per day to one dose per week).
- Cosmetically acceptable—that is, a transparent, white hue.
- Easy packaging, meaning the bare minimum of pouches and steps needed to implement the method Simple release liner removal (i.e., for patients who are elderly or toddlers)
- Sufficient skin adherence, so there won't be any falls off between doses and removal will be simple and painless.
- No leftover material, such as ?cold flow? around the patch's perimeter when it's being stored, applied to skin, or removed.
- No undesirable skin reactions, such as erythema,Itching, burning, stinging, skin sensitization, phototoxicity, and contact dermatitis, etc.
- Uniform biopharmaceutical performance, meaning the ability to precisely measure the necessary pharmacokinetic and pharmaco-dynamic response in the same individual throughout time, as well as in different individuals.
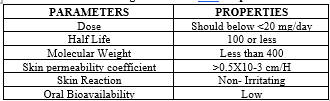
DRUG CRITIRIA OF TDDS
For developing a TDDS the drug was to be chosen with great the following are some of the desirable prop of a drug suitable for transdermal delivery.[21-22]
Table 02: Drug Parameters And Properties
THE DEVELOPMENT OF TRANSDERMAL DRUG DELIVERY TECHNOLOGY SYSTEM:
The technologies may be categorized using four fundamental methods.
- Polymer membrane partition controlled TDDS
- Polymer matrix diffusion controlled TDDS
- Drug reservoir gradient controlled TDDS
- Micro reservoir dissolution controlled TDDS
MEMBRANE PERMEATION – CONTROLLED SYSTEMS
This kind of system completely encloses the supply of drugs in a shallow compartment made of a metallic laminate made of plastic that is impervious to drugs and a rate-controlling polymeric membrane, such as vinyl acetate ethylene (EVA) copolymer, which has a specific medication permeability characteristic. An illustration of this system's cross section. The rate-controlling membrane is the only barrier that allows The medication molecules to escape. The drug particles are either suspended in a thick, insoluble liquid medium, like silicone liquid to create a pastelike suspension, or they are disseminated in a solid polymer matrix inside the drug storage tank compartment. To create a close Interaction of the transdermal system with the skin surface, a thin coating of drug-compatible, hypoallergenic adhesive polymer, such as silicone or polyacrylate glue, can be placed to the rate-regulating membrane's exterior surface. By adjusting the adhesive's thickness, permeability coefficient, and polymer composition, the ratelimiting membrane's and the administration of drugs system's rate can be customized. The main benefit within the membrane permeability controlled transdermal interface is the drug's consistent release rate. There is, however, an uncommon possibility that a dosage dumping or quick release of the full drug content could occur from an inadvertent rupture of the ratecontrolling membrane.[23]

Figure 3: controlled membrane permeation system
ADHESIVE DISPERSION TYPE SYSTEMS
The controlled membrane permeation system is represented by this streamlined version. In order to create a thin drug reservoir layer, The medication is first dispersed directly in an adhesive polymer, such as poly (isobutylene) or poly (acrylate) adhesive. The medicated adhesive is then spread via hot melt or solvent casting onto the a single sheet of drug-impermeable backing made of metallic plastic. To create an adhesive diffusion-controlled delivery system, slender layers of nonmedicated, rate-controlling adhesive polymer with a certain permeability and consistent thickness are put above the medication reservoir layer.[24]
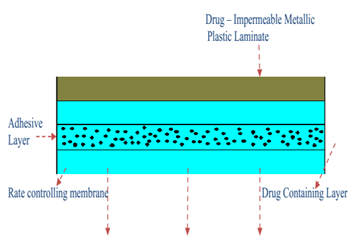
Figure 4:system of adhesive dispersion type
SYSTEMS WITH MATRIX DIFFUSION CONTROL
Using this method, drug particles are uniformly dispersed within a matrix of hydrophilic or lipophilic polymers to create the drug reservoir. After that, a medicated polymer with a regulated thickness and A certain section of surface is shaped into the shape of a medicated disc. One of two methods can be used to disperse the drug particles in the polymer matrix: either mix the finely ground drug particles uniformly with a liquid polymer or a very viscous base polymer, then cross link the polymer chains; or mix the drug solids uniformly with a rubbery polymer at a high temperature. Another method for creating the medication storage is to dissolve the medication and polymer in a common solvent, then evaporate the solvent in a mold either under vacuum or at a high temperature.After pasting the drug reservoir-containing polymer disc onto an occlusive polymer and spreading it around the perimeter, an adhesive rim strip is formed around the medicated disc.[25]

Figure 5: Matrix Diffusion-Controlled System.
MICRO-RESERVOIR TYPE OR MICRO-SEALED DISSOLUTION CONTROLLED SYSTEMS:
This can be viewed as a hybrid drug delivery device that combines matrix diffusion with reservoir technology. Here, The supply of drugs is created by distributing the medication suspension uniformly within a lipophilic polymer, such as silicone elastomers, using a high energy dispersion technique. This creates a number of distinct, unleachable tiny spheres that serve as drug repositories. First, The medication-solidified materials are suspended in a water-based aqueous solution soluble liquid polymer. By instantly cross-linking the in situ polymer chains, the Dispersion that is thermodynamically unstable happens fast stabilized, yielding a An polymer disk with a fixed thickness and constant surface area. A coating of biocompatible polymer can be added to the device in order to change the mechanism and rate of medication release, depending on the physiochemical property of the medication and the intended speed at which drugs are released. By centering the disk with medication and encircling it using a glue rim, a trans-dermal therapeutic system is created.[26-27]
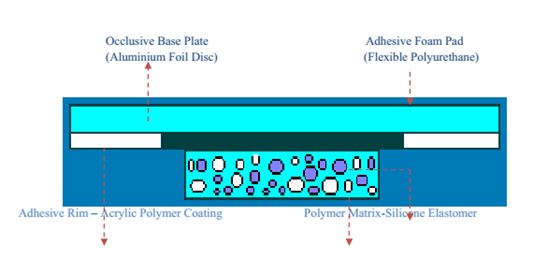
Figure 6: Micro-Reservoir Type Controlled System
TYPE OF TRANSDERMAL DRUG SUPPLER
- Single layer medication in an adhesive:
This kind has the prescription drug implanted in the sticky layer. The medicine is released via the skin via the adhesive layer, which also acts as a glue to hold the other layers together. There is a backer and a temporary liner all around the adhesive layer.
- Multi -layer drug in adhesive:
This kind is comparable to the single layer kind as well, but in addition to the adhesive layer, it has two additional layers: one for controlled release and the other for immediate medication release. The medicine is released due to the action of the sticky stratum. This patch also features an ongoing support and a transient liner-layer.
- Vapour patch:
The coating of glue in this kind of patch has two functions: it releases vapour and holds the different layers together. Vapour patches, which are relatively fresh on the market, are frequently used to release the essential oils to relieve congestion. There are numerous other kinds of vapor patches on the market that are intended to lessen the symptoms associated with cigarette smoking and enhance the quality of sleep.
- Reservoir system
This system embeds the drug reservoir between a rate-controlling membrane and an impermeable backing layer. Only the rate-regulating membrane—which might be or might not be microporous—allows the medication to release. The medication may take the shape of a gel, suspension, solution, or disseminated across a strong matrix of polymers within the supply of drugs compartment. A drug-compatible polymeric membrane on the outside made of The hypoallergenic polymer adhesive can be used.
- Matrix system:
- Drug-in-adhesive system:
This kind of framework creates a drug reservoir through drug distribution into a glue polymer, which is subsequently put on an impermeable backing layer by melting or solvent casting (if hot-melt adhesives).Unmediated sticky Layers of polymers are put to the reservoir's top for protection.
- Matrix-dispersion system:
In this kind, the medication is uniformly distributed within a matrix made of lipophilic or hydrophilic polymers. This medication-containing A polymer disk is installed in a compartment made of an impermeable medication backing layer, fixed to an absorbing foundation plate. To create a sticky rim strip, the adhesive is not applied on the drug reservoir's face; rather, it is dispersed around its circumference.
f. Micro reservoir system:
This kind of medication administration mechanism combines a matrix-dispersion mechanism with a reservoir. For To create many thousands of impenetrable, little spheres of medication reservoirs, first suspended in a watery mixture of a water-soluble polymer and then uniformly spread over a lipophilic polymer. Cross-linking the polymer in situ right away using cross-linking agents stabilizes This unstable thermodynamic dispersion.[28-34]
DIVERSE APPROACHES TO PREPARATION TDDS:
Unbalanced TPX membrane technique:
A heat-sealable film made of polyester (type 1009, 3m) with a 1cm diameter concave can be utilized to manufacture a prototype patch. This film will serve as the membrane that supports. The A drug sample is injected via the concave membrane, sealed with an adhesive, was enveloped in an asymmetric TPX {poly(4-methyl-1-pentene)} membrane.
[(Preparing an Asymmetric TPX membrane):
The wet/dry inversion method is accustomed to create them. To create a polymer solution, TPX has been eliminated. at 60°C in a combination of nonsolvent ingredients and solvent (cyclohexane). Using a Gardner knife, The solution of the polymer is cast to a predetermined thickness atop a glass dish after being maintained for 24 hours at 40°C . Following a 30-second The casting film vanishing at 50°C, the plate made of glass must be submerged right away in a coagulation bath at a temperature maintained at 25°C. Ten minutes of soaking is enough time to remove the membrane. and allowed to air dry for 12 hours at 50°C in a flow oven.
Teflon mold process in a circle:
A solvent that is organic is used to dissolve solutions that include polymers in different ratios. Half as much of the similar organic solvent is used to dissolve the computed quantity of medication. The second To dissolve, half of the organic solvent is utilized. enhancers at varying concentrations before adding them. Regarding the medication polymer solution, di-N-butyl phthalate is added as a plasticizer. After 12 hours of stirring, the entire mixture should be put into a Teflon circle mold. To regulate solvent vaporization in a model of laminar flow hood with 0.5 m/s of air velocity, the molds must be set placed level and covered with an inverse funnel. For a whole day, The evaporator is left to evaporate. Before evaluation, the dried films must be held for a further twenty-four hours kept in a desiccator at 25±0.5°C filled with silica gel to prevent aging effects. Within a week following their preparation, the kinds of movies have to be assessed.
Mercury Substratum Technique:
This approach dissolves the medication inside a polymer mixture with the plasticizer. To create a uniform dispersion, the aforementioned solution should be mixed for ten to fifteen minutes. Then, It ought to be poured over a leveled mercury surface, then covered with a funnel that is upside down to prevent evaporation of solvent.
Through the use of “IPM membranes” technique:
Using a magnetic stirrer, the medication dissolves in a solution of propylene glycol and water that contains carbomer 940 polymer, and the mixture is then shaken for a duration of 12 hours. Triethanolamine is going to be included in the dispersion To be able to neutralize it and finish it viscous. If the medication is soluble in aqueous solution is extremely low, solution gel can be created using pH 7.4 buffer. The membrane of the IPM will incorporate The gel that's produced.
Through the use of “EVAC membranes” technique:
EVACC (ethylene vinyl acetate copolymer) with polyethylene (PE) membranes, and 1% reservoir gel of carbopol can all be utilized as rate-regulating membranes to get ready for goal transdermal medication system. Gel is made with propylene glycol Should the medication is insoluble in water. The usage of ropylene glycol is dissolve the drug. Carbopol resin is then added to the mixture and neutralized using asodium hydroxide solution, 5% w/w. The medication (in gel form) is applied to a sheet of backing layer covering the designated area. To create a leak-proof device, a rate-regulating membrane will be positioned on top of the gel, with the edges being heated to seal.
Aluminium backed sticky film technique:
Should the loading dosage for There is more than 10 mg in a transdermal medication delivery device., unstable matrices may be produced. The adhesive film approach with aluminum backing is appropriate. Since the majority of medications and adhesives are contained in chloroform, It is the preferred solvent for preparing the same. Adhesive substance is added and dissolved in the medication solution once The medication dissolves in chloroform. One uses aluminum foil to line a specially constructed aluminum former, and cork blocks that fit firmly are used to blank off the ends. TDDS preparation with proliposomes:The film deposition approach is employed in the carrier method to get ready for the pro-liposomes. An optimal dosage of lecthin in a 0.1:2.0 ratio can be obtained from the previous reference medication. To create the pro-liposomes, Powdered mannitol, 5 mg is added to a 100 milliliter round-bottom flask that is held at 60–70°C. The flask is in then swirled at 80–90 rpm and vacuum-dried for thirty minutes. The water bath's temperature is brought down to 20–30°C after drying. AThe round-bottomed flask is filled with 0.5 ml aliquot of the organic solution at 37°C following the medication and lecithin have been dissolved in an appropriate organic solvent mixture. After the solution has completely dried, another 0.5 ml aliquot of the remedy has to be included. Following the final filling, the flask that held the drug-loaded powdered mannitol (proliposomes) is linked in a lyophilized manner. The overnight desiccator treatment is followed by a sieving via 100 mesh screen.The The powder that has been collected is put into a glass bottle and kept at freezing temperature until it is characterized.
By employing the free film technique:
Casting on a mercury surface creates a free cellulose film acetate. Chloroform is to be applied to create a 2% w/w polymer solution. Plasticizers must be added at a 40% weighttoweight ratio of the polymer. A ring made of glass set over the surface of mercury in a glass petri dish dish was filled with five milliliters in the polymer solution. A funnel that is upside-down placed above the petri dish regulates the solvent's rate of evaporation. After the solvent has completely evaporated, the mercury surface is examined to detect the creation of a layer. Before being used, The film that has dried will be removed preserved in a desiccatoin between the wax paper sheets. It is possible to produce free films with varying thicknesses by altering the polymer's volume solution.[35-40]
MATERIAL EMPLOYED OF TDDS
TDDS BASIC COMPONENTS
- Mesh matrix / Medication container Drug
- Permeation enhancers
- Pressure sensitive adhesive (PSA)
- Backing laminates
- Release liner
- Other excipients like plasticizers and solvents Polymer matrix / Drug reservoir
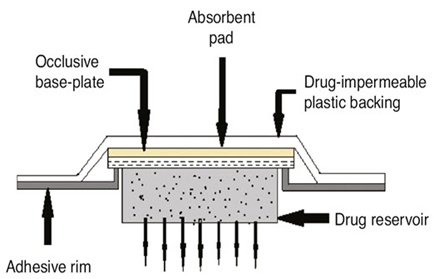
Figure 7: Fundamental TDDS Components
DRUG RESERVOIR/ POLYMER MATRIX
The core TDDS is polymers, which regulate the drug's release from the apparatus. Drugs can be dispersed synthetic polymer bases in a liquid or solid state to create polymers matrix. The polymers employed in TDDS devices should be stable, compatible with the drug and other parts of the system, and capable of releasing the drug effectively and safely throughout the device.
The following types of polymers are utilized in TDDS:
- Natural polymers
Include things like zein, gelatin, chitosan, waxes, gums, natural rubber, and derivatives of cellulose.
- Synthetic elastomers
Include butyl rubber, nitrile, acrylonitrile, silicon rubber, hydrin rubber, neoprene, and polybutadiene.
Polyvinyl alcohol, polyacrylate, polyvinyl chloride, polyethylene, polypropylene, polyamide, polyuria, polyvinylpyrrolidone, polymethylmethacrylate, and other synthetic polymers are examples.
Matrix type TDDS is made of polymers such as ethyl cellulose, polyethylene glycol, eudragits, polyvinylpyrrolidone, and hydroxypropyl methylcellulose. Polymers such as silicon rubber, polyurethane, and EVA are utilized as TDDS rate controllers.
DRUG
The physicochemical qualities of the medicine are what determine which drug is best for TDDS. The following drugs are better suited for transdermal drug delivery systems:
- Large first-pass metabolic process and a small window for therapy.
- Short half-life that results in frequent dosing and non-compliance.
- Dose ought to be fewer than 25 mg. per day.
- Minimal molecular weight, weighing less than 500 Daltons.
- Sufficient soluble in both water and oil (log P between 1-3).
- Minimal melting point (below 200°C).
PERMEATION ENHANCERS
By interacting with lipids or proteins within the stratum corneum, these chemicals can raise the stratum corneum's permeability and achieve increased therapeutic dosages of the medicine. They affect the stratum corneum's lipid and protein packing, which chemically modifies How the barrier works and increases permeability. Propylene glycol with dimethyl sulfoxide, 2-Pyrrolidone, Isopropyl myristate, Laurocapram (Azone), NaOH, Sorbitan monolaurate, Pluronic, Cardamom, Caraway, Lemon, Menthol, Limonene, and Linoleic acid are a few examples.
PRESSURE SENSITIVE ADHESIVE (PSA)
The Transdermal medication administration method is securely attached to the epidermis using pressure-sensitive adhesive (PSA). It ought to be forceful and persistently sticky, attach with only finger pressure, and make a powerful gripping force. It ought to be easily removed off the glossy surface without disappearing any trace. Adhesives have to be suitable with the skin, providing the least amount of sensitivity or discomfort, and easily removed without causing physical harm or leaving behind residue. They must also be able to dissolve the drug and excipient in enough amounts to have the intended pharmacological effect without losing their skin-tolerability and adhesive qualities. Transdermal systems that are sold commercially use PSAs such as polyacrylate, polyisobutylene, and polysiloxane. Polyacrylates, are most frequently employed. All acrylic adhesives are typically polar in nature, which enables them to easily absorb moisture and hold onto damp skin. They also effectively dissolve the majority of medications, allowing polyacrylate matrices to have significant drug loading. Polyisobutylenes (PIBs), are distinguished, in contrast, by a low drug-solvent capability. In membrane-controlled systems, where the first burst of medication released from the glue layer should be restricted, PIBs are frequently utilized. High and low molecular weight polymer combinations, which give cohesion and tackiness, respectively, are used in PIB-based adhesives. Each system can have its own unique cold flow and adhesiveness by modifying the PIB formulation's composition. Silicone, Low allergenicity is a characteristic of adhesives. Like PIBs, silicones control tackiness and cohesiveness by adjusting the size of their polymers, and they dissolve most medicines poorly. However, since medications with amine groups can stimulate further polymerization in silicone adhesives that retain residual silanol groups, controlling the molecular weight of silicones can be challenging when storing drug-adhesive compositions. In order to solve this issue, unique silicones have been created that end-cap silanol functional groups, making them resistant to amine-catalyzed condensation.
Hot Melt Pressure Sensitive Adhesives (HMPSA), HMPSA are
thermoplastic materials that melt to a viscosity that is suitable for coating, but they usually remain in an ideal state after cooling. Compounded HMPSA include paraffin waxes, ethylene vinyl acetate copolymers, low density polypropylene, styrene-butadiene copolymers, and ethylene-ethacrylate copolymers. Uncompounded HMPSA include polyesters, polyamides, and polyurethanes.
BACKING LAMINATE
It is necessary for backing materials to have strong tensile strength and to be flexible. Materials including polyolefin, polyesters, and metallized or transparent pigmented elastomers are frequently utilized. Compared to less flexible materials like polyester, elastomeric materials—such low-density polyethylene—adjust more easily to skin movement and offer superior adherence. Low water vapour transmission rates are particularly important for backing materials because they encourage higher skin hydration and, thus, higher skin permeability. For systems that store medication in a liquid or gel, the backing material must be heat-sealable To be able to enable form-fill-seal, or fluid-tight, packaging of the medication reservoir. The backing with the highest rate of moisture vapour transmission, good oxygen transmission, minimum modulus or great flexibility is going to be the most comfortable. Vinyl, polyester films, polyesterpolypropylene films, polypropylene resin, polyethylene resin, polyurethylene, Co Tra film, ethylene-vinyl acetate, and aluminum plastic laminate are a few examples of backing materials.
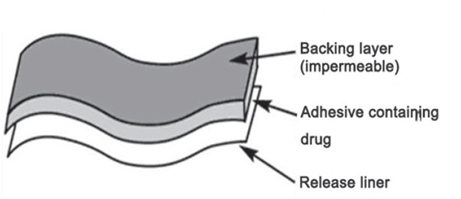
Figure 8: Backing Film Material.
RELEASE LINER
A protective liner that covers the patch while it is being stored is taken off right away and let out before the patch is applied to the skin. As a result, it is thought of as a component of the main packing material as opposed to the drug's dose form. The liner must, however, adhere to strict guidelines about chemical permeability and inertness medication, penetration enhancer, and water because it is in close contact with the delivery system. Release liners are usually comprised of two layers: a base layer, which can be either occlusive (such as paper cloth) or non-occlusive (such as polyethylene, polyvinyl chloride), and a coating layer composed of Teflon or silicon. Metalized laminates and polyester foil are additional materials utilized in TDDS release liners.
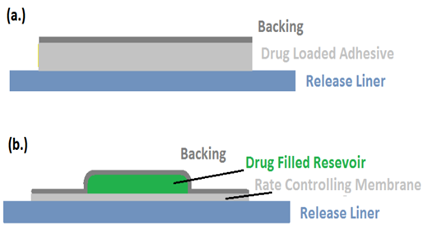
Figure 9: (A)Transdermal Patch With Release Liner And Figure (B)Patch With Reservoir
OTHER EXCIPIENTS
Drug reservoirs are prepared using a variety of solvents, including dichloromethane, acetone, methanol, and chloroform. In order to administer the transdermal patch additional flexibility, plasticizers such propylene glycol, triethylcitrate, polyethylene glycol, and dibutylpthalate are used.[37-42]
EVALUATION PARAMETERS:
The transdermal dosage form evaluation techniques fall into the following categories.
- Physicochemical evaluation
- In vitro evaluation
- In vivo evaluation
- Stability studies
Physicochemical evaluation:
Interaction Studies:
Practically every pharmacological dose form includes excipients as a necessary component. Among other things, the drug's appropriateness with the excipients determines a formulation's stability. Finding any potential chemical or physical interaction between the medicine as well as the excipients is essential because it might impact the medication's stability and bioavailability. A stable product depends on the medication and its excipients working well together. Compatibility studies are crucial to formulation development Should the excipients be novel and haven't been used in combinations containing the active ingredient before. Thermal analysis, FTIR, UV, and chromatographic techniques are frequently used in interaction investigations, wherein their physicochemical characteristics are compared.
Density of the Patch:
To guarantee the prepared material's thickness patch, The breadth of the drug-loaded Patch dimensions are given. at various spots using a digital micrometer, which also calculates the standard deviation and average thickness of the same.
Weight Disparity:
Before testing, the ready-made patches must dry for four hours at 60°C. A predetermined patch area needs to be divided into several sections, then weighed using a digital balance. The individual weights needs to be utilized for get the standard deviation and average weight values.
Putting Endurance in Folds:
It is necessary to cut a strip of a certain region uniformly and fold it at the same spot repeatedly until it breaks. The importance of folding endurance was determined by counting how many how many times the film could be folded in the same direction without breaking.
Moisture Content as a Percentage: The produced films must be weighed separately and stored for 24 hours at room temperature in desiccators filled with fused calcium chloride. The films must be reweighed after 24 hours in order to calculate the moisture content percentage using the formula below.
Percentage moisture content = [Initial weight- Final weight/ Final weight] ×100
The percentage of moisture absorbed:
To maintain 84% relative humidity, the films that were weighted must be stored within a desiccator with a saturated potassium chloride solution for 24 hours at room temperature. The films must be reweighed after 24 hours in order to calculate the percentage of moisture absorption using the formula below.
Percentage moisture uptake = [Final weight- Initial weight/ initial weight] ×100
Assessing Water Vapour Permeability (WVP): The You can employ the foam dressing approach. to measure the Permeability of water vapor of an Oven with air force that is replaced with an oven with natural air circulation. The following formula can be utilized to find the WVP: WVP equals W/A. where An is the surface region of the openness tests stated in m2, W represents the quantity of vapor that entered the patch and was represented in gm/24hrs, and The unit of WVP is gm/m. per 24 hours.
Drug Content:
A predetermined patch area needs to dissolve in a predetermined volume of an appropriate solvent. After that, the mixture must pass across a filter material for filtering before the drug's content is examined using the appropriate technology (UV or HPLC). The average of three separate samples is shown by each value.
Content Uniformity Test:
Ten patches are chosen, and each patch's content is decided. Transdermal patches pass the content uniformity test if nine out of ten have material that falls between 85% and 115% of the given value, and one patch has content that falls between 75% and 125% of the specified value. However, 20 more patches are examined for drug content if three of the patches have content between 75% and 125%. The transdermal patches pass the test if the range of these 20 patches is between 85% and 115%.
The homogeneity of the dosage unit test :
To fully extract the medication from the patch, chop up a precisely weighed amount of the patch, move it to a volumetric flask of a certain capacity, dissolve it in an appropriate solvent, sonicate the mixture, and add more medication as needed. After letting the resultant solution settle in for perhaps sixty minutes, the supernatant was appropriately diluted with the right kind of solvent to achieve the required concentration. The solution was examined using an appropriate analytical method (HPLC or UV), and the amount of medication in each piece was calculated.
Polariscope Examination:
This test will be carried out using a polariscope to look at the medication crystals originating from the patch. To determine if the patch contains the medication in an amorphous or crystalline form, a particular surface area of the object must be kept on the object slide and examined for drug crystals.
Test for Shear Adhesion:
This test's objective is to determine an adhesive polymer's cohesive strength. The molecular mass, degree of cross-linking, polymer composition, kind, and quantity of tackifier used can all have an impact. A stainless steel plate is covered with adhesive-coated tape, and to cause the tape to pull along a path that is parallel to the plate, a predetermined weight is suspended from it. The duration of time required to remove the recording from the plate is used to calculate shear adhesion strength. The shear strength increases with the length of time required for removal.
Adhesive Studies:
It is the polymer's capacity to stick to a substrate with minimal contact pressure. The quantity of tack in a polymer is established by its molecular weight, composition, and usage of tackifying resins.
Well, it is a qualitative test used to determine the adhesive's tack properties. All that is needed to determine the relative tack property of the glue is pressing the thumb down on it.
A length of Using tape, a surface in this test, and it is subsequently lifted from the surface in a predetermined fashion. The variables that affected the peel adhesion properties were the adhesive polymer's molecular weight and the kind and quantity of additives. The force needed for a specific tape width is reported as a consequence of the analysis. A single piece of tape is put to a membrane for backing or stainless steel plate. It is then extracted from the substrate at a 180-degree the force, and the angle needed to remove The recording is calculated.
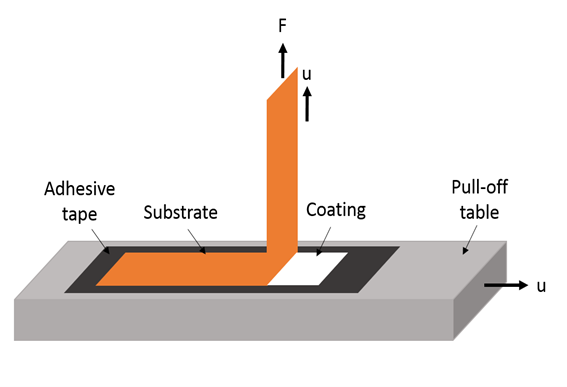
Figure 10: Test For Peel Adhesion
Flatness Test: :
Each film needs to have three horizontal slits cut out of it, two from the middle, two from the left, and one among the right. Each strip's length was measured, and the percentage of constriction—0% constriction being equal to 100% flatness—was used to calculate the length variation resulting from non-uniformity in flatness.
% constriction = I1 – I2/ I1X 100
Where, I1 = Initial length of each strip. I2 = final length of each strip.
Test for Rolling Ball Tacks:
This test quantifies a polymer's talk-related softness. A 7/16inch-diameter Ball made of stainless steel is thrown into an inclined track throughout this examination, causing it to roll Come on down into touch using a glue that faces upward and horizontally. The tack measurement, measured in inches, is derived from how far the ball moves along the adhesive.

Figure 11: Rolling Ball Tack Examination
Peel-Stick (Quick stick tack) Test:
The tape is pulled in this test at a speed of 12 inches per minute and 90ºC a distance from the substrate. The value of a tack is given ounces or grammes per inch width, is a measurement and record of the force of the peel needed to sever the binding between the glue and substrate.
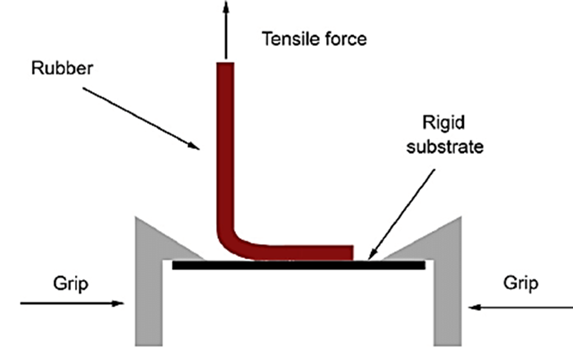
Figure 12: Quick Test Or Peel- Tack Test
Probe Tack Test:
The purpose of the experimental method known as probe tack is to evaluate a film's adhesive qualities during extremely brief contact periods. This test involves contacting the adhesive film that has been applied to a hard substrate with a flatended cylindrical probe. After that, the probe is kept contained for a predetermined amount of contact time under a regulated pressure. It breaks mechanically when the probe is removed later. Tack is the unit of measurement for the force needed to remove the probe from the adhesive at a set pace. It is represented in grams.
Figure 13: Probe Tack Test
Test for Percentage Elongation Break:
The length immediately preceding the breaking point should be noted To be able to calculate the lengthening percentage break. This can be done using the formula below.
Percentage of elongation = L1-L2/L2 ×100
where L1 is each strip's ultimate length and L2 is each strip's starting length.
Shear strength properties or creep resistance: The adhesive's cohesive strength polymer, or the degree to which a device should not slip when applied, is measured by shear strength, which is obtained by timing how long the time required to remove an adhesive-coated tape from one stainless plate. The apparatus used in the test was made in accordance with the PSTC-7 (pressure sensitive tape council) specification.[43]
Figure 14: Shear Strength Or Creep Resistance
In Vitro Evaluation
Studies on drug release in vitro:
The drug release from the produced patches can be evaluated using the method of paddle over disc (USP equipment V). arid layers of a given thickness need to be weighed, cut into a specific form, and adhered to a dish made of glass using a glue. After equilibrating the apparatus to 32±0.5°C, The plate of glass was submerged in 500 mL of the pH 7.4 phosphate buffer or dissolving medium. Next, moved at a pace of 50 revolutions per minute and positioned 2.5 centimeters apart plate. S from the window amples taken out5 ml portions can be at predetermined intervals for hours, and analysis can be p HPLC or a UV spectrophotometer erformed. The goal of The experiment must be carried out in triplicate for up to 24 minutes in order to calculate the mean value.
Studies on in vitro skin penetration:
A diffusion cell can be used to conduct a penetration in vitro research. Completely developed abdomen skin in 200–250 grams. Before beginning the procedure, the dermal side of the skin was carefully cleansed with distilled water to get rid of any clinging tissues or blood vessels. experiment. It was then equilibrated in phosphate buffer or dissolving media for one hour at pH 7.4. and diffusion. The abdominal region's hair should be carefully positioned on a magnetic stirrer equipped with a tiny magnetic needle for even utilizing an electric removed clipper. The thermostatically regulated heater was utilized to maintain the cell's temperature at 32 ± 0.5°C. The piece of isolated rat Skin is to be installed in between the diffusion cell's with the donor compartment with the epidermis facing up. A predetermined volume of sample has to be removed from the receptor-containing area and replaced with an equivalent amount of fresh medium. Samples must pass through a filtering media before being subjected to HPLC or spectrophotometric analysis. The permeability coefficients were derived simply dividing the drug load in the beginning by the flux mg cm, and flux can be directly estimated as the angle of the curve between the drug's steady-state values penetrated mg cm2 vs. hours of time. The horizontal-type skin permeation system has been extensively employed in the assessment of drug penetration through the skin. The cell is separated into receptor and donor compartments, each of which has a tiny membrane area (0.64 cm2) and a low solution volume (3.5 ml). An identical pair of star-head magnets that rotate at 600 rpm constantly agitate them. Water with a thermostat is used to operate the system via a water jacket that encloses the two compartments.
Franz diffusion cell:
The donor and receptor compartments make up the two parts of the cell. The receptor compartment has an effective surface area of 1–5 cm2 and a capacity of 5–12 ml. A magnetic bar continuously stirs the diffusion buffer at 600 rpm. A water jacket enclosing the receptor compartment allows thermostated water to circulate, maintaining the temperature in the majority of the solution.
Flow-through diffusion cell:
One benefit of using flow through diffusion cells is that they can be applied in situations when the drug's solubility in the receptor compartment is reduced. This cell can be immediately connected to an HPLC and is fully automated. They have a large capacity donor chamber to allow for proper compound loading and a tiny volume (0.3 ml) receiver chamber to guarantee quick penetrant removal at comparatively low pumping rates.[44-47]

Figure 15: Flow Through Diffusion Cell
In Vivo Evaluation Studies
In vivo Assessment: An accurate representation of a drug's performance can be found in in vivo assessments.In vivo research allow for the full exploration of variables that are not possible to account for in vitro experiments.
The following methods can be used to evaluate TDDS in vivo:
- Animal models
- Human volunteers
- Biophysical models
Animal models:
Small-scale studies involving animals are chosen over human subjects because they require less time and funding. The most often utilized animal species for transdermal drug delivery system evaluations include guinea pigs, mice, hairless rats, dogs, and hairless rhesus monkeys. We conclude from a variety of investigations that hairless animals are preferable to hairy animals in both in vitro and in vivo settings. Among the most trustworthy models for assessing transdermal medication distribution in humans in vivo is the rhesus monkey.
Human models:
After The repair is made to human volunteers, the last phase within a transdermal device's development entails gathering pharmacokinetic and pharmacodynamic data. Clinical trials have been carried out to evaluate the effectiveness, risk, adverse effects, patient compliance, and other factors. Phase I clinical trials are carried out primarily to ascertain volunteer safety, while phase II clinical trials are carried out primarily to ascertain patient effectiveness and shortterm safety. Phase IV studies at post-marketing surveillance are carried out for commercialized patches to detect adverse medication reactions, while phase III trials show safety and efficacy in a wide patient population. Even though they cost a lot of money, human studies are the most effective way to evaluate a drug's effectiveness.
Biophysical Models:
The literature has given models based on the steady-state mass balance equation, the device's solution of Fick's second law of diffusion, the stratum corneum and viable epidermis, and linear kinetics. It is clear that there is room for improvement despite the multitude of methods for transdermal system invivo evaluation that have been proposed. The age-related changes in the skin's barrier function, skin metabolism, the in-vivo activity of penetration enhancers, and other unresolved concerns are among them.
Skin Soreness study:
Testing for skin irritation and sensitization can be done on rabbits who are in good health. weighing between 1.2 and 1.5 kg on average. The rabbit's dorsal surface (50 cm2) needs to be cleansed. The hair should be shaved off of the clean region, and rectified spirit can be utilized to clean the surface before representative formulations are put on the skin. After 24 hours, The intended use of the patch is taken off, moreover, the skin is to be examined, with the extent of the skin injury being divided into 5 grades.[48-50]
Stability Studies
In accordance with the ICH standards, stability tests must be completed by keeping the examples of TDDS for six months at 75±5% and 40±0.5°C relative humidity. The specimens were taken out at0,30,60,90, and 180 days, and their drug content was appropriately analyzed.[51-52]
CURRENT TRENDS
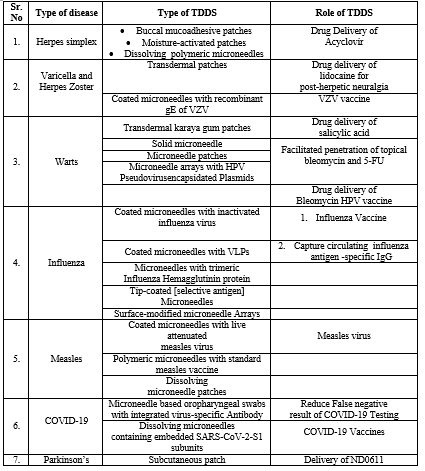
DISEASE CURED/TREAT VIA TRANSDERMAL ROUTE
Herpes simplex
The most common types of herpes simplex, caused by the herpes simplex virus, are vaginal herpes and herpes labialis. Cold sores, sometimes called herpes labialis, are caused by the type 1 and type 2 herpes simplex viruses; however, genital herpes more commonly affects the vaginal region than HSV-1.
Varicella and herpes zoster
VZV is the primary cause of both varicella and herpes zoster, and it also causes both of them. Individuals with compromised immune systems are more susceptible to complications such meningitis, pneumonia, cranial nerve palsies, myelitis, hepatitis, and widespread infection.
Warts
The human papillomavirus, or HPV, is the cause of cutaneous viral infections that manifest as warts or verrucas. They appear as varying-sized papules or plaques that often have a rough, scaly appearance. Localized spread of skin lesions is quite common. Based on their anatomical locations or morphologies, warts can be classified into four basic categories: common warts (Verruca vulgaris), fat warts (Verruca plana), plantar and palmar warts (Condyloma acuminatum), and anogenital warts (Verruca vulgaris). Immunomediated therapies or the physical elimination of infected epithelial cells are common wart treatment strategies. Currently the most popular approach is cryotherapy, which uses liquid nitrogen to freeze and eliminate wart lesions. However, some patients might not be able to tolerate further treatments because to the extreme pain of cryotherapy.
Influenza
Influenza is a respiratory illness that spreads easily and is caused by influenza viruses. A person may experience minor flu symptoms like fever, headaches, sore throats, and runny nose, or more severe symptoms like pneumonia that may require hospitalization or even result in death. Patients who are elderly or immunosuppressed are considerably more prone to experience major complications and ultimately pass away. The best defense against influenza and its spread throughout the population is the influenza vaccination.
Measles
When aerosols or droplets are inhaled, the highly contagious measles virus spreads through the respiratory system. Despite the availability of a secure and reliable vaccination, it remains the primary cause of disease and death for children globally.
COVID-19
SARS-CoV-2 is a novel coronavirus that is the cause of the devastating COVID-19 epidemic. It is a member of the family Coronavidae and genus Beta-Coronavirus, making it the seventh known coronavirus. 25 89,208 cases, 6 77,959 active cases, 18 60,672 recovered cases, and 50,085 deaths had been reported in India as of August 15, 2020.
Parkinson’s disease
The progressive deterioration of the nigrostriatal neuron and the subsequent reduction in striatal dopamine are the neurochemical basis of Parkinson's disease [PD]. The discovery in 1960 that the post-mortem brains of Parkinson's disease patients had a striatal dopamine shortage was the catalyst for the creation of dopamine replacement treatment.[53-55]
Table 2: Variety Of Disease That Cured By Transdermal Drug Delivery System , The Different Type Of Patches Used In That Disorder And Role Of Transdermal Drug Delivery System.
RECENT ADVANCES IN TRANSDERMAL DELIVERY SYSTEM
The following summarizes recent findings in the realm of transdermal patches:
- Patch technology for protein delivery:
One innovative and fascinating delivery technique for big proteins is transdermal distribution. Trans Pharma supplements its Thru Derma delivery method with transdermal protein delivery using its own printed patch technology. It is hypothesised that the interstitial fluid released from the skin through the RF-MicroChannels dissolves the highly water soluble proteins, creating an in situ highly concentrated protein solution. The dissolved chemicals are subsequently diffused over a sharp concentration gradient into the skin's living tissues through the use of the RF-Micro Channels.
- Testosterone transdermal patch system in young women with spontaneous premature ovarian failure:-
About half of the 300 ?g of testosterone produced daily by premenopausal women comes from the adrenal glands and the other half from the ovaries. When compared to women who ovulate normally, young women with spontaneous premature ovarian failure (sPOF) may have lower amounts of testosterone. The purpose of the testosterone transdermal patch (TTP) is to supply testosterone at the regular rate of ovarian production.
- Transdermal patch of oxybutynin used in overactive bladder: -
Approved in the US under the trade name Oxytrol and in Europe under the brand name Kentera, the medicine is a transdermal patch containing oxybutynin hydrochloride. Twice a week, OXYTROL is a transparent, thin, and flexible patch that is put to the belly, hip, or buttocks. It delivers oxybutynin continuously and consistently over a period of three to four days.
- Nanotechnology gaining hold: -
With this method, the benefits of both transdermal patches and needles are combined. The devices consist of hundreds of hollow microneedles that range in length from 100 to 1,000 micrometers, and are made of polymer the size of a dime. The medicine can easily pass through the top layers of skin thanks to these tiny needles.
- Pain relief: -
Transdermal patch technology frequently helps with pain relief. The Duragesic patch is familiar to the majority of readers. One for post-herpetic neuralgia is Lidoderm, a % lidocaine patch. A fascinating development in pain management is the E-Trans fentanyl HCl patch. This patch, the size of a credit card, is an active delivery system that uses a self-contained battery to administer powerful opioid pulses called fentanyl HCl. This simulates the usage of costly intravenous self-controlled analgesia systems.
- Poke with patch approach: -
Involves making a puncture in the skin, then applying a medication patch to the treated area.
- Coat and poke approach: -
After the drug-coated needles are placed into the skin, the medication dissolves and is released.
- Biodegradable micro needles: -
Consists of encasing the medication inside biodegradable polymeric microneedles that are injected into the skin.
- Hollow micro needles: -
Involves administering the medication with a hollow-bored needle.[56-57]
CONCLUSION & FUTURE ASPECTS
Future applications of first-generation patch technology are probably going to involve the transport of a tiny molecule medications Using the suitable combination that of qualities, particularly those currently being supplied both via injection and oral means and about to expire from patent. It is recommended that second-generation chemical augmentation be kept in usage as excipients in formulations in certain systemic patches for small molecule medications as well as topical creams for dermatology and ointments. Since the most potent chemical enhancers typically disperse beyond the stratum corneum and cause deeper irritation tissue, they most likely won't possess a noteworthy impact on the delivery of hydrophilic medications and macromolecules. Though they remain in the early phases of development, focused, third-generation combinations of chemical amplifiers and biochemical techniques provide tactics for more focused augmentation. Iontophoresis, a second-generation physical enhancement technique, has already had a significant therapeutic impact, particularly in terms of quick, targeted skin administration. The iontophoresis has a unique feature that are suitable for patient-controlled dosage and other intricate delivery profiles: its electronic control over delivery rates. Nevertheless, unless it is combined with other techniques that raise skin permeability, iontophoresis does not appear to possess a noteworthy impact on the distribution of macromolecules or vaccines since it does not significantly alter the skin barrier. Similarly, non-cavitational ultrasound has shown useful in the physical therapy setting for transdermal administration of anti-inflammatories; however, it does not seem to be appropriate for the delivery of big molecules. By rupturing the stratum corneum at the nanoscale, third-generation physical enhancement employing cavitational ultrasound and electroporation improves transdermal delivery. Cavitational ultrasound is already licensed for transdermal distribution of lidocaine; peptides and other tiny macromolecules may be allowed in the future. Applications of cavitational ultrasonography may be restricted, despite its effectiveness, by the requirement for an advanced technology that solely enhances skin permeability at the nanoscale scale, potentially limiting its applicability to macromolecules and vaccinations.
Third-generation physical improvement techniques such as thermal ablation, microdermabrasion, and microneedles can cause micron-scale disruptions in the skin. These techniques hold particular promise as they seem to be widely capable of delivering macromolecules and vaccines in addition to small compounds. Published evidence indicates that these approaches can be safe and successful, and unpublished clinical trials seem to produce encouraging results. Other microneedle and thermal ablation technologies are undergoing advanced clinical studies, and a microneedle product for vaccine delivery has been submitted for regulatory approval in Europe.[58-59] Diffusion of big substances via micron-scale disturbances is limited by the strong inverse relationship between diffusivity and molecule size. Therefore, it could take a while for an inactivated virus particle vaccine to diffuse through, even though it can fit through a micronsized hole with ease. Using microneedles that actively push medications and macromolecules into the skin may be preferred when speedy delivery is required, as may combining micron-scale disruption with an additional driving force like iontophoresis. All things considered, transdermal medication distribution presents strong chances to overcome the limited ability of many oral medications, the discomfort additionally agony a injections, and the restricted possibilities for both types of controlled release. Third-generation physical enhancers, such as ultrasound, heat ablation, and microneedles, may make it possible to deliver macromolecules and vaccines transdermally while building on the achievements of firstgeneration transdermal patches. Second-generation Both iontophoresis and chemical boosters are increasing transport properties for tiny molecules. Transdermal drug administration is now at a fresh degree of capability that will permit targeted disruption of the corneum stratum while sparing deeper tissues. These technical and scientific advancements Place transdermal medication delivery in a an increasingly broad impact on medicine.[60-61]
REFERENCES
- Ali S, Shabbir M, Shahid N. The structure of skin and transdermal drug delivery system-a review. Research journal of pharmacy and technology. 2015;8(2):103-9.
- https://encrypted-tbn0.gstatic.com/images?q=tbn:ANd9GcRqB7pZDxw_W4o-s928J_eOtfPidJFUB6WNSA&usqp=CAU
- Ale I, Lachapelle JM, Maibach HI. Skin tolerability associated with transdermal drug delivery systems: an overview. Advances in therapy. 2009 Oct;26:920-35.
- https://encrypted-tbn0.gstatic.com/images?q=tbn:ANd9GcTcFbROirssPHuxXwk9rbk_ZnmUHRRVpzy5Zse9G3Wd2yuhKj3ccJx56mlqdacGj6Q2eAE&usqp=CAU
- Sahu DK, Ghosh G, Rath G. Introduction to transdermal drug delivery system. InDrug Delivery Devices and Therapeutic Systems 2021 Jan 1 (pp. 309-323). Academic Press.
- Prausnitz MR, Langer R. Transdermal drug delivery. Nature biotechnology. 2008 Nov;26(11):1261-8.
- Kesarwani A, Yadav AK, Singh S, Gautam H, Singh HN, Sharma A, Yadav C. Theoretical aspects of transdermal drug delivery system. Bull. Pharm. Res. 2013;3(2):78-89.
- Guy RH. Transdermal drug delivery. Drug delivery. 2010:399-410.
- Reddy YK, Reddy DM, Kumar MA. Transdermal drug delivery system: a review. Indian Journal of Research in Pharmacy and Biotechnology. 2014 Mar 1;2(2):1094.
- Purushotham K, Vijetha KA. A review on transdermal drug delivery system. GSC Biological and Pharmaceutical Sciences. 2023;22(2):245-55.
- Levin CY, Maibach HI. 11 Transdermal Drug Delivery System: An Overview. Dermatotoxicology. 2007 Nov 26:101.
- Ahmed A, Karki N, Charde R, Charde M, Gandhare B. Transdermal drug delivery systems: an overview. International Journal of Biomedical and Advance Research. 2011;2(1):38-56.
- Nagdev SA, Agrawal O, Usman MR. Transdermal drug delivery system: An overview. Research Journal of Pharmacy and Technology. 2022 Mar 1;15(3):1371-7.
- Ghulaxe C, Verma R. A review on transdermal drug delivery system. The Pharma Innovation. 2015 Mar 1;4(1, Part A):37.
- Rastogi V, Yadav P. Transdermal drug delivery system: An overview. Asian Journal of Pharmaceutics (AJP). 2012;6(3).
- Ranade VV. Drug delivery systems. 6. Transdermal drug delivery. The Journal of Clinical Pharmacology. 1991 May;31(5):401-18.
- Bala P, Jathar S, Kale S, Pal K. Transdermal drug delivery system (TDDS)-a multifaceted approach for drug delivery. J Pharm Res. 2014 Dec;8(12):1805-35.
- Raza R, Mittal A, Kumar P, Alam S, Prakash S, Chauhan N. Approaches and evaluation of transdermal drug delivery system. Int J Drug Dev Res. 2015 Jan;7(1):222-33.
- Gaikwad AK. Transdermal drug delivery system: Formulation aspects and evaluation. Compr J Pharm Sci. 2013 Feb;1(1):1-0.
- Keleb E, Sharma RK, Mosa EB, Aljahwi AA. Transdermal drug delivery system-design and evaluation. International journal of advances in pharmaceutical sciences. 2010 Jul 1;1(3).
- Bathe RS, Kapoor R. Transdermal drug delivery system: formulation, development and evaluation-An overview. International Journal of Biomedical and Advance Research. 2015 Jan 30;6(1):1-0.
- Kadam AS, Ratnaparkhi MP, Chaudhary SP. Transdermal drug delivery: An overview. International Journal of Research and Development in Pharmacy & Life Sciences. 2014 Jul 15;3(4):1042-53.
- John L. Review on transdermal drug delivery system. International Journal of Pharma Research and Health Sciences. 2014;2(4):261-72.
- Yeoh T. Current landscape and trends in transdermal drug delivery systems. Therapeutic Delivery. 2012 Mar;3(3):295-7.
- Maurya VB, Kumar V, Kumar R. An Overview on Transdermal Drug Delivery System. Journal of Drug Delivery and Therapeutics. 2019 Aug 30;9(4-A):773-8.
- https://ars.els-cdn.com/content/image/3-s2.0-B9780128211854000142-f07-04-9780128211854.jpg
- https://www.researchgate.net/publication/317179461/figure/fig3/AS:499996145717248@1496219778084/Cross-Section-of-Adhesive-Controlled-TDDS-3-Matrix-Diffusion-Controlled-Systems-In-this.png
- https://www.researchgate.net/publication/317179461/figure/fig4/AS:499996145717249@1496219778126/Cross-Section-of-Matrix-Dispersion-TDDS-4-Micro-reservoir-Type-or-Micro-sealed.png
- https://www.researchgate.net/publication/317179461/figure/fig5/AS:499996145717250@1496219778208/Cross-Section-of-Micro-reservoir-TDDS.png
- Monika B, Amit R, Sanjib B, Alisha B, Mihir P, Dhanushram T. Transdermal drug delivery system with formulation and evaluation aspects: overview. Research Journal of Pharmacy and Technology. 2012 Sep 1;5(9):1168.
- Bird D, Ravindra NM. Transdermal drug delivery and patches—An overview. Medical Devices & Sensors. 2020 Dec;3(6):e10069
- Patel D, Kavitha K. Formulation and evaluation aspects of transdermal drug delivery system. International journal of pharmaceutical sciences review and research. 2011;6:1-2.
- Mali AD, Bathe R, Patil M. An updated review on transdermal drug delivery systems. International journal of advances in scientific research. 2015 Jul 30;1(6):244-54.
- Bariya SH, Gohel MC, Mehta TA, Sharma OP. Microneedles: an emerging transdermal drug delivery system. Journal of Pharmacy and Pharmacology. 2012 Jan;64(1):11-29.
- https://lh3.googleusercontent.com/9_qHHnzXLZx1Hh6gHfey-4ElCHh3Ib9y1-N-CgnbRqQPebjjVCZqYqSP4s_we59R57APzN9VbGhbURthCagw7YVpoXwEG1_pTUYs01jkMELf1Vs7KhyOURIrlKmmzXx1x_chkYcoyCKx7NswE7rYRJExRL3sJeDZ-w7uLXJmm5Fi0H3CsK_2XK61DA
- https://lh6.googleusercontent.com/NYK2v-wcRHNHj1gjg7v6yz9HmtMTTOq7hr_nAQWTTsl2pB-e9tQgL082dwyKrK-mshrdDbD665A6LydokXVPnPazJ8nfHVp-6Wnvz7todeG55J6bPGQ6RzUnZ3_wZsYQCxxA0IVgwJlZNO0Sipiop5mSs9NNlyau43G0bhhohCY0-iHlRbfDdAH32A
- https://lh4.googleusercontent.com/mtvs5DKFxj5fFaautcb5PRQtE2myp4tT44aSQl5kwTfGcbRnnWu3XiqVNwS31EX72akuqJVlV-0BdUl0nxziVD1bFxu95NOA-MmPcrCTOug6-hBcleeUGnvAEaiyhLn3uX27HE8C0jRqqgYmIIcDnLDlJrv5eVcJaogc9Rjlu3f3_XQq_pA1QtPyTw
- Hanumanaik M, Patil U, Kumar G, Patel SK, Singh I, Jadatkar K. Design, evaluation and recent trends in transdermal drug delivery system: a review. International Journal of pharmaceutical sciences and research. 2012 Aug 1;3(8):2393.
- Tanwar H, Sachdeva R. Transdermal drug delivery system: A review. International journal of pharmaceutical sciences and research. 2016 Jun 1;7(6):2274.
- Patel AV, Shah BN. TRANSDERMAL DRUG DELIVERY SYSTEM: A REVIEW. Pharma Science Monitor. 2018 Jan 1;9(1).
- Sudam KR, Suresh BR. A Comprehensive Review on: Transdermal drug delivery systems. Int. J. Biomed. Adv. Res. 2016;7:147-59.
- Sharma A, Saini S, Rana AC. Transdermal drug delivery system: a review. International Journal of research in pharmaceutical and biomedical sciences. 2013 Jan;4(1):286-92.
- Ramteke KH, Dhole SN, Patil SV. Transdermal drug delivery system: a review. Journal of Advanced Scientific Research. 2012 Feb 10;3(01):22-35.
- https://www.mvm.kit.edu/img/content/am/Peel-Test.png
- https://onlinelibrary.wiley.com/cms/asset/377399ee-a609-4b3f-bbe3-95650834a200/mfig003.jpg
- https://www.researchgate.net/profile/Costantino-Creton/publication/248923099/figure/fig1/AS:669979150127111@1536746885450/a-Overall-schematics-of-the-probe-tack-apparatus-and-b-Detailed-schematics-of-the_Q320.jpg
- https://5.imimg.com/data5/ANDROID/Default/2021/11/HA/RU/IQ/12427460/product-jpeg-500x500.jpg
- Dhiman S, Singh TG, Rehni AK. Transdermal patches: a recent approach to new drug delivery system. Int J Pharm Pharm Sci. 2011;3(5):26-34.
- Bhowmik D, Pusupoleti KR, Duraivel S, Kumar KS. Recent approaches in transdermal drug delivery system. The pharma innovation. 2013 May 1;2(3, Part A):99.
- Hafeez A, Jain U, Singh J, Maurya A, Rana L. Recent advances in transdermal drug delivery system (TDDS): an overview. J Sci Innov Res. 2013;2(3):733-44.
- Brown MB, Martin GP, Jones SA, Akomeah FK. Dermal and transdermal drug delivery systems: current and future prospects. Drug delivery. 2006 Jan 1;13(3):175-87.
- Ramadon D, McCrudden MT, Courtenay AJ, Donnelly RF. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug delivery and translational research. 2021 Jan 20:1-34.
- Jeong WY, Kwon M, Choi HE, Kim KS. Recent advances in transdermal drug delivery systems: A review. Biomaterials research. 2021 Dec;25:1-5.
- Soni D, Prakash K, Shakeel K, Kesharawani P. Current Trends and Recent Development of Transdermal Drug Delivery System TDDS. Asian Journal of Pharmaceutical Research and Development. 2023 Jun 30;11(3):181-9.
- Arunachalam A, Karthikeyan M, Kumar DV, Prathap M, Sethuraman S, Ashutoshkumar S, Manidipa S. Transdermal drug delivery system: a review. Journal of Current Pharma Research. 2010 Oct 1;1(1):70.
- Upadhyay G, Verma S, Parvez N, Sharma PK. Recent trends in transdermal drug delivery system-a review. Advances in biological research. 2014;8(3):131-8.
- SHINGADE GM. Review on: recent trend on transdermal drug delivery system. Journal of drug delivery and therapeutics. 2012 Jan 19;2(1).
- Yadav V. Transdermal drug delivery system. International journal of pharmaceutical sciences and research. 2012 Feb 1;3(2):376.
- Rawat A, Bhatt GK, Kothiyal P. Review on transdermal drug delivery system. Indo Am J Pharm Sci. 2016 May 1;3:423-8.
- Chaithanya N, Amaravathi V, Venkatesh P, Kalarini DH, Prema R. A Review Article of Transdermal Drug Delivery System (TDDS). International Journal of Research in Engineering, Science and Management. 2009;2(11):111-6.
Mantry S, Sindhuja N, Kumar SA. International Journal of Innovative Pharmaceutical Sciences and Research.


 Drasti Pandya* 1
Drasti Pandya* 1


















 10.5281/zenodo.11453521
10.5281/zenodo.11453521