Abstract
In the process of finding new drugs, analytical development and validation are crucial. It is a continuous and inter-dependent task linked with research and development, quality assurance and quality control department. Analytical method development and validation helps to demonstrate that the technique developed it precise, accurate, linear, and robust, as well as specific to analyse a drug in pharmaceutical industry. The essential part of a study is developing the analytical methodology. Advancement in analytical instruments lead to recent development in analytical methods. The excipients, active pharmaceutical ingredient (API), degradation products, residual solvents and other related substances are developed and validated using analytical techniques. To provide accurate results, analytical method validation used. This review is focusing on the important concepts, criteria, strategies, and importance of developing analytical method and validating the developed method.
Keywords
Analytical method development, Spectroscopy, Analytical method, Chromatography, Validation.
Introduction
In analytical method validation, sample is analysed for its chemical makeup. Analytical chemistry is of great importance. The main concern is finding the qualitative and quantitative makeup is the main concern. To identify atomic and molecular species or functional groups in a sample, qualitative approach is used, but to know the proportional of these elements, quantitative approach is used. Analytical chemistry studies how to analyse a sample to find out its chemical makeup. Finding the materials qualitative and quantitative makeup is its main concern. The functional group’s atomic or molecular structure can be obtained by a qualitative approach. On the other hand, a quantitative approach offers numerical data regarding the proportion of one or more of these elements. It is a subdivision of science to study the structure, chemical makeup and behaviour of a matter. To comprehend the identity, configuration of chemical entities, concentration. It is used in drug development processes, ranging from quality control, safety, marketing, storage. A dosage form developed for human consumption should be of highest purity without harmful impurities. Analytical techniques are used to analyse such dosage forms, since it could have a harmful effect upon consumption. Analytical methods are foundation for analytical chemistry. In order to analyse samples various analytical techniques are developed and validated [1]. Other scientific fields which require analytical data are zoology, biology, arts, space exploration, archaeology. Apart from medical diagnosis, other fields using analytical chemistry are biological, clinical research, pollutant management, applied sciences, geological testing [2]. Analytical chemistry also consists of evaluating natural and artificial materials containing more than one or one component and separating, measuring and identifying them for any chemical additives present. Qualitative and quantitative evaluation are two primary classes of analytical chemistry.
Analytical Method
A predefined detailed procedure to analyse one or more than one analytes in quantitative, qualitative or structural way is known as analytical method [3].
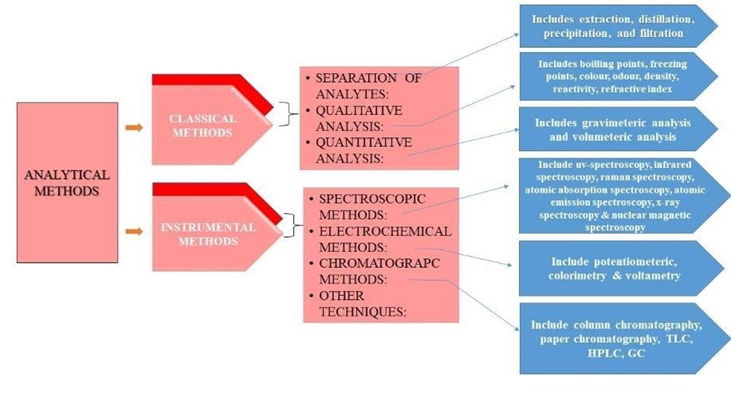
Fig 1: Analytical Methods
Typical techniques and instrumentation in analytical chemistry
In 1860 caesium (Cs) and rubidium (Rb) were discovered, Robert Bunsen and Gustav Kirchhoff invented Flame emissive spectrometry and it is the first instrumental analysis done.6 The properties of the analyte to be analyzed determine the classification of the instrumental techniques used for chemical analysis. such as solubility, specific gravity, density, transparency, turbidity, colour, freezing point, boiling point, moisture content can also be determined. Both physical and chemical changes are brought by chemical reactions [6] [7] [8].
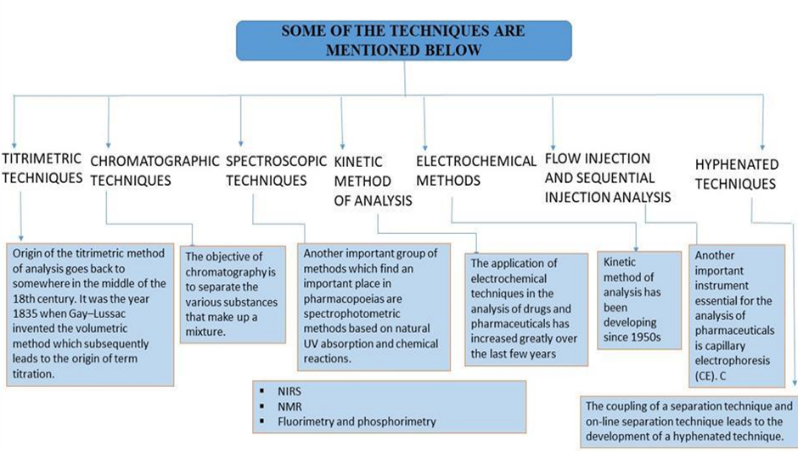
Fig 2: Techniques and Instrumentation in Analytical Methods.
Fundamentals Of Spectroscopy
It is defined as a scientific term to study the interaction between matter. Radiation is emitted during absorption or emission of energy by matter. Emission and absorption are two kinds of spectroscopy. The electromagnetic radiation absorbed by the sample are studied using absorption spectroscopy such as UV-visible, infrared, microwave, nuclear magnetic resonance, radio wave spectroscopy. Flame photometry, fluorimetry, emission spectroscopy are techniques used to analyse electromagnetic radiation emitted by a sample. Various samples are studies for their atomic and molecular structure using spectroscopy. Atomic spectroscopy includes atomic absorption and flame photometry. It explains how electromagnetic radiation interacts with atoms thereby producing energy fluctuations at atomic level. Molecular spectroscopy includes ultraviolet and infrared region of spectroscopy; it explains how radiation interact with molecules to cause a change in their energy. [4]
Ultraviolet-visible spectrophotometry
The most frequently used technique in pharmaceutical analysis is UV-visible spectroscopy. It is employed to quantify the quantity of visible or ultraviolet light that the compound in a solvent absorbs. It calculates the function or ratio of two UV-visible light beams' intensities. A spectrophotometer is used to qualitatively analyse and identify organic substances and to measure the amount of radiation absorbing species, qualitative spectrometry can be used if any existing data is available. Small amount of samples can be analysed in spectrophotometric analysis, it is easy to use, time saving and specific. Beer and lambert’s law governs the principles involved in spectrophotometry. [5]
- Beer’s law: It states that the quantity of absorbing molecules causes an exponential drop in the intensity of a parallel monochromatic radiation beam. In accordance with Beer’s law, absorbance stands as function of concentration.
- Lambert’s law: It state that as a beam of parallel monochromatic radiation travels through a medium with a uniform thickness, its intensity drops exponentially. The Beer-Lambert law results from the confluence of these two laws.
Table 1: Regions of Electromagnetic Spectrum
|
Region
|
Wavelength
|
|
Far (or vacuum) ultraviolet
|
10-200nm
|
|
Near ultraviolet
|
200-400nm
|
|
Visible
|
400-750nm
|
|
Near infrared
|
0.75-2.2µm
|
|
Mid infrared
|
2.5-50µm
|
|
Far infrared
|
50-1000µm
|
UV Method Development
Everyday an increasing number of new medications are introduced into the market. A delay occurs in the drug commercialisation and when it is included in the official pharmacopoeias, this results in flaws in current usage, recently reported toxicities, patients developing resistance, expanded usage and competitors developing advanced medicines. In these circumstances, pharmacopoeias will not have scientific methods for such medicines. This calls to develop a new theoretical framework for such medications. Quality is an essential element in all products and services, since drugs deal with people’s lives [9].
The normal values for a medicine are evaluated using official analytical methods instead of using the administrative systematic methods, it is suggested to use logical methodology, an important step when developing new pharmaceutical medicine is security testing [10].
Degradation products produced and dynamic medication test are used to determine the usability of pharmaceutical items that are to be used in realistic timeline. The following are some explanations for the advancement in drug analysis techniques:
When any drug or combination of drug is not included by any pharmacopoeias as official, following could be the causes:
- An expository approach for a treatment might not be available in writing due to patent regulations.
- Using listed excipients in analytical systems may not be always feasible in medications.
- Analytical systems for drugs combined with other treatments could be inaccessible [11].
Various techniques for UV spectrophotometric multicomponent analysis.
- Difference spectrophotometry
- Derivative spectrophotometry (DS)
- Isobestic “isoabsorptive” point method
- Dual wavelength method
- Successive ratio
- Simultaneous equation method
- Q-absorbance ratio method
- Absorptivity factor method
- Multivariate chemo metric method
- Derivative ratio spectra method
- Double divisor ratio spectra derivative method [12]
Instrumentation of UV-Visible spectroscopy
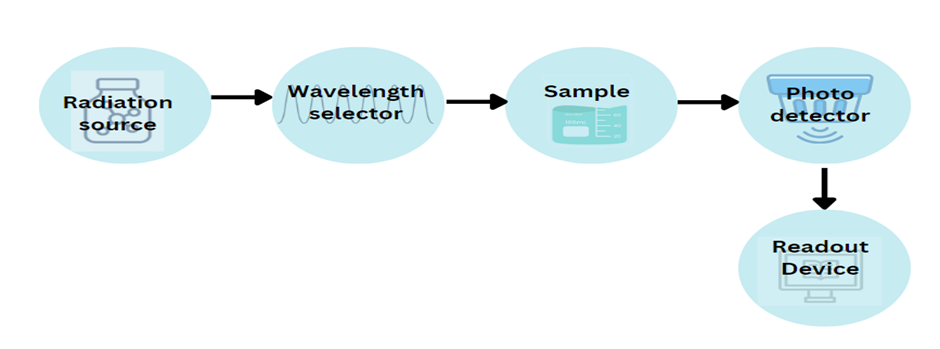
Fig 3: Instrumentation of UV-Visible spectroscopy
Radiation source: Tungsten, deuterium discharge, hydrogen discharge, xenon discharge, and mercury lamps are commonly used as the radiation sources. (figure 3)
- Wavelength selector: The radiation is dispensed by monochromator which selectively chooses desired wavelength. An exit slit, input slit and a dispensing element are three fundamental components of a monochromator.
- Sample cell: Also referred to as cuvettes, sample cells are containers used for analysis of samples. The material of these is quartz.
- Photo detector: Barrier layer cell, photomultiplier tube, and photocell are most commonly used detectors in UV Spectrophotometer [13].
Introduction To Chromatography
Separating mixtures by physicochemical technique is chromatography. It is a technique that separates mixture of substances into its component parts using both, mobile phase and stationary phase [14] [15].
HPLC
The most popular tool in analytical chemistry is HPLC (high performance liquid chromatography). It is used in distinguishing, recognize, and measure the liquid soluble chemicals. The most accurate analytical techniques quantitative and qualitative spectroscopy are frequently applied to drug product analysis [16]. HPLC is a separation technique in which a minor quantity of liquid sample volume is injected into a tube packed with small particles, referred to as the stationary phase, that are 3 to 5 microns in diameter. Liquid (mobile phase) is injected with the help of high pressure provided by pump, which moves individual components of sample down the column packing. Column packing has different chemical or physical interactions within the molecules and packed particles, keeping the components separated from each other.
Instrumentation of HPLC
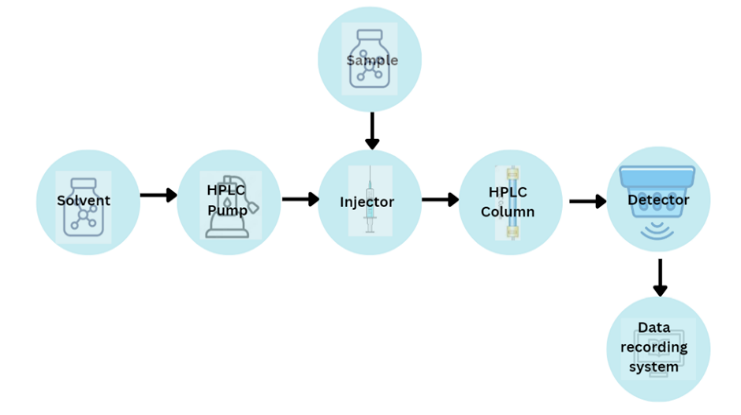
Fig 4: Instrumentation of HPLC
Mixing system, solvent reservoir and degassing system:
The solvent reservoir is used to store the mobile phase. These containers are compound stainless steel or glass. The mostly used solvent reservoirs are made of glass [17]. The pump also needs to combine solvents with extreme precision and accuracy along with distributing the mobile phase. Low pressure mixing and high pressure mixing are the two primary types of mixing units [18]. The trapped air bubbles are eliminated from the solvent using the degassing system. Ultra-sonication and filtration are the degasser procedures which assist with the degassing.
2. High pressure pump:
A liquid is pushed with the help of pump to provide a particular flow rate. Millilitres per minute (ml/min) is the unit for measurement of flow rate [19]. Pump pressure typically ranges from 400 to 600 bar (6000 to 9000 psi), and 1-2 ml/min is the normal flow rate.
Mobile phase is sucked by the pump from the reservoir and is forced into the column and then is passed to the detector. The operating pressure is 40000 KPa. Particle size, flow rate, column dimensions, and mobile phase composition are a few operating parameters [20].
3. The sample injector:
The sample injector is used to introduce the sample liquid into the mobile phase. The sample valve is positioned between the pump and the column. An injector, also called an auto sampler, is used to inject the sample into the continuously running mobile phase that feeds the sample onto the HPLC column. The sample quantities range from 5 to 20 microliters (µl) [21]. Automatic and manual are two different types of injectors.
4. Column:
The actual components separation occurs in the column. Columns are made of stainless steel and length is ranging from 5 to 25 cm with its internal diameter is 2 to 4 cm.
5. The detector:
Each component that elutes from the column is identified by the detector, which transforms the data into an electrical signal. Bulk property detectors and specialized detectors are the two types of detectors that are employed. UV-VIS, fluorescence, spectrometric detectors and photo diode array are examples of specific detectors. Refractive index, light scattering and electrochemical detectors are examples of bulk property detectors.
6. Data recorder
The output is captured as a series of peaks, and a computer is used to automatically compute the data by determining the area beneath each peak.
Developing Analytical Method
The process to develop analytical procedure which is accurate, to determine the composition of a formulation is known as analytical method development. Analytical method development is the process of demonstrating that an analytical technique is suitable for use in a laboratory. Protocols should be followed while developing analytical methods meeting acceptance criteria according to the ICH guidelines and utilized only in GMP and GLP environments. Q2 (R1) [24].
Techniques, method, procedure, protocol and the steps of analytical method development
Criteria to develop new analytical method
The foundation for determining the product is drug analysis. Due to a significant delay between a drug's release date in the market and the date it is included to pharmacopeia, it causes potential ambiguities in ongoing and increased use of such medications. Reporting of new toxicities, patients develop resistance, launching of more effective medications by the competitors pose a major concern while developing an efficient analytical method [22]. There could be a possibility that no pharmacopoeia has accepted the new medication or medication combination because of patent regulations, a proper analytical process might not be published in the literature for the medicine and analytical techniques might not be accessible in the form of formulation excipients. There might not have been development of analytical techniques for a medication when used with other medications. It is possible that the current analytical techniques require costly solvents and reagents. It could include laborious extraction and separation techniques, which might not be dependable [23].
Necessity to develop a method
The drug evaluation includes identification, characterisation, and resolution of the medications in combination, such as dosage forms and in organic fluids. The goal of developing analytical method is to produce data regarding efficiency (which may be closely linked to the requirement for a specific dosage), impurity (safety of the medication), stability (identifies breakdown product), bioavailability (includes crystal type, drug homogeneity, and medication release), and manufacturing impact parameters to confirm that the medicinal product being produced is steady.

Fig 5: Steps involved in method development
Need to develop analytical method and its validation
The requirement to develop and validate analytical technique arose from global rivalry, keeping the standards of highly valuable items in the commercial domains, market domains, and ethical considerations. A few of the well-known groups are in charge of the quality standards:
Validation
FDA (food and drug administration) states validation as a process and production technique intended to guarantee the uniqueness, excellence, potency, and purity of the pharmaceutical products. to demonstrate that the procedure and the system fulfil the necessary specifications. The concept of validation was developed in 1978 in United States. Over the years, the concept of validation has broadened to encompass a variety of activities, such as computerized systems for clinical trials, labelling or process control, and analytical methods to guarantee the quality of medical products and therapeutic substances. It is advised to think validation as an essential component of cGMP. It refers to the assessment of validity or the process of demonstrating efficacy. Validation is a collaborative effort which involves individuals from several plant branches. A higher level of certainty that the equipment or product will meet the goals of the analytical application is provided by the "process of establishing documented evidence," sometimes referred to as "method validation." [25] [26] [27]
According to USP: Analytical method
- Category I: Analytical techniques used to determine the amounts of active compounds, such as preservatives, in pharmaceutical products.
- Category II: Analytical techniques to identify contaminations in bulk or to identify contaminants in bulk or to identify substances that causes product degradation in pharmaceutical finished goods.
- Category III: Analytical techniques to figuring out performance attributes such as disintegration and medication release.
- Category IV: Identification tests.
According to ICH method
"Establishing a documented evidence, which provides a high degree of assurance that a specific activity will consistently produce desired result or product meeting its predetermined specifications and quality characteristics" is defined as validation by the International Committee of Harmonization. For the trace impurity assay, a distinct methodology and set of acceptance standards are required than for the major component assay. Some aspects of the HPLC process may be significant because of differences in the backgrounds and abilities of employees, as well as in the HPLC instrumentation, lab supplies, and reagents. The methods may need to be adaptable in order to create different forms of the same medication with different potencies or physical characteristics. System appropriateness, linearity, accuracy, precision, specificity, robustness, limit of detection, limit of quantification, and stability of samples, reagents, and equipment are all important factors in method validation study [28].
Method validation parameters
- Accuracy
- Precision
- Linearity
- Limit of detection
- Limit of quantitation
- Specificity
- Range
- Robustness
1. Accuracy:
Accuracy is the degree to which the measured value closely resembles the true value. To match the true value, a sample with a known true value is analyzed using a highly accurate method. Recovery studies are determined by accuracy.
- Accuracy can be verified using 3 ways.
- Assessing in accordance with the benchmark.
- Spiking analyte in sample and later recovering.
- Analyte is added as usual and total contaminants should be identified specifically.
2. Precision:
It is an indicator of how closely different values from the same sample are related. RSD (relative standard deviation) is the way it is expressed. To obtain precise results under explanatory conditions, several homogenous samples are tested.
%RSD = Standard deviation/Mean ×100
- Repeatability: Accuracy with the same operational conditions and for a brief period of time, using the same analyst.
- Intermediate precision: Several days, tools, analytes, etc., are used to test the procedure to study reproducibility among different laboratories.
According to the ICH Guidelines, repeatability must appropriately conform to at least nine determinations within a specified range or a minimum of six determinations at 100 % of the test concentration.
3. Linearity:
The potential to obtain a linear response which is directly proportional to concentration of the sample in solution. Expressed as confidence limit around the slope of the regression line.
4. limit of detection (LOD):
The lowest amount of analyte in a sample that can be detected but not quantified. It is expressed as concentration at specific signal to noise ratio. LOD= 3.3 × S/SD
5. limit of quantitation (LOQ):
Lowest concentration of analyte that can be quantified is known as limit of detection. Signal to noise ratio of 10:1 is recommended by ICH.
LOQ= 10 × S/SD
6. Specificity:
The ability to provide interference free signals is one of the notable feature of HPLC. The capability of analytical technique and distinguish and detect the analyte in mixture is referred to as specificity according to ICH is defined as the ability to evaluate the analyte in the presence of several substances that may potentially by present. These can be contaminants, matrix, degradants and other things. The following implications follow from the definition:
- Identification test: Identification tests should distinguish between mixtures of closely related structures to ensure the quality of a test subject.
- Purity test: Ensures that the scientific methodology used enables an accurate detect the number of pollutants present in an analyte, such as related chemicals, solvent residue, significant metals.
- Assay: This provides an accurate report on the amount or strength of analyte in a sample and help to arrive at a precise result.
7. Range: It refers to the range of the analyte levels that have been determined with appropriate accuracy, precision and linearity, and the lower level. It is calculated by linear response curve or non-linear response curve. Unit is same as in test findings.
8. Robustness: Ability to remain unchanged due to minor, intentional alterations in parameters such as column temperature, flow rate, pH, composition, or mobile phase is termed as robustness [29] [30]
Summary Of Literature Survey Of Different Drug.
Table No: 2 Summary of literature survey of different drugs
|
Sr.no.
|
Drug
|
Instrument
|
Column
|
Flow rate
|
Mobile phase
|
Retention time
|
Linearity
|
Ref
|
|
1
|
Ivabradine Hydrochloride
|
HPLC
|
C18 (250×4.6mm;5µm)
|
0.8 mL/min
|
Phosphate buffer: Methanol (60:40 v/v)
|
6.55±0.05 min
|
r2=0.9998
|
[31]
|
|
|
|
HPLC
|
C18
(250mm×4.6mm;5µm)
|
1 mL/min
|
Methanol: ACN (80:20: v/v)
|
5.8 ml/min
|
r2=0.998
|
[32]
|
|
|
|
HPLC
|
ODS-3V (250mm×4.6mm)
|
0.7 mL/min
|
0.5% Formic acid (pH 7.0): Acetonitrile (65:35 v/v)
|
8.67±0.0376
|
r2=0.999
|
[33]
|
|
2
|
Brivaracetam
|
HPLC
|
Agilant column (4.6×150mm; 5µm)
|
1.0 mL/min
|
Phosphate buffer: Methanol (25:75 v/v)
|
2.182 Min
|
r2=0.992
|
[34]
|
|
|
|
RP HPLC
|
ODS RPC18,5mm, 15mm x 4.6mm
|
1.0 mL/min
|
phosphate buffer: methanol: acetonitrile (30:35:35% v/v)
|
2.183 min
|
r2=0.999
|
[35]
|
|
3
|
Pregabalin
|
HPLC
|
Hypersil BDS, C8, (150×4.6mm, 5µm)
|
1 mL/min
|
Phosphate buffer (pH 6.9): ACN (95:05)
|
***
|
r2=0.9998
|
[36]
|
|
|
|
HPLC
|
C18 Column (250×4.6mm; 3.5 µm)
|
0.8 mL/min
|
Acetonitrile: Methanol (80:20)
|
***
|
r2=0.999
|
[37]
|
|
|
|
HPLC
|
Inertsil ODS 3V, (250× 4.6mm ID, 5µm)
|
1 mL/min
|
Phosphate buffer: Acetonitrile: Methanol (80:10:10)
|
4.7 min
|
r2=0.999
|
[38]
|
|
4
|
Levetericetam
|
HPLC
|
Prontosil C18 Column (150×4.6 mm; 5µm)
|
1.2 mL/min
|
Buffer solution (pH 2.8): Acetonitrile (90:10)
|
4 min
|
r2=0.9999
|
[39]
|
|
|
|
UV
|
-
|
-
|
H2O
|
***
|
r2=0.9982
|
[40]
|
|
|
|
HPLC
|
Flexit C18 Column (150×4.6mm; 5µm)
|
1 mL/min
|
Water: Methanol (75:25)
|
***
|
r2=0.9989
|
[41]
|
|
5.
|
Lamotrigine
|
LCMS
|
Chromolith, C18 (50×4.6mm; 4µm)
|
0.500 mL/min
|
Phosphate buffer (pH2.5) Acetonitrile (80:20)
|
***
|
r2=0.9987
|
[42]
|
|
|
|
HPLC
|
Intersil C8 column (250mm length, 4,6mm)
|
0.8 mL/min to 1.2 mL/min
|
ACN: Methanol (60:40 v/v)
|
2.542 min
|
r2=0.9905
|
[43]
|
|
|
|
HPLC
|
C8 (4.6mm ID×150mm. 3.5µm, Make:XTerra)
|
0.8 mL/min
|
Acetonitrile: potassium dihydrogen phosphate buffer of pH= 7.0 (60:40)
|
2.797 min
|
r2=0.998
|
[44]
|
|
6.
|
Ketoprofen
|
HPLC
|
C18 Column (5µm)
|
1.0 mL/min
|
Acetonitrile: Milli Q water acidified by 0.1 % (v/v) Formic acid
|
3.06 min
|
r2=0.9997
|
[46]
|
|
|
|
UV
|
|
|
NaHCO3
|
|
r2=0.998
|
[47]
|
|
|
|
HPLC
|
C18 Column, 5µm (25×4.6 mm
|
1mL/min
|
Methanol: Water (70:30)
|
<10>
|
r2=0.9999
|
[48]
|
|
7.
|
Omeprazole pellets
|
Ultra fast gradient LC method
|
Column (50×20mm id)
|
1.0 mL/min
|
0.15% (v/v) trifluoracetic acid (TFA) in water & 0.15% (v/v) TFA in acetonitrile
|
0.74 min
|
r2=0.998
|
[50]
|
|
|
|
RP HPLC
|
RP?C18 column
|
1.5 mL·min?1
|
Phosphate buffer (pH 7.4) and acetonitrile (70:30 v/v)
|
5 min
|
r2=0.9995
|
[51]
|
|
|
|
LC-MS/MS
|
RP-C8 (50 X 4.6mm, 5µm particle size)
|
0.6 mL min-1
|
Methanol: water (containing 0.5% Formic acid) 8:2
|
***
|
r2=0.9999
|
[54]
|
Based on the literature survey of various analytical methods for different pharmaceutical compounds, I have selected Ketoprofen and Omeprazole pellets for the current study. analytical methods used for estimating ketoprofen and omeprazole in pharmaceutical formulations. Based on your literature survey, it seems there is limited research available on the use of UV, HPLC, HPTLC, and stability-indicating RP-HPLC specifically for the combination of ketoprofen and omeprazole. This observation could indicate a gap in the existing literature, and it may provide a unique opportunity to explore and develop an analytical method for this combination.
The following outlines the detailed breakdown of the research work.
Ketoprofen and Omeprazole
Ketoprofen is a nonsteroidal anti-inflammatory drug (NSAID), commonly used for pain relief and inflammation.
Omeprazole is a proton pump inhibitor (PPI) used to treat gastroesophageal reflux disease (GERD) and other acid-related disorders.
Since these two drugs often appear together in combination formulations (to reduce the gastrointestinal irritation caused by NSAIDs), developing a reliable and robust analytical method for their simultaneous estimation in pharmaceutical dosage forms would be highly valuable.
Existing Literature Insights
UV Spectroscopy: UV-Vis spectrophotometry is a common method for drug quantification but has limitations in terms of selectivity and sensitivity, especially for combinations. There is a need to explore whether the wavelengths used for each drug could offer a distinct signature for ketoprofen and omeprazole.
HPLC: High-performance liquid chromatography (HPLC) is widely used for the separation and quantification of drug mixtures. The literature suggests that there is some work done on estimating ketoprofen and omeprazole individually or in combination with other drugs, but possibly with some method limitations in terms of sensitivity, selectivity, or reproducibility.
HPTLC: High-performance thin-layer chromatography (HPTLC) might be less common in this specific context but could be explored as a potential technique due to its lower operational costs and ease of use in pharmaceutical analysis.
Stability-Indicating RP-HPLC: Stability-indicating methods are essential for assessing the stability of drugs under various conditions (like temperature, humidity, and light). Since ketoprofen and omeprazole might undergo degradation, developing a stability-indicating RP-HPLC method for the simultaneous estimation of these drugs could be a significant contribution.
Potential Research Directions
Development of a Novel Analytical Method:
Work could be done on optimizing an RP-HPLC method that can separate and quantify both ketoprofen and omeprazole simultaneously. You may need to fine-tune the mobile phase composition, flow rate, and column type to achieve the required resolution and sensitivity.
Stability-Indicating Method:
Develop a stability-indicating RP-HPLC method for the combination drug. This would involve subjecting the drug to stress conditions (e.g., heat, light, oxidation) and ensuring that the method can distinguish the drug from its degradation products.
Validation:
To ensure that the method developed adheres to validation criteria (e.g., specificity, accuracy, precision, linearity, limit of detection, limit of quantification, robustness) to ensure its suitability for routine analysis.
Exploring Analytical Alternatives:
Explore the possibility of using other techniques, like HPTLC, for simpler and more cost-effective analysis. Although HPTLC may not be as sensitive as HPLC, it might be suitable for routine quality control if combined with suitable detection systems (e.g., densitometry).
Table 3: Summary of literature survey of Ketoprofen and Omeprazole pellets
|
Sr.no.
|
Drug
|
Instrument
|
Column
|
Flow rate
|
Mobile phase
|
RT
|
Linearity
|
Ref
|
|
1
|
Ketoprofen
|
HPLC
|
C18 Column (5µm)
|
1.0 mL/min
|
Acetonitrile: Milli Q water acidified by 0.1 % (v/v) Formic acid
|
3.06 min
|
r2=0.9997
|
[45]
|
|
|
|
UV
|
|
|
NaHCO3
|
|
r2=0.998
|
[46]
|
|
|
|
HPLC
|
C18 Column, 5µm (25×4.6 mm)
|
1mL/min
|
Methanol: Water (70:30)
|
<10>
|
r2=0.9999
|
[47]
|
|
|
|
HPLC
|
Shim-pack column vp-ODS (250×4.6 mm)
|
1mL/min
|
Ethanol: Phosphate buffer (80:20 v/v)
|
10 min
|
r2=0.9999
|
[48]
|
|
|
|
RP-HPLC
|
C18 Type MG column (250×4.6mm)
|
1.0 mL/min
|
Acetonitrile: 0.02 M Potassium dihydrogen orthophosphate buffer (40:60)
|
***
|
r2=0.999
|
[49]
|
|
2
|
Omeprazole Pellets
|
Ultra fast gradient LC method
|
Column (50×20mm id)
|
1.0 mL/min
|
0.15% (v/v) trifluoracetic acid (TFA) in water & 0.15% (v/v) TFA in acetonitrile
|
0.74 min
|
r2=0.998
|
[50]
|
|
|
|
RP-HPLC
|
RP?C18 column
|
1.5 mL·min?1
|
Phosphate buffer (pH 7.4) and acetonitrile (70:30 v/v)
|
5 min
|
r2=0.9995
|
[51]
|
|
|
|
RP-HPLC
|
C18 (150×4.6mm; 5µm)
|
0.5mL/min
|
Acetonitrile: Phosphate buffer solution (60:40 v/v)
|
7.71 min
|
r2=0.9988
|
[52]
|
|
|
|
RP-HPLC
|
C18, (250 x 4.6 mm, 5µ)
|
1.0 mL/min
|
Phosphate buffer (pH 7.4) and acetonitrile in the ratio of 60:40, v/v
|
7.71 min
|
r2=1.0000
|
[53]
|
|
|
|
LC-MS/MS
|
RP-C8 (50 X 4.6mm, 5µm particle size)
|
0.6 mL min-1
|
Methanol: water (containing 0.5% Formic acid) 8:2
|
-
|
-
|
[54]
|
|
3
|
Ketoprofen
+ Omeprazole pellets
|
HPLC
|
Phenomenex Luna C18 (2) column (150 × 4.6 mm, 5 ?m)
|
0.8 mL/min
|
Mobile phase A consisted of 0.01 M KH2- PO4 and 3 mL of TEA (pH 7.0 adjusted with H3PO4). Mobile phase B contained a mixture of Milli Q water and ACN in the ratio of 10:90 (v/v).
|
3.9 min.
|
r2=>0.999
|
[55]
|
|
|
|
HPLC
|
LiChrosorb C18, 125 mm x 4.6 mm, 5 ?m
|
1.5 ml/min.
|
Phosphate buffer (pH adjudted to 4.6 with ortho-phosphoric acid) and methanol (25:75v/v)
|
3.05 and 6.96 min
|
Ketoprofen- r2=0.9999 Omeprazole- r2=0.9998
|
[56]
|
|
|
|
HPTLC
|
-
|
-
|
Chloroform: methanol 9:1 (v/v)
|
-
|
Ketoprofen-
r2=0.999
Omeprazole-
r2=0.999
|
[57]
|
CONCLUSION
In conclusion, this review outlines essential guidance on method development, validation, and its critical role in pharmaceutical analysis. It highlights the importance of detailing various validation types, and executing precise methods to ensure their effectiveness for intended applications. The primary goals of analytical technique development identification. The primary goals of analytical technique development identification, purification, and qualification are fundamental in verifying that pharmaceutical products meet quality standards. Ensuring the reliability and accuracy of methods in the pharmaceutical industry is crucial, as it supports the production of high-quality medicines and materials, ultimately protecting consumer health and safety.
REFERENCES
- Harvey DT. Analytical Chemistry for Technicians: 3rd Edition (John Kenkel). Journal of Chemical Education 2005; 82 (1): 39
- Kissinger PT. Instant Notes Analytical Chemistry: Clinical Chemistry 2002; 48 (12): 14-99.
- Kachave R, Jadhav K. Analytical Method Development and Validation: Journal of International Journal of Creative Research Thoughts. 2021; 9 (8), 2320–2882.
- Khokrale S, Lulla J, Borse L. A Review of the UV-Visible Spectroscopy’s Method Development and Validation: International Journal of Pharmaceutical Sciences 2024; 2 (5); 527-538
- Davidson AG. Ultraviolet-visible absorption spectrophotometry: In BeckettAH, Stenlake JB, (4thEdition), Practical Pharmaceutical Chemistry. CBS Publishers and Distributors, New Delhi, 275-278
- Yoshiko A. Basic Education in Analytical Chemistry (Analytical Sciences, 17 Supplement: 2014; 9 (2): 501-506.
- Willard H, Merritt J, Dean JA. Instrumental Methods of Analysis. Journal of Instrumental Methods of Analysis, 1988.
- Day RA, and Underwood AL1, Quantitative Analyses, (5th Ed., Prentice Hall, New Delhi: (1986).
- Nimje HM, Oswal RJ, Kshirsagar SS, Chavan M. Spectrophotometric Analysis for Estimation of Felodipine in Tablet Dosage Form by Calibration Curve Method: Research Journal of Pharmacy and Technology. 2011; 4 (12): 1805–1806.
- Kumarswamy U, Kumar S. Applications of Fourier Transform Infrared and UV-Visible Spectroscopy for the Demonstrating Sameness of Ganirelix Peptide in Liquid Injection Formulation: Research Journal of Pharmacy and Technology. 2022; 15 (4); 1709–1712.
- Sasikala M, Mohan S, Gokilambal V, Mymoona S, Hari P. Validated Uv-Visible Spectroscopic Analytical Method Development and Stability Studies on Oseltamivir: Research Journal of Pharmacy and Technology. 2020; 13 (9); 4323-4328.
- Paithane PR, Gore KR, Waghmare S, Kamble DR. H. (n.d.). A review on UV-visible spectroscopy: International Journal of Innovative Research in Technology. 2024; 11 (1): 171-177.
- Chatwal GR, Anand SK. Instrumental Methods of Chemical Analysis. Himalaya Publishing House. (2002)
- Vidushi Y, Meenakshi B. A Review on HPLC Method Development and Validation: Research Journal of Life Sciences, Bioinformatics, Pharmaceutical and Chemical Sciences 2017; 2 (6): 166-178.
- Luxminarayan L, Sharma N, Viswas A, Khinchi M. P. A review on chromatography techniques: Asian Journal of Pharmaceutical Research and Development, (2017) 1–8.
- Pallavi M, Patil N. HPLC Method Development: A review. SGVU Journal of Pharmaceutical Research & Education 2017; 1 (2): 243–260.
- Aggarwal R, Chamoli A, Rawat M, Nautiyal A. (n.d.). HPLC Instrumentation and Validation. https://doi.org/10.35629/7781-0801
- Jena A. HPLC: Highly Accessible Instrument in Pharmaceutical Industry for Effective Method Development. Pharmaceutical Analytical 2012; 03 (01). 1-9.
- Agilent Technologies. The LC Handbook Guide to LC Columns and Method Development. Agilent Technologies. (2016); 16.
- Rina R, Baile M, Jain A. Systematic Review Pharmacy 450 a Review: Analytical Method Development and Validation. 2021; 12(8): 450-454.
- Hamilton RJ, Sewell PA. Introduction to high performance liquid chromatography. Springer. 1982; 1-12.
- Dhanshri P, Fand: A Review on Analytical Method Development: IJCRT 2022; 10 (1): 2320-2882.
- Malviya R, Bansal V, Pal OP, Sharma PK: High performance liquid chromatography: a short review. J Glob Pharma Technol. 2010; 2(5): 22-26.
- Conner’s KA: A Text Book of Pharmaceutical Analysis, CBS Publishers and Distributors Pvt. Ltd., New Delhi, 3rd edition 2001, 3-6.
- Jay BR, Kelvin J, Pierre B. Understanding and Implementing Efficient Analytical Methods Development and Validation, 2003.
- Chauhan A, Mittu B, Chauhan P. Analytical method development and validation: a concise review. J Anal Bioanal Tech. 2015; 6(1): 1-5.
- Lavanya G, Sunil M, Eswarudu MM, Eswaraiah MC, Harisudha K, Spandana BN. Analytical Method Validation: An updated review. Int J Pharm Sci Res. 2013; 4(4):1280-1286.
- Luxminarayan L, Sharma N, Viswas A, Khinchi MP. A review on chromatography techniques: Asian Journal of Pharmaceutical Research and Development. 2017; 5(2): 1-8
- Chavan D, Mahendra D. Analytical Method Validation: A Brief Review. World Journal of Advanced Research and Reviews 2022; 16 (2): 389–402.
- Mohite VI, Malviya K, Ghodke D, Gupta V. Analytical method development and validation of some topical antimicrobial formulations according to ICH guidelines: Journal of Population Therapeutics and Clinical Pharmacology 2023; 29 (04): 1123–1136.
- Seerapu, S. Developement and Validation of RP-HPLC Method for the estimation of lvabradine Hydrochloride in tablets: Indian Journal of Pharamaceutical Sciences 2010, 72(5), 667.
- Thete PG, Saudagar, Bhanudas R. Analytical Method Development and Validation for the Determination of Ivabradine HCl by RP-HPLC in Bulk and Pharmaceutical Dosage Form: Asian Journal of Pharmacy and Technology. 2019; 9 (2): 89-92.
- Maheshwari S, Khandhar A, Jain A. Quantitative Determination and Validation of Ivabradine HCL by Stability Indicating RP-HPLC Method and Spectrophotometric Method in Solid Dosage Form: Eurasian J. Anal. Chem 2010; 5 (1): 53–62.
- Spandana K, Subhashini N. Analytical Method Development and Validation of Brivaracetam in Bulk and Pharmaceutical Dosage Form by RP-HPLC Method. IOSR Journal of Pharmacy 2021; 11, (1): 26–33.
- Bhadru B, Kumar PN, Sujatha D, Renukuntla P, Bogula N. Analytical Method Development and Validation of Brivaracetam in API and Marketed Formulation by RP-HPLC: Asian Journal of Pharmaceutical Research and Development 2024; 12 (2): 154–159.
- Kasawar G, Farooqui M. Development and Validation of HPLC Method for the Determination of Pregabalin in Capsules: Indian J Pharm Sci. 2010; 72(4): 517–519.
- Balaji J, Ramchandra B.,et al. Analytical RP-HPLC Method for Development And Validation Of Pregabalin In Bulk And The Determination Of Pregabalin In Capsule Dosage Form: International Journal of Innovative Research in Science, Engineering and Technology. 2014; 3. (4): 11094-11098
- Seema A, Jeeja P. Development and Validation of HPLC Method for Estimation of Pregabalin in Bulk & Capsule Dosage Form: Pharmaceutical Analytical Acta. 2016; 7, (6): 1-6.
- Valamathy J, Samuelijoshus L. RP-HPLC Method Developemnt and Validationa for Assay of Levetiracetam in tablet dosage form: Research Journal of Pharmacy and Technology. 2008; 1,(4): 395-397.
- Ravisankar P, Niharika, A. et al. A simple validated UV spectrophotometric method for quantitative analysis of Levetiracetam in pharmaceutical dosage form: Indian Journal of Research in Pharmacy and Biotechnology. 2015; 3(5): 380-385.
- Masud A, Arefeen MD.; et al. Development and validation of a RP-HPLC Method for the Estimation of Levetiracetam in Bulk and Pharmaceutical Formulation: Journal of Chemical and Pharmaceutical Research. 2011; 3(1): 324-329.
- Kumar A, Joshi M.; et al. Development and validation of analytical method for the estimation of lamotrigine in human plasma: World journal of pharmacy and pharmaceutical sciences. 2017; 6, (8): 2658-2669.
- M, Mahapatra, D.; et al. Analytical quality-by-design (AQBD) approach for the development and validation of RP-HPLC method for the estimation of lamotrigine in bulk and tablet formulation: Journal of medical pharmaceutical and allied sciences. 2021; (10) 5: 3591-3596.
- Ahmad R, Soni P.; et al. Development and validation of a stability indicating rphplc assay method for determination of lamotrigine in tablet formulation: Asian Journal of Pharmaceutical Education and Research. 2018; (7) 4: 57-67.
- Oleksandra Vozniuk, Zden?k Kejík, Kate?ina Veselá, Markéta Skali?ková, Petr Novotný, Róbert Hromádka, Hajduch J, Pavel Martásek, Jakubek M: A Fast HPLC/UV Method for Determination of Ketoprofen in Cellular Media: ChemistryOpen 2023; 13 (3): 1-9.
- Hassan AA, Shantier SW. Development and validation of uv-visible spectrophotometric method for estimation of ketoprofen in capsule and tablet dosage forms: Zenodo (CERN European Organization for Nuclear Research 2019: 9(01): 1685-1870.
- Zafar F, Shoaib MH, Naz A, Yousuf RI, Ali H. Determination of Ketoprofen in Human Plasma by RP-HPLC: American Journal of Analytical Chemistry 2013; 04 (05): 252–257.
- Tadjuddin N, Wahyu RS, Ilas A, Dali S, Baharuddin H. Validation of Analysis Method for Determining Ketoprofen Concentration in Pharmaceutical Dosage Form Using High Performance Liquid Chromatography: European Journal of Chemistry 2013; 4 (1): 58–60.
- Srivastava M, Kohli K, Ali M. Stability Indicating RP-HPLC Method for Analysis of Ketoprofen in Bulk Drug and Eugenol Containing Nanoemulsion Gel (NEG) Formulation Using Response Surface Methodology: Current Pharmaceutical Analysis 2014; 10 (2): 135–144.
- Borges KB, Sánchez AJ, Pupo MT, Bonato PS, Collado IG. Ultra-Fast Gradient LC Method for Omeprazole Analysis Using a Monolithic Column: Assay Development, Validation, and Application to the Quality Control of Omeprazole Enteric-Coated Pellets: Journal of AOAC International 2010; 93 (6): 1811–1820.
- Murakami FS, Cruz AP, Pereira R, Valente B, Antonio M. Development and Validation of a RP?HPLC Method to Quantify Omeprazole in Delayed Release Tablets: Taylor and Francis 2007; 30 (1): 113–121.
- Mohamed AM. Development and Validation for RP-HPLC Method of Assay of Omeprazole Capsules Formulation: American Journal of Chemical Engineering 2018; 6 (1): 1-6.
- Nataraj KS, Mohammad BD, Pragallapati K, Dussa KK. Development and Validation of RP-HPLC Method for the Estimation of Omeprazole in Bulk and Capsule Dosage Forms: International Current Pharmaceutical Journal (2012), 1 (11), 366–369.
- Vijayaraghavan R, Jayababu G, Prasad R, Thirugnanam PE, Kumar GR: bio-analytical method development and validation for omeprazole using lc-ms/ms. 2015: 2(9):2475-81.
- Koppala S, reddy V, Anireddy JS: Development and Validation of a Novel Stability-Indicating RP-HPLC Method for the Simultaneous Determination of Related Substances of Ketoprofen and Omeprazole in Combined Capsule Dosage Form. 2016; 54 (5): 765-775.
- Maslarska, V., et al.: "High pressure liquid chromatographic method for simultaneous determination of ketoprofen and omeprazole." Pharmacia 65 (2018), 3-8.
- Tambe V, Vichare V, Kolte R,S', P. Development and Validation of an HPTLC Method for Simultaneous Estimation of Omeprazole and Ketoprofen in a Developed Tablet Formulation: Journal of International Journal of PharmTech Research CODEN 2014: 6 (2): 634–640


 Mukesh Patil*
Mukesh Patil*
 Ashish Jain
Ashish Jain
 Roshani Dhumal
Roshani Dhumal
 Shrusti Kadave
Shrusti Kadave
 Aishwarya Patil
Aishwarya Patil




 10.5281/zenodo.14506358
10.5281/zenodo.14506358