Abstract
Transdermal drug delivery systems (TDDS), sometimes known as “patches,” are dosage forms designed to disperse a therapeutically active dose of medication across a patient’s skin in order to create systemic effects. Transdermal drug delivery devices are used to administer medications topically. These are pharmacological preparations of different sizes that include one or more active compounds. They are meant to be administered topically to the intact skin and prevent first pass metabolism by delivering the active component after it has passed through the skin barriers. Currently, 74% of medications are taken orally and are not as effective as intended. Transdermal medication delivery systems were developed to increase efficacy. With TDDS, the medication enters the skin and travels to the target spot with ease. Transdermal drug delivery devices were created as a solution to the issues with oral medication administration. Since 1981, these systems have been used as trustworthy and safe drug delivery methods. Due to hepatic first pass metabolism and the propensity to cause both high and low blood level spikes, the typical oral dose forms have substantial downsides. These include poor bioavailability, which necessitates high and/or frequent dosing, which can be both expensive and inconvenient. Transdermal drug delivery systems (TDDS) have been developed to address these issues. By delivering medications to particular sites within the body, TDDS can reduce dosage size and increase safety while also improving therapeutic efficacy. TDDS is one such delivery method that has been thoroughly investigated with positive treatment outcomes during the past 25 years. TDDS is the perfect treatment for conditions that require ongoing care. Compared to the traditional oral dosing type, topical medication delivery has numerous benefits. The restriction of hepatic metabolism, improvement of therapeutic efficacy, and preservation of the drug's constant plasma level are significant benefits of TDDS.
Keywords
Polymer Matrix, Diffusion, First-generation, Second-generation, and Transdermal Drug Delivery Systems (TDDS) improvers of penetration, Microneedles with Iontophoresis.
Introduction
One technology that falls under the category of controlled drug delivery is the transdermal drug delivery system (TDDS), whose goal is to distribute the medication through the skin at a predetermined and regulated rate. TDDS are defined-surface adhesive drug-containing devices that release a fixed dose of medication on to undamaged skin at a predetermined rate in order to enter the systemic circulation. Transdermal delivery offers a significant advantage over oral and injectable methods due to its ability to prevent first-pass metabolism and increase patient compliance, respectively. The transdermal route has been in competition with oral treatment to be the most successful innovative drug delivery research area. This is because oral treatment involves introducing a fixed dose at regular intervals to maintain the drug concentration in the body within a therapeutically effective range, which increases the risk of side effects or therapeutic failure. Additionally, a significant amount of drug is lost in the vicinity of the target organ, necessitating close monitoring of therapy to prevent overdosing. Transdermal drug administration through intact skin can closely mimic the benefits of intravenous drug infusion, without the potential risks, and overcome the limitations of the oral route. This includes the ability to avoid hepatic “first pass” hepatic elimination (HEPE) and maintain constant, prolonged, and therapeutic effective drug levels in the body. A transdermal patch, often known as a skin patch, regulates the speed at which the liquid medication inside the reservoir of the patch can permeate the skin and enter the bloodstream using a unique membrane.
These transdermal patches are classified into three types:
- Drug in adhesive (the drug is directly dispersed into the adhesive polymer),
- Reservoir (consists of a drug reservoir between a backing membrane and Rate-controlling membrane, with a skin-contacting adhesive layer)
- matrix (consists of a drug reservoir in the centre with a peripheral adhesive ring around the edges) (Tan, Pfister, 1999; Subedi et al., 2010).
? Definition:
An adhesive patch that has been medicated and applied to the skin to deliver a specified dosage of medication through the skin and into the bloodstream is called a transdermal patch.
? Drug Selection Criteria
- Dose should be low i.e. < 20mg>
- Half life should be 10h or less.
- Molecular weight should be <400>
- Partition coefficient Log Po/w should be between 1.0 and 4.
- Skin permeability coefficient should be 0.5x10-3 cm/h.
- Drug should be non-irritating and non-sensitizing to the skin.
- Oral bioavailability should be low.
- Therapeutic index should be low.
? Basic components of TDDS:
1.Polymer matrix
2.Drug
3.Permeation enhancers
4. Pressure sensitive adhesives
5. Backing laminates
6. Release liner
7. Miscellaneous expients
1. Polymer matrix:
The polymer regulates the drug's release from the apparatus.
The following polymers could be helpful for transdermal devices:
a. Natural polymers: such as natural rubber, starch, zein, gelatin, shellac, waxes, proteins, and gums and their derivatives.
b. Synthetic elastomers: such as Neoprene, Polybutadieine, Hydrin, Silicone, Nitrile, Acrylonitrile, Butyl, and Styrene butadieine rubber.
c. Synthetic polymers: such as polymethyl methacrylate, epoxy, polyvinyl alcohol, polyvinyl chloride, polyethylene, polypropylene, polyacrylate, polyamide, polyurea, and polyvinyl pyrrolidone,
2. Drug:
For successful development of a transdermal drug delivery system, the drug should be choosing with great care.
The properties of a drug for transdermal delivery are given below:
Chemical and Physical Properties:
• The medicine being formulated in TDDS need to have a molecular weight of no more than roughly 1000 Daltons.
• The medication needs to be affinities for hydrophilic and lipophilic phases. Maximum partitioning properties do not support effective topical medication administration.
• There should be a low melting point for the medication in TDDS.
• The medication must be strong, have a brief half-life, and not irritate or cause inflammation.
3.Penetration Enhancers:
These are substances that increase skin permeability by changing the skin’s ability to act as a barrier to the flow of a desired penetrant.
Enhancers of penetration are added to a mixture to increase the medication’s solubility and diffusivity through the skin that would cause the skin’s barrier resistance to reversibly decrease. Of the TDDS The Drone’s release Vices include: Zein, Gelatin, and Vatives their offspring, Natur Rubbe Hydrin Azone and its derivatives, alcohol and glycols, water, pyrolidones, fatty acids and alcohols, essential oils, terpenes and derivatives, sulfoxides and their derivatives, urea, and surfactant are among them.
Ideal properties of penetration enhancers:
? Controlled and reversible enhancing action
? Chemical and physical compatibility with drug and other pharmaceutical excipients
? Should not cause loss of body fluids, electrolytes or other endogenous materials
? Non toxic, non allergic, non irritating
? Pharmacological inertness
? Ability to act specifically for predictable duration
? Odorless, colorless, economical and cosmetically acceptable
These may conveniently be classified under the following main headings:
Solvents:
These are the compounds increases penetration possibly by Swallowing the polar pathway and/or by fluidizing lipids. Ex; water, alcohols – methanol and ethanol; alkyl methyl sulfoxides - dimethyl sulfoxide, alkyl homologs of methyl sulfoxide dimethyl acetamide and dimethyl formamide; pyrrolid- ones- 2 pyrrolidone, N-methyl, 2-purrolidone; laurocapram (Azone), miscellaneous solvents- propylene glycol, glycerol, silicone fluids, isopropyl palmitate.
Surfactants:
These compounds are enhances polar pathway transport, especially of hydrophilic drugs i.e drugs having low solubility. The ability of a surfactant to change penetration is a function of the polar head group and the hydrocarbon chain length.
? Anionic Surfactants:
e.g. Sodium lauryl sulphate, Decodecyl methyl sulphoxide, Dioctylsulphosuccinate, etc.
? Nonionic Surfactants:
e.g. Pluronic F127, Pluronic F68, etc.
? Bile Salts:
e.g. Sodium deoxycholate, Sodium tauroglycocholate Sodium mstaurocholate.
? Binary system:
These systems apparently open up the heterogeneous multilaminate pathwayas well as the continuous pathways. e.g. Propylene glycol-oleic acid and 1, 4-butane diol-linoleic acid.
Lipid action
Some enhancers interact with the organized intracellular lipid structure of the stratum corneum so as to disrupt it and make it more permeable to drug molecules. Some solvents act by extracting the lipid components and thus make the horny layer more permeable.
Protein modification
Ionic surface active molecules in particular tend to interact well with the keratin in the corneocytes, to open up the dense keratin structure and make it more permeable. The intracellular route is not usually prominent in drug permeation, although drastic reductions to this route could open up an alternative path for drug penetration.
Partitioning promotion
Many solvents can enter the stratum corneum, change its solvent properties and thus increase the partitioning of a second molecule into the horny layer. This molecule may be a drug, a co enhancer or a co-solvent. C.g. Ethanol has been used to increase the penetration of the drug molecules nitroglycerin and estradiol.
- Miscellaneous chemicals:
These include a hydrating and keratolytic agenturea; N, N-dimethyl-m-toluamide; calcium thioglycolate; anticholin- ergic agents.
4. Pressure Sensitive Adhesives (PSA):
A PSA can be used to adhere any transdermal device to the skin. It should be placed on the device’s face or at its rear and extend outward.
? The first strategy is the creation of novel polymers, such as polyurethanes and hydrogel hydrophilic polymers.
? The second strategy involves altering the chemistries of the PSAs that are currently in use, such as acrylates and silicones, either chemically or physically.
Physical modification is the process of creating base adhesives with special additives that work in concert with the medicine and excipients in the system formulation to improve skin adhesion and promote drug distribution.
To increase drug delivery rates, chemical modification entails grafting or chemically adding functional monomers to the traditional PSA polymers.
5. backing laminates:
Laminating backings are chosen for look, adaptability, and occlusion requirement. Aluminium vapour coated layers, polyester, polyethylene, and polyolefin films are a few types of backings. Additional assiduities include the backing’s additives seeping out and the medicine or compositions diffusing through it. Overemphasising chemical resistance can frequently result in stiffness and high occlusivity to air and moisture vapour. When used repeatedly, it raises the TDDS and could irritate the skin.
6. Release liner:
During storage the patch is covered by a protective Liner that is removed and discarded before the application of the Patch to the skin. Since the liner is in intimate contact with the TDDS, the liner should be chemically inert. The release liner is Composed of a base layer which may be non-occlusive (e.g. paper Fabric) or occlusive (e.g. polyethylene, polyvinylchloride) and a Release coating layer made up of silicon or Teflon. Other materials Used for TDDS liners include, polyester foil and metalized Laminate that protects the patch during storage. The liner is Removed prior to use.
7. Other excipients:
Various solvents such as chloroform, Methanol, acetone, isopropanol and dichloromethane are used to Prepare drug reservoir. In addition, plasticizers such as dibutyl- Phthalate, trietyl citrate, polyethylene glycol and propylene glycol Are added toprovide plasticity to the transdermal patch.
A) Adhesive:
The glue is typically used to secure the transdermal dose form. Polyisobutylenes, acrylics, and silicones are the polymers employed in the adhesives. The glue needs to meet these requirements.
• Avoid causing skin irritation or sensitisation.
• It should be simple to remove;
• It should adhere to the skin during the dosing time.
• It must not leave any residue that cannot be cleaned. The following requirements should be met by the face adhesive system.
• It must be in harmony with the medication, excipients, and enhancers both chemically and physiologically.
• There shouldn’t be any impact on the drug’s penetration. R) Reservation lining:
• There shouldn’t be any changes to the permeation enhancers’ dispersion.
- Backing Membrane:
They are adaptable, offer a strong attachment to the medication reservoir, stop the medication from escaping the dosage form through the top, and allow printing. When applied to the skin, the product is shielded by an impenetrable membrane. It has formulation both during the wearing duration and the shelf life. It needs to work with formulations that aren't adsorbent, including metallic plastic laminate and plastic backing with an adhesive foam pad that absorbs moisture.
Plasticizer and solvents:
Plasticizer
In transdermal systems, plasticizers are used to improve the brittleness of the polymer and to Provide flexibility. They are generally non-volatile organic liquids or solids with low melting Temperature and when added to polymers, they cause changes in definite physical and mechanical Characteristics of the material. Upon addition of plasticizer, flexibilities of polymer Macromolecules or macromolecular segments increase as a result of loosening of tightness of
Intermolecular forces. Many of polymers used in pharmaceutical formulations are brittle and Require the addition of plasticizer into the formulation. The plasticizers with lower molecular Weight have more molecules per unit weight compared to the plasticizers with higher molecular Weight. These molecules can more easily penetrate between the polymer chains of the film Forming agent and can interact with the specific functional groups of the polymer. By adding plasticizer to a polymeric material, elongation at break, toughness and flexibility are expected to Increase; on the other hand, tensile stress, hardness, electrostatic chargeability, and glass Transition temperature (Tg) are expected to decrease.
Solvents
Various solvents are used to solve or disperse the polymer and adhesive or drug used in preparation of transdermal system. Among those chloroform, methanol, acetone, iso propanol and dichloromethane are used frequently.
? Factors affecting permeation:
Skin is multi-layered organ composed of many histological layers. The major divisions of the skin, from top to bottom, are the epidermis, the dermis and the hypodermis.
• Stratum Corneum (SC) forming the outer most layer of theepidermis, exposing to the external environment. This is the most important layer to Transdermal delivery as its composition allows it to keep water within the body and foreign substances out
• The boundary between dermisand epidermis layer is called Dermal-Epidermal junction which provides a physical barrier for the large molecules of drug and cells.
• When a molecule reaches intact skin, it contacts with the cellular debris, normal flora of microorganisms, sebum and other materials.
• The major and principle transport mechanism across mammalian skin is by passive diffusion through primarily the trans
epidermal route at steady state or through trans-appendageal route at initially, non-steady state.
• The factors that affect the permeability of the skin are classified into following three categories:
- Physicochemical properties of the permeate molecule
- Physicochemical properties of the drug delivery system
- Physiological and pathological condition of the skin
- Physicochemical properties of the permeate molecule
1. physicochemical properties of the permeate molecule:
i. Partition co-efficient:
Drug possessing both water and lipid solubility are favorably absorbed through the skin. Transdermal permeability co-efficient shows a linear dependence partition coefficient. Varying the vehicle may also alter a lipid/water partition co-efficient of a drug molecule. The partition co-efficient of a drug molecule may be altered by chemical modification without affecting the pharmacological activity of the drug.
ii. Molecular size:
There is an inverse relationship existed between transdermal flux and molecular weight of the molecule. The drug molecule selected as candidates for transdermal delivery tend to lie within narrow range of molecular weight (100-500 Dalton).
iii. Solubility / Melting point:
Lipophilicity is a desired property of transdermal candidates as lipophilic molecules tend to permeate through the skin faster than more hydrophilic molecules. Drugs with high melting points have relatively low aqueous solubility at normal temperature and pressure.
iv. pH condition:
The pH mainly affects the rates of absorption of acidic and basic drugs whereas unchanged form of drug has better penetrating capacity. Transport of ionizable species from aqueous solutions shows strong pH dependence. According to pH partition hypothesis, only the unionized form of the drugs can permeate through the lipid barrier in significant amounts.
2. Physicochemical properties of the drug delivery system
1. The affinity of the vehicle for the drug molecules:
It can influence the release of the drug molecule from the carrier. Solubility in the carrier determines the release rate of the drug. The mechanism of drug release depends on whether the drug is dissolved or suspended in the delivery/carrier system and on the interfacial partition co-efficient of the drug from the delivery system to skin tissue.
ii. Composition of drug delivery system:
Composition delivery system may affect not only the rate of drug release but also the permeability of the SC by means of hydration. Of drug
iii. Enhancement of transdermal permeation:
Due to the dead nature of the SC the release of the drug from the dosage form is less. Penetration enhancers thus can cause the physicochemical or physiological changes in SC and increase the penetration of the drug through the skin. Various chemical substances are found to possess such drug penetration enhancing property
3. Physiological and pathological condition of the skin:
i. Skin age:
Foetal and infant skin appears to be more permeable than mature adult skin and therefore percutaneous absorption of topical steroids occurs more rapidly in children than in adults whereas, water permeation has shown to be same in adults and in children
ii.Lipid film:
The thin lipid film on skin surface is formed by the excretion of sebaceous glands and celllipids like sebum and epidermal cell which contain emulsifying agent may provide a protective film to prevent the removal of natural moisturizing factor from the skin and help in maintaining the barrier function of the SC.
iii.Skin hydration :
Hydration of SC can enhance transdermal permeability. The rate of penetration study of salicylic acid through skin with dry and hydrated corneum showed that when the tissues were hydrated, the rate of penetration of the most water-soluble esters increased more than that of the other esters.
iv. Skin temperature :
Raising skin temperature results in an increase in the rate of skin permeation. Rise in skin temperature may also increase vasodilation of blood vessels, which are in contact with skin leading to an increase in percutaneous absorption.
V) Cutaneous drug metabolism:
After crossing the SC barrier, some of the drug reaches the general circulation in active form and some of this in inactive form or metabolic form, because of the presence of metabolicenzymes present in the skin layers. It was reported that more than 95% of testosterone absorbed was metabolized as it present through the skin.
Vi.Species differences:
Mammalian skin from different species display wide differences in anatomy in such characteristics as the thickness of SC, number of sweat glands and hair follicles per unit surface area.
Vii. Pathological injury to the skin:
Injuries to the skin can cause the disturbance in the continuity of SC and leads to increase in skin permeability.
? Formulation of transdermal drug delivery system:
Various components of a transdermal drug delivery system are:
Drug substance:
For successfully developing a transdermal drug delivery system, the drug should be chosen with great care. The following are some of the desirable properties of a drug for transdermal delivery.
Physicochemical properties:
- The drug should have a molecular weight less than 1000 Daltons.
- The drug should have affinity for both lipophilic and hydrophilic phasca. Extreme partitioning characteristics are not conductive to successful drug delivery via the skin.
- The drug should have low melting point.
- Along with these properties the drug should be potent, having short half life and be non- irritating.
Biological Properties:
- Drug should be very potenti.e. it should be effective in few mg/day
2. The drug should have short biological half life.
3. The drug should not be irritant and non allergic to human skin.
4. The drug should be stable when contact with the skin.
5. They should not stimulate an immune reaction to the skin.
6. Tolerance to the drug must not develop under near zero order release profile of transdermal delivery.
7. Dose is less than 50 mg per day, and ideally less than 10 mg per day.
8. The drug should not get irreversibly bound in the subcutaneous tissue.
9. The drug should not get extensively metabolized in the skin.
Polymer matrix:
Polymers are the backbone of transdermal drug delivery system. System for transdermal delivery
Are fabricated as multi layered polymeric laminates in which a drug reservoir or a drug polymer matrix is sandwiched between two polymeric layers, an outer impervious backing layer that prevents the loss of drug through the backing surface and an inner polymeric layer that functions as an adhesive, or rate controlled membrane.
Ideal properties of a polymer to be used in a transdermal system:
? Molecular weight, chemical functionality of the polymer should be such that the specific drug diffuses properly and gets released through it.
? The polymer should be stable,
? The polymer should be nontoxic
? The polymer should be easily of manufactured
? The polymer should be inexpensive
? The polymer and its deaggration product must be non toxic or non-antagonistic to the host.
? Large amounts of the active agent are incorporated into it.
? The polymers utilized for TDDS can be classified as:
Natural polymers cellulose derivatives, zein, gelatin, shellac, waxes, gums, natural rubber Chitosan, starch, etc.
Synthetic elastomers polybutadiene, polyisobutylene, silicon rubber, nitrile, acrylonitrile. Styrene-butadiene rubber, neoprene, butylrubber, polysiloxane, etc.
Synthetic polymers polyvinyl alcohol, polyvinylchloride, polyethylene, polypropylene, polyacrylate, polyamide, polyurea, polyvinylpyrrolidone, epoxy polymethylmethacrylate, ethyl cellulose, hydroxy propyl cellulose etc. The polymers like cross linked polyethylene glycol, eudragits, ethyl cellulose and hydroxyl propyl methylcellulose are used as matrix formers for TDDS. Other polymers like EVA (Ethyl vinyl acetate), silicon rubber and polyurethane are used as rate controlling membrane.
? Structure-Based Enhancement Techniques
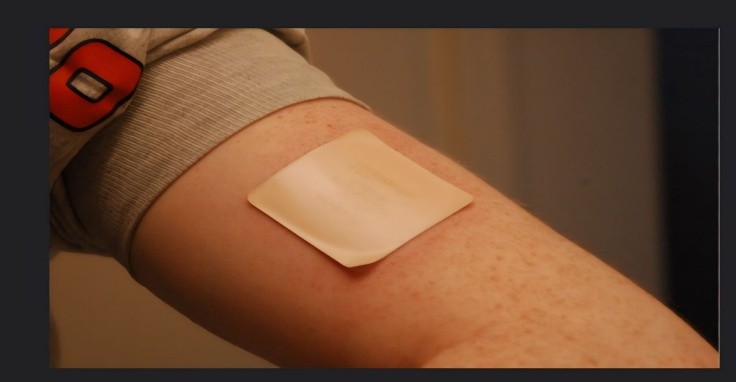
Transdermal Patches
A transdermal patch or skin adhesive patch is that device which is loaded with drug candidate And usually applied on the skin to transport a specific dose of medication across the skin and into The blood circulation. An adhesive serves two functions: It is glue in nature that keeps the patch Adhered to the skin, and it acts as the suspension that holds the drug. The problems associated
With this is the concentration of the drug within the adhesive directly affects the “stickyness” of The adhesive so if the large quantities of drug is to be administered, either the size of the patch Have to be increased or the patch needs to be reapplied again and again. Several usually combined with substances, like alcohol, within the patch to improve their penetration via Skin in order to improve absorption.
Components of Transdermal Patches:
1. Liner – Protects the patch during storage. The liner should be removed before its use.
2. Drug-Drug solution is in direct contact with release liner.
3. Adhesive- It serves to adhere the components of the patch together along with adhering the Patch to the skin. E.g. – Acrylic, polyisobutylene (PIB), and silicone are the adhesives have many Pharmaceutical applications. For applications in which the adhesive, the drug, and perhaps Enhancers are compounded, the selection of a PSA is more complex (e.g., a matrix design).
4. Membrane- It controls the release of the drug from the reservoir and multi-layer patches.
5. Backing- The film protects the patch from the outer environment
Microfabricated Microneedles
These are the devices which are having the features of both the Hypodermic needle and transdermal patch that can deliver the drug that transports the drug Effectively across the memberane. The systems consists of a drug reservoir and some projections (microneedles) extending from the reservoir, these helps in penetrating the stratum cornea and Epidermis to deliver the drug.NG TDDS Poke with patch approach- Involves piercing into the Skin followed by application of the drug patch at the site of treatment. Coat and poke approach- Needles coated with the drug are inserted into the skin and release of medicament is then occurs
By dissolution.
? Biodegradable microneedles: Involves encapsulation of the drug within the biodegradable, Polymeric microneedles, which is then inserted into the skin.
? Hollow microneedles: Involves injecting the drug through the needle with a hollow bore.
Macroflux
These are devices having an area of around 8cm as well as 300 micro projections per cm2 with The length of individual micro projection less than 200μm. Three types of Macroflux have been Designed. They include, Dry-Coated Macroflux system-this is used for short period delivery that Consists micro projection array coated with medicament that adhered to a elastic polymer Adhesive backing.
Metered-Dose Transdermal Spray (MDTS)
It is a liquid preparation in the form of solution that are used topically which is made up of a Vehicle that is volatile come non volatile in nature, which consists the completely dissolved Medicament in solution. The use of MDTS reaches the sustained level and better permeation of The drug via skin.
The MDTS has the following potential advantages:
? Improves delivery potential without skin irritation due to its non-occlusive nature.
?Increased acceptability.
?Dose flexibility
? Simple manufacture
? Electrically-Based Enhancement Techniques
Iontophoresis
It involves passing of current (few milliamperes) to skin limited to a certain area using the Electrode remains in contact with the formulation which is to be administered. Pilocarpine Delivery can be taken as example to induce sweat in the diagnosis of cystic fibrosis and Iontophoretic delivery of lidocaine is considered to be a nice approach for rapid onset of Anesthesia.
Ultrasound
In this technique, there is a mixing of drug substance with a coupling agent (usually with gel, Cream or ointment) that causes ultrasonic energy transfer from the system to the skin. This Involves rupturing the lipids present in stratum cornea, which allows the medicament to permeate Via biological barrier.
Photomechanical Waves
Photomechanical waves significantly led to the stratum cornea highly permeable to drug Substance through a possible permeabilisation mechanism due to development of transient Channels.
Electroporation
It this method, short and high-voltage electrical pulses are applied to the skin thus the diffusion Of drug is improved with the increasing permeability. The electrical pulses are considered to form Small pores in the stratum cornea, through which transportation of drug occurs. For the safe and Painless administration, the electrical pulses introduced by closely spaced electrodes to reserved The electric field within the stratum cornea.
Electro-Osmosis
To the porous membrane which is having some charge, a voltage difference is applied to it, thus A bulk fluid or volume flow takes place with no concentration gradients. This process is known as electro-osmosis.
? Velocity Based Enhancement Techniques
Needle-Free Injections
?Intraject
? Implaject
?Jet Syringe
? Iject
?Mini-ject
Powder Ject Device
The solid drug particles are propelled across the skin with the aid of high-speed gas flow. This Consists of a gas canister that allows helium gas at high pressure to enter a chamber at the end of Which drug cassette containing powdered drug between two polycarbonate membranes. After Release, the instantaneous rupturation of both membranes usually seen that results in the gas to Expand quickly which forms a strong motion like a wave that travels down the nozzle. This takes Place at the speed of 600-900 m/s.
Other Enhancement Techniques
Transfersomes
This device penetrates the skin barrier along the skin moisture gradient. Transfersome carriers Can create a drug depot in the systemic circulation that is having a high concentration of drug. Transfersomes contain a component that destabilizes the lipid bilayers and thus leading to the Deformable vesicles.
Medicated Tattoos
Medical Tattoos is a modification of temporary tattoo which contains an active drug substance For transdermal delivery. This technique is useful in the administration of drug in those children Who are not able to take traditional dosage forms.
Skin Abrasion
This involves direct removal or disruption of the upper layers of the skin to provide better Permeation of topically applied drug substance. In general, one approach is adopted to create Micro channels in the skin by eroding the impermeable outer layers with sharp microscopic metal Granules are generally known as Microscissuining.
Controlled Heat Aided Drug Delivery (CHADD) System
It facilitates the transfer of drug substance to the blood circulation by applying heat to the skin That increases the temperature and ultimately led to increase in microcirculation and permeability In blood vessel. CHADD system consists of small unit that is used for heating purpose, placed on Top of a conventional patch device. An oxidation reaction occurs within the unit which tends to Form heat of limited intensity and duration.
Laser Radiation
This involves the exposure of the skin to the laser beam that results in the ablation of the stratum Cornea without damaging the epidermis which remains in contact with it. Removal of the stratum Cornea by this technique is considered to improve the delivery of lipophilic and hydrophilic Drugs.
Magnetophoresis
The effect of magnetic field on diffusion flux of drug substance was found to enhance with Increasing applied strength.
? Evaluation of transdermal patches:
The transdermal patches can be characterized in terms of following parameters
1. Physicochemical evaluation
2. In vitro evaluation
3. In vivo evaluation
Physicochemical evaluation:
Transdermal patches can be physicochemically evaluated in terms of these parameters:
Thickness: The thickness of transdermal film is determined by travelling microscope, dial gauge, screw Gauge or micrometer at different points of the film.
Uniformity of weight: Weight variation is studied by individually weighing 10 randomly selected patches and Calculating the average weight. The individual weight should not deviate significantly from the Average weight.
Drug content determination: An accurately weighed portion of film (about 100 mg) is dissolved in 100 mL of suitable solvent In which drug is soluble and then the solution is shaken continuously for 24 h in shaker Incubator. Then the whole solution is sonicated. After sonication and subsequent filtration, drug In solution is estimated spectrophotometrically by appropriate dilution.
Content uniformity test:
10 patches are selected and content is determined for individual patches. If 9 out of 10 patches Have content between 85% to 115% of the specified value and one has content not less than 75% To125% of the specified value, then transdermal patches pass the test of content uniformity. But if 3 patches have content in the range of 75% to 125%, then additional 20 patches are tested for Drug content. If these 20 patches have range from 85% to 115%, then the transdermal patches
Pass the test.
Moisture content:
The prepared films are weighed individually and kept in a desiccators containing calcium Chloride at room temperature for 24 h. The films are weighed again after a specified interval until They show a constant weight. The percent moisture content is calculated using following formula.
% Moisture content = Initial weight – Final weight X 100
Moisture Uptake:
Weighed films are kept in a desiccator at room temperature for 24 h. These are then taken out and Exposed to 84% relative humidity using saturated solution of Potassium chloride in a desiccator Until a constant weight is achieved. % moisture uptake is calculated as given below.35 %
Moisture uptake = Final weight – Initial weight X 100
Flatness:
A transdermal patch should possess a smooth surface and should not constrict with time. This can Be demonstrated with flatness study. For flatness determination, one strip is cut from the centre And two from each side of patches. The length of each strip is measured and variation in length is Measured by determining percent constriction. Zero percent constriction is equivalent to 100
Percent flatness.
% constriction = I1 – I2 X 100
I2 = Final length of each strip
I1 = Initial length of each strip.
Folding Endurance:
Evaluation of folding endurance involves determining the folding capacity of the films subjected To frequent extreme conditions of folding. Folding endurance is determined by repeatedly folding The film at the same place until it break. The number of times the films could be folded at the Same place without breaking is folding endurance value.
Tensile Strength:
To determine tensile strength, polymeric films are sandwiched separately by corked linear iron Plates. One end of the films is kept fixed with the help of an iron screen and other end is Connected to a freely movable thread over a pulley. The weights are added gradually to the pan Attached with the hanging end of the thread. A pointer on the thread is used to measure the Elongation of the film. The weight just sufficient to break the film is noted.
Tack properties:
It is the ability of the polymer to adhere to substrate with little contact pressure. Tack is Dependent on molecular weight and composition of polymer as well as on the use of tackifying Resins in polymer.
Thumb tack test:
The force required to remove thumb from adhesive is a measure of tack.
Rolling ball test:
This test involves measurement of the distance that stainless steel ball travels along an upward Facing adhesive. The less tacky the adhesive, the further the ball will travel.
Quick stick (Peel tack) test:
The peel force required breaking the bond between an adhesive and substrate is measured by Pulling the tape away from the substrate at 90 at the speed of 12 inch/min.
Probe tack test:
Force required to pull a probe away from an adhesive at a fixed rate is recorded as tack.
In vitro release studies:
Transdermal patches can be in vitro evaluated in terms of Franz diffusion cell the cell is Composed of two compartments: donor and receptor. The receptor compartment has a volume of 5-12ml and effective surface area of 1-5 cm2. The diffusion buffer is continuously stirred at 600rpm by a magnetic bar. The temperature in the bulk of the solution is maintained by Circulating thermostated water through a water jacket that surrounds the receptor compartment. The drug content is analyzed using suitable method, maintenance of sink condition is essential.33
In vivo Studies:
Transdermal patches can be in vivo evaluated in terms of In vivo evaluations are the true Depiction of the drug performance. The variables which cannot be taken into account during in Vitro studies can be fully explored during in vivo studies. In vivo evaluation of TDDS can be Carried out using animal models human volunteers.
Animal models:
Considerable time and resources are required to carry out human studies, so animal studies are Preferred at small scale. The most common animal
Species used for evaluating transdermal drug delivery system are mouse, hairless rat, hairless Dog, hairless rhesus monkey, rabbit, guinea pig etc. Various experiments conducted leads to a Conclusion that hairless animals are preferred over hairy animals in both in vitro and in vivo Experiments. Rhesus monkey is one of the most reliable models for in vivo evaluation of Transdermal drug delivery in man.
Human model
The final stage of the development of a transdermal device involves collection of
Pharmacokinetic and pharmacodynamic data following application of the patch to human Volunteers. Clinical trials have been conducted to assess the efficacy, risk involved, side effects, Patient compliance etc. Phase I clinical trials are conducted to determine mainly safety in Volunteers and phase II clinical trials determine short term safety and mainly effectiveness in Patients. Phase III trials indicate the safety and effectiveness in large number of patient Population and phase IV trials at post marketing surveillance are done for marketed patches to Detect adverse drug reactions. Though human studies require considerable resources best to Assess the performance of the drug.
REFERENCES
- Han T., Das D.B. Potential of Combined Ultrasound and Microneedles for Enhanced Transdermal Drug Permeation: A Review. Eur. J. Pharm. Biopharm. 2015; 89:312-328. Doi: 10.1016/j.ejpb.2014.12.020.
- Schoellhammer C.M., Blankschtein D., Langer R. Skin Permeabilization for Transdermal Drug Delivery: Recent Advances and Future Prospects. Expert Opin Drug Deliv. 2014; Doi: 10.1517/17425247.2014.875528.
- Wilkosz, M.F., Transdermal Drug Delivery: Part I. U.S. Pharmacist. Jobson publication, 2003: 04.
- Kharat Rekha Sudam and Bathe Ritesh Suresh, A Comprehensive Review on: Transdermal drug delivery Systems, International Journal of Biomedical and Advance Research, ISSN: 2229-3809 (Online); 2455-0558
- Rajesh Mujoriya” and Kishor Dhamande, A Review on Transdermal Drug Delivery System. Research J. Science and Tech. 3(6): Sept.-Oct. 2011: 227-231 GSC Biological and Pharmaceutical Sciences, 2023, 22(02), 245-255
- Jalwal P, Jangra A, Dhaiya L, Sangwan Y, Saroha R. A review on transdermal patches. Pharm Res. J. 2010; 3:139- 149.
- Kharat Rekha Sudam and Bathe Ritesh Suresh, A Comprehensive Review on: Transdermal drug delivery systems, International Journal of Biomedical and Advance Research ISSN: 2229-3809 (Online); 2455-0558
- Loyd V. Allen Jr. Nicholas G. Popovich, Howard C. Ansel. Pharmaceutical dosage forms and drug delivery systems, Bth Edition, Wolter Kluwer Publishers, New Delhi, 2005 pp. 298-299.
- Rachel B. Geevarghese and Satish V. Shirolkar, formulation development of rosuvastatin calcium drug in adhesive transdermal system, JPSR, 2020; Vol. 11(8): 3902-3911
- Front Line Strategic Consulting Inc. Alternative Drug Delivery Systems Series: Transdermal Drug Delivery Systems. 2002, Front Line Strategic Consulting Inc.
- Mehta R. Topical and transdermal drug delivery: what a pharmacist needs to know. InetCE. 1st Ed., [ Arizona:2004:1-10.
- Jain.N. K, Controlled and novel drug delivery, first edition, CBS publishers and distributors, New Delhi. 1997.
- Mathiowitz.Z. E. Chickering, Lehr.C.M, Bio adhesive drug delivery systems; fundamentals, novel approaches and Development, Marcel Dekker, Inc. New York. Basel
- Sharma N, Parashar B, Sharma S, Mahajan U. Blooming Pharma Industry with Transdermal Drug Delivery System. ndo Global Pharm. Sci. 2012; 2(3): 262-278.
- Chad RW. Development and Selection of Components for Transdermal Drug Delivery Systems, [Internate).


 Magar Anuradha *
Magar Anuradha *
 Ulka mote
Ulka mote

 10.5281/zenodo.14209796
10.5281/zenodo.14209796