Abstract
The study focuses on traditional medicines, specifically the use of medicinal plants for health maintenance and illness treatment. It emphasizes the importance of using plant based treatments to mitigate issues associated with conventional therapies. Collect bulbs from Payangadi and prepare both aqueous and ethanolic extracts. The research involves Pharmacognostic evaluations, including microscopic and macroscopic analysis, followed by phytochemical studies. Finally assess the antimicrobial activity of the extracts. This approach highlights the potential of traditional remedies and the role of medicinal plants in modern healthcare.
Keywords
medicinal plants, phytochemical studies, Ethanolic Extract of Allium Sativum.
Introduction
Infection can be defined as the multiplication of microbes in the tissues of the host. The host may or may not be symptomatic. Microorganisms can cause diseases without actually come in contact with the host by virtue of toxin production. The pathogenic organisms are highly adapted to the pathogenic state and have developed characteristics that enable them to be transmit, attach to surfaces, invade tissue, avoid host defenses, and thus cause disease. Approximately 53 million deaths worldwide in 2009, at least a third were due to infectious diseases [1]. Recently, antibiotics and most drugs on the market have shown unwanted symptoms and the emergence of resistant pathogenic microorganisms, toxic effects related to these drugs, and withdrawal issues restricting their use in many countries [2].
Garlic is the most commonly used food and flavoring agent. When used as a food product, garlic is not produce any health benefits or side effects. When used as a medicinal product, garlic may produce both desired and unwanted effects in the body. Garlic products sold as health supplements may vary in the amount of allicin, the active ingredient in garlic. Allicin is unstable and can be reduced in garlic products when they are aged to reduce odor. Odorless garlic may contain little to no allicin. The lower the amount of allicin cause less effectiveness the product might be. Garlic taken orally has been used as alternative medicine in treating high blood pressure, coronary artery disease , circulation problems in the legs, high cholesterol, stomach ulcers caused by H. pylori, stomach cancer, colon cancer , rectal cancer etc. Garlic applied to the skin is used in treating fungal skin infections like ringworm, jock itch, or athlete's foot. Preventing the common cold and reversing difficulty urination in patients with prostatomegaly are uses not proved by research till now [3].Garlic is one of the edible part which have generated a lot of interest throughout human history as a medicinal plant. A wide range of microorganisms such as bacteria, fungi, protozoa ,viruses etc have been shown to be sensitive to crushed garlic preparations [4]. Allicin [S-(2-propenyl)-2-propene-1-sulfinothioate], the most biologically active sulfur-containing compound of garlic, is responsible for its smell and taste [5]. Alliin (S-allyl-L-cysteine sulfoxide) is the main precursor of allicin, which represents about 70% of total thiosulfinates existing in the crushed cloves [6].

Fig. 1

Fig. 2
Garlic has a variety of bioactive compounds, including organosulfur compounds, saponins, phenolic compounds, and polysaccharides . The major active components of garlic are its organosulfur compounds, such as diallyl thiosulfonate (allicin), diallyl sulfide (DAS), diallyl disulfide (DADS), diallyl trisulfide (DATS), E/Z-ajoene, S-allyl-cysteine (SAC), and S-allyl-cysteine sulfoxide (alliin) . In general, organosulfur compounds in raw garlic have higher digestibility than those in cooked garlic . In addition, saponins were found to be more stable in
the cooking process . The total amount of saponin in purple garlic was almost 40 times higher than that in white garlic, and several saponin compounds were only found to exist in purple garlic, such as desgalactotigonin-rhamnose, proto-desgalactotigonin, proto-desgalactotigonin- rhamnose, voghieroside D1, sativoside B1-rhamnose, and sativoside R1 . Moreover, garlic contained more than 20 phenolic compounds, with higher contents than many common vegetables . The main phenolic compound was ?-resorcylic acid, followed by pyrogallol, gallic acid, rutin, protocatechuic acid, as well as quercetin [7] . Recently garlic has been proven to be effective against a plethora of gram- positive, gram-negative and acid-fast bacteria and fungi. It has been documented that garlic exerts a differential inhibition between beneficial intestinal microflora and potentially harmful enterobacteria [8]. Garlic contains more than 200 chemical compounds with multiple properties. It is 65% water, 28?rbohydrates, 2.3% organosulfur compounds, 2% proteins, 1.2% free amino-acids, and 1.5% fiber. It also contains fat-soluble vitamins (vitamin A, vitamin K, and vitamin E), water-soluble vitamins (vitamin C, B-complex vitamins: B1, B2, B3, B6, and B8), and minerals (Ca, Fe, Mg, P, K, Na, and Zn). Organosulfur compounds give garlic its characteristic taste and odor as well as its pharmacological properties [9].
|
Biologically active compound
|
Biological functions
|
|
Alliin
|
Antioxidant, Antimicrobial
|
|
Allicin
|
Anticancer, Anti-inflammatory, Antimicrobial, Antioxidant, Cardio protective, Immunomodulatory
|
|
Allyl sulfide
|
Anticancer, Antimicrobial, Antioxidant, Antithrombotic
|
|
DADS
|
Anti-inflammatory, Antioxidant, Anticancer, Regulation of Metabolism, Detoxifying effects, Antimicrobial Activity, Antifungal Activity, Antiviral Activity, Cardiovascular protection, Neuroprotection
|
|
1,2-vinyldithiin
|
Antimicrobials, Antioxidants, Antithrombotic
|
|
Ajeons
|
Anticancer, Antimicrobial, Antioxidant, Cardio protective
|
|
DATS
|
Antioxidant
|
Antibacterial activity
Various garlic extracts (aqueous, chloroform, methanolic, and ethanolic extracts) were reported to inhibit the growth of several pathogenic bacteria with varying degrees of susceptibility [11]. Allicin’s antimicrobial activity is due to its chemical interaction with enzymes containing thiol e.g., thioredoxin reductase, RNA polymerase, and alcohol dehydrogenase by oxidizing protein cysteine or glutathione residues under physiological conditions [12].
Antifungal activity
The antifungal activity of various A. sativum extracts namely aqueous, ethanolic, methanolic, and petroleum ether against human pathogenic fungi such are Trichophyton verrucosum, T.mmentagrophytes, T.rubrum, Botrytiscinerea, Candida species, Epidermophyton floccosum, Aspergillus niger, A. flavus, Rhizopus stolonifera, Microsporum gypseum, M. audouinii, Alternaria alternate, Neofabraea alba, and Penicillium expansum. The garlic extract acted by affecting the fungal cell wall and causing irreversible ultrastructural changes in the fungal cells, which lead to loss of structural integrity and affected the germination ability. These changes in the cytoplasmic content lead to nucleus and cell organelles damage that ultimately leads to cell death [13].
Antiprotozoal activity
The aqueous, ethanolic, and dichloromethane A. sativum extracts exhibited anthelmintic activity against Haemonchus contortus and the ethanolic extract was the most effective one, while aqueous garlic extract showed potent activity against Trichuris muris and Angiostrongylus cantonensis . Garlic was also examined in vivo and in vitro against Taenia taeniaeformis, Hymenolepis microstoma, H. diminuta, Echinostoma caproni, and Fasciola hepatica [14].
Antiviral activity
In vivo experiment exhibited the antiviral activity of garlic extract and they reported that garlic showed protective activity against influenza viruses by improving the production of neutralizing antibodies when given to mice and this activity was based on the presence of several phytochemicals namely, ajoene, allicin, allyl methyl thiosulfinate, and methyl allyl thiosulfinate. Allicin acts by preventing several thiol enzymes, while ajoene’s antiviral activity was due to the prevention of adhesive interaction and fusion of leukocytes [15].
Disc diffusion method
Disc diffusion by the Kirby-Bauer method is a standardized technique for testing rapidly growing pathogens. It is the most flexible antimicrobial susceptibility testing method. The method consists of placing paper disks saturated with antimicrobial agents on a lawn of bacteria seeded on the surface of an agar medium, incubating the plate overnight, and measuring the presence or absence of a zone of inhibition around the discs. Because reproducibility depends on the log growth phase of organisms, fresh subcultures are used. Filter paper disks impregnated with a standardized concentration of an antimicrobial agent are placed on the surface, and the size of the zone of inhibition around the disk is measured after overnight incubation [16].
MATERIALS AND METHODS
Plant Material: The garlic bulb was collected from Payangadi town of Kerala during the month of December and authentified by botanist.
Bacterial strain
Escherichia Coli were used in the study. Pure culture was collected from Government Medical College, Pariyaram, Kannur, Kerala.
Preparation of Extract: The garlic bulb (150gm) was peeled, washed with distilled water thoroughly. 100 gm of garlic was weighed and partially crushed using clean mortar and pestle, 10 ml of distilled water was added to it and homogenized again with mortar and pestle and filtered through a clean muslin cloth. To this residue 5 ml distilled water was added and filtered again. The ethanolic extract was prepared using the same procedure with the exception of solvent which was 95% ethanol instead of distilled water. All the extracts were stored in a refrigerator.
Phytochemical screening
The extracts of Allium sativum bulb was analyzed for the presence of phytochemical constituents such as steroids, triterpinoids, carbohydrates, alkaloids, glycosides, phenolic compounds, flavonoids, saponins, tannins, amino acids and sulphur with the standard qualitative phytochemical methods.
|
Sl
No.
|
Qualitative Test
|
Aqueous Extract
|
Ethanolic Extract
|
-
|
Test for Steroids
|
|
|
|
|
Liebermann-Burchard test
|
+
|
+
|
|
|
Salkowski test
|
+
|
+
|
-
|
Test for Triterpenoids
|
+
|
-
|
-
|
Test for Carbohydrates
|
|
|
|
|
Molisch’s test
|
+
|
+
|
|
|
Fehling’s test
|
+
|
+
|
|
|
Benedict’s test
|
+
|
+
|
|
|
Barfoed’s test
|
+
|
+
|
-
|
Test for Alkaloids
|
|
|
|
|
Mayer’s test
|
-
|
+
|
|
|
Dragendroff’s test
|
+
|
+
|
|
|
Wagner’s test
|
+
|
+
|
|
|
Hager’s test
|
-
|
+
|
-
|
Test for Glycosides
|
|
|
|
|
Keller Kiliani test
|
+
|
+
|
-
|
Test for Phenolic compounds
|
|
|
|
|
Ferric chloride test
|
-
|
-
|
|
|
Lead acetate test
|
-
|
-
|
-
|
Test for Flavanoids
|
|
|
|
|
Shinoda test
|
+
|
-
|
|
|
Sodium hydroxide test
|
+
|
-
|
-
|
Test for Saponins
|
|
|
|
|
Foam test
|
-
|
+
|
-
|
Test for Tannins
|
|
|
|
|
Ferric chloride test
|
-
|
-
|
|
|
Lead acetate test
|
-
|
-
|
-
|
Test for Amino acids
|
|
|
|
|
Ninhydrin test
|
+
|
+
|
-
|
Test for Sulphur
|
|
|
|
|
Lead acetate test
|
+
|
+
|
|
|
Sodium Nitroprusside test
|
+
|
+
|
Antimicrobial Screening
Preparation of nutrient broth
Suspend 8.4 grams of Muller Hinton agar in 300 ml of distilled water. Add 2.5 mg of agar-agar as a solidifying agent. Autoclave the mixture at 1210C for 15 minutes. Once the nutrient agar has been autoclaved , allow it to cool but not solidify. When temperature reduces to 400C transfer a pure culture of organism to the agar broth. Pour the nutrient agar into 5 different petri dishes and leave the plates on a sterile surface until the agar has solidified.
Preparation of standard drug solution
Ciprofloxacin tablets (500mg) were powdered using a mortar and pestle.1g of the powdered tablets was weighed and dissolved in 100ml of distilled water.1ml of this solution was pipette out and transferred into a 10ml volumetric flask. The flask was then filled up to the 10 ml mark with distilled water.
Preparation of discs
Sterilize filter paper discs by autoclaving them at 1210C for 15 minutes. Immerse the sterilized discs in the aqueous extract of Allium sativum. Immerse another set of discs in the ethanolic extract of Allium sativum. Soak other sets of discs in the Ciprofloxacin solution and soak the remaining discs in distilled water as a control. Allow the soaked discs to dry.
Disc diffusion method
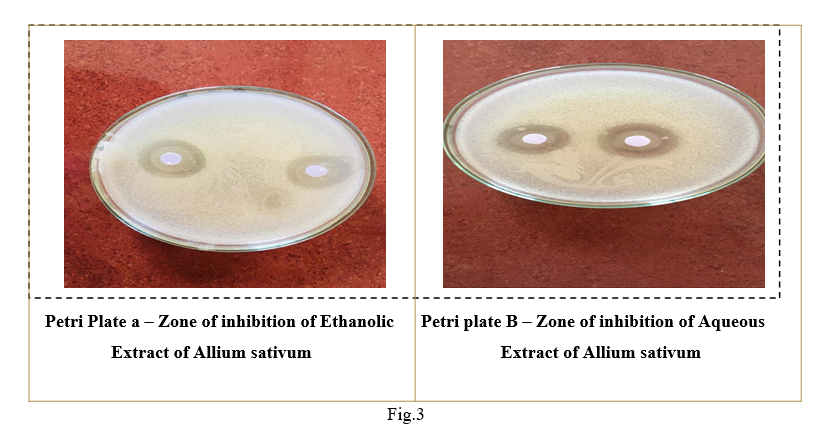
Using sterile forceps, each disc is placed on the surface of the inoculated and dried plate. Without moving the disc, fix it lightly to ensure complete contact. The disc should be positioned so that the minimum center-center distance is 24 mm and no closer than 10 to 15 mm from the edge of the petri dish. Four petri plates were prepared : Petri plate A (Ethanolic extract), Petri plate B (Aqueous extract), Petri plate C (Ciprofloxacin) and Petri plate D (control). All four prepared petri plates were observed for zone of inhibition is measured by using ordinary ruler.
|
Sl. No.
|
Samples
|
Zone of Inhibition (cm)
|
|
Disc-1
|
Disc-2
|
-
|
Ethanolic extract
|
1.7
|
1.8
|
-
|
Aqueous extract
|
1.9
|
2.0
|
-
|
Ciprofloxacin
|
3.5
|
3.4
|
-
|
Control
|
0
|
0
|
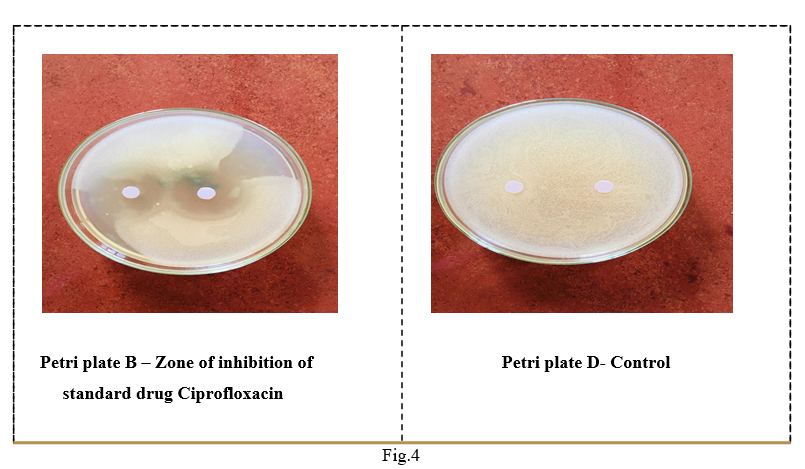
DISCUSSION
There’s a growing trend towards herbal remedies as people seek natural alternatives for health and wellness. With the rising issue of antimicrobial resistance due to the overuse of antibiotics, natural products like garlic have generated attention for their potential therapeutic effects and minimal side effects. Garlic, known for its numerous health benefits, contains a compound called Allicin, which exhibits significant antimicrobial properties. Research indicates that both ethanolic and aqueous extracts of garlic can inhibit microbial growth, but the aqueous extract shows a greater zone of inhibition compared to the ethanolic extract. This suggest that garlic may be a viable option for addressing certain infections while reducing reliance on conventional antimicrobial drugs.
CONCLUSION
This study provides valuable insights into the antibacterial properties of Allium sativum ( garlic bulb ) extracts. It’s clear that both aqueous and ethanolic extracts exhibit inhibitory effects on the tested bacteria, although they are less effective than Ciprofloxacin. The observation that the aqueous extract demonstrates more significant activity than the ethanolic one is particularly noteworthy. For future research, exploring the interactions between garlic extracts and standard antibiotics could yield important information about potential synergistic effects. Additionally, assessing the toxicity of theses extracts on human cells is crucial for understanding their safety profile. Standardizing dosages will also be essential for clinical applications. Overall, while garlic shows promise as an antimicrobial agent, further investigations are necessary to fully elucidate its potential role in infection treatment.
REFERENCES
- Machael Scheld W. Introdution to microbial disease : Host- pathogen interactions. Goldman’s Cecil Medicine, 24 (2), 2012, 1761-1762.
- Batiha G.E.S, Beshbishy A.M, Tayebwa D.S, Adeyemi O.S, Shaheen H, Yokoyama N, Igarashi I. Evaluation of the inhibitory effect of ivermectin on the growth of Babesia and Theileria parasites in vitro and in vivo. Tropical Medicine and Health, 2019, 42-47. doi: 10.1186/s41182-019-0171-8.
- https://www.drugs.com/mtm/garlic.html
- https://www.researchgate.net/publication/238069512 Potential Health Benefits of Garlic Allium Sativum A Narrative Review
- Rahman M.S. Allicin and other functional active components in garlic : Health benefits and bioavailability. International Journal of Food Properties. 10, 2007, 245–268. doi: 10.1080/10942910601113327
- Kaye A.D, De Witt B.J, Anwar M, Smith D.E, Feng C.J, Kadowitz P.J, Nossaman B.D. Analysis of responses of garlic derivatives in the pulmonary vascular bed of the rat. Journal of Applied Physiology. 89, 2000, 353–358. doi: 10.1152/jappl.2000.89.1.353.
- Ao Shang, Shi-Yu Cao, Xiao-Yu Xu, Ren-You Gan, Guo-Yi Tang, Harold Corke, Vuyo Mavumengwana, Hua-Bin Li. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods. 8(7), 2019, 246. https://doi.org/10.3390/foods8070246.
- S Ankri, D Mirelman. Antimicrobial Properties of Allicin from Garlic. Microbes and Infection. 1(2), 1999, 125-129.
- Lucía Melguizo-Rodríguez, Enrique García-Recio,Concepción Ruiz,Elvira De Luna-Bertos, Rebeca Illescas-Montes and Víctor J. Costela-Ruiz. Biological properties and therapeutic applications of garlic and its components. Food and Function. 13, 2022, 2415-2426. DOI: 10.1039/D1FO03180E .
- Gaber El-Saber Batiha, Amany Magdy Beshbishy, Lamiaa G. Wasef, Yaser H. Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review. Nutrients. 12(3), 2020, 872 doi: 10.3390/nu12030872.
- Mikaili, P.; Maadirad, S.; Moloudizargari, M.; Aghajanshakeri, S.; Sarahroodi, S. Therapeutic uses and pharmacological properties of garlic, shallot, and their biologically active compounds. Iran. J. Basic Med. Sci. 2013, 16, 1031–1048.
- Gruhlke, M.C.; Nwachwukwu, I.; Arbach, M.; Anwar, A.; Noll, U.; Slusarenko, A.J. Allicin from garlic, effective in controlling several plant diseases, is a reactive sulfur species (RSS) that pushes cells into apoptosis. In Proceedings of the Modern fungicides and antifungal compounds VI. 16th International Reinhardsbrunn Symposium, Friedrichroda, Germany, April 2010, 25-29.
- Fufa B. Anti-bacterial and anti-fungal properties of garlic extract (Allium sativum): A review. Microbiology Research Journal International 2019, 28:1–5. doi: 10.9734/mrji/2019/v28i330133.
- Zhen H., Fang F., Ye D.Y., Shu S.N., Zhou Y.F., Dong Y.S., Nie X.C., Li G. Experimental study on the action of allitridin against human cytomegalovirus in vitro: Inhibitory effects on immediate-earlygenes. Antiviral Research, 2006,72:68–74. doi: 10.1016/j.antiviral.2006.03.017.
- Sawai T., Itoh Y., Ozaki H., Isoda N., Okamoto K., Kashima Y., Kawaoka Y., Takeuchi Y., Kida H., Ogasawara K. Induction of cytotoxic T-lymphocyte and antibody responses against highly pathogenic avian influenza virus infection in mice by inoculation of a pathogenic H5N1 influenza virus particles inactivated with formalin. Immunology. 2008,124:155–165. doi: 10.1111/j.1365-2567.2007.02745.x.
- Mounyr Balouiri, Moulay Sadiki and Saad Koraichi Ibnsouda. Methods for in vitro evaluating antimicrobial activity : A review. Journal of Pharmaceutical Analysis. 2016, 6(2), 71–79


 Jaseela K. P.*
Jaseela K. P.*
 Anusree Sreedharan
Anusree Sreedharan



 10.5281/zenodo.14325465
10.5281/zenodo.14325465