Abstract
Patients with untreated illnesses and disorders now have more options because to recent developments in stem cell technology. Human pluripotent stem cells (hPSCs) and multipotent mesenchymal stem cells (MSCs) are two examples of stem cell-based therapy that has recently become a major force in regenerative medicine. Self-renewing cell types known as hPSCs are capable of differentiating into the three germ layers and other cellular phenotypes found in the human body. According to the International Society for Cell and Gene Therapy (ISCT), MSCs are multipotent progenitor cells with the capacity to differentiate into mesenchymal lineages and self-renew (although limited in vitro).Stem cell treatment has emerged as a very promising and cutting-edge area of scientific study in recent years. High expectations have been raised by the advancement of treatment techniques. The discovery of various stem cells and possible treatments based on these cells are the main topics of this review study. Stem cell genesis is followed by regulated stem cell culture and derivation in the lab. Assays for teratoma formation and quality control are crucial steps in evaluating the characteristics of the stem cells under investigation. Setting up the right environmental conditions for regulated differentiation requires the use of culture media and derivation techniques. Because of their adaptability, the utilization of graphene scaffolds and the promise of extracellular vesicle-based therapies warrant study among the many different kinds of stem tissue applications.
Keywords
Multipotent, mesenchymal lineages, progenitor cells, teratoma formation, stem cell
Introduction
In India, stem cell therapy is a rapidly developing field with encouraging developments in medical care. It entails utilizing stem cells to replace, regenerate, or repair cells, tissues, or organs that have been harmed or damaged by a variety of illnesses. Numerous ailments, such as neurological disorders, cardiovascular diseases, orthopedic problems, diabetes, and more, are being treated in India with stem cell therapy. 1,2,3The nation is seeing an increase in the number of specialist stem cell centers and research institutes that investigate the potential of stem cell treatments through studies and clinical trials. The safe and ethical use of stem cells for therapeutic reasons is governed by laws and regulations. Compared to many Western nations, patients seeking stem cell therapy in India enjoy the benefit of a somewhat reduced treatment cost. Furthermore, India is home to talented researchers and medical professionals that are advancing stem cell therapy and research.4,5,6 India is positioned as a potential center for stem cell research and therapy thanks to ongoing studies, partnerships with global specialists, and ongoing advancements in healthcare infrastructure, which give patients with a range of illnesses hope for better treatment options and results. We give a broad summary of the process of bringing stem cell therapies from concept to clinical use in this article. The initial stage in implementing a new treatment approach is to comprehend the detailed knowledge that underpins the conversion of concepts into medical services.
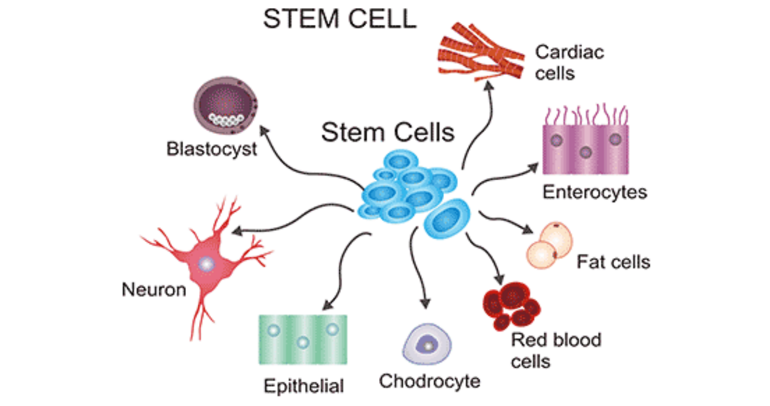
Fig.1: Stem Cell
History of stem cell:
- In the 1950s, teratocarcinomas and tumors with a diverse (or heterogeneous mix) of tissue types served as an uncommon source for the identification of stem cells. These consist of completely separate, specialized structures like hair and teeth.The observation of malignant growth and transplantability suggested the presence of a proliferative (highly dividing) and undifferentiated (non-specialised and capable of producing a range of cell types) cell. This cell was named the embryonal carcinoma (EC) cell.7,8,9
- A pioneering experiment demonstrated that the injection of EC cells in the adult mouse brain resulted in teratocarcinomas; this provided concrete evidence that EC cells can produce all cellular constituents of the teratocarcinoma. Corroborating evidence for their ability to propagate tumors and self-renew was indicated by their ability to be transplanted10,11.
- Germ cells and embryonal carcinoma cells-The origins of EC cells are primarily testicular in humans and mice. Leroy Stevens discovered that the teratocarcinomas in 129 strains of inbred mice arose from germ cells, those of the sperm and egg. Paradoxically, germ cells do not give rise to tumors, nor do they differentiate into other cell types.
- This quality is termed potency, of which there are four types, in the hierarchal order:Totipotency– the potential to give rise to all the cell types in the embryo and adult (e.g. the fertilized egg), thus a totipotent cell can give rise to the whole organism
Pluripotency – the potential to give rise to cells of all body lineages, but not a whole organism
Multipotency – the potential of a cell to give rise to a limited number of cell types in the body
Unipotency – the ability of a cell to give rise to only a single cell type
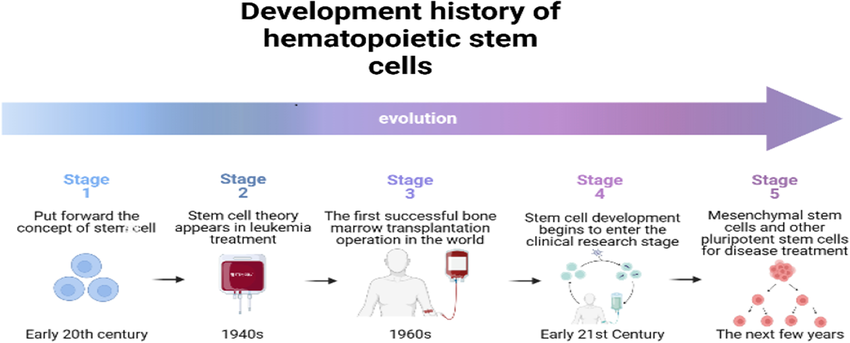
Fig.2: development history of hematopoietic stem cell
Characteristics that identify stem cells:
Pluripotent
The mouse model is used by many scientists since federal funding for human embryonic stem cells is limited in the United States. Human and mouse pluripotent stem cells have many characteristics, including the capacity to self-renew indefinitely and develop into cell types of all three germ layers. 12,13Considering that mammals share intracellular structure and genetic sequences, it should come as no surprise that so many pluripotency features are preserved across species. Human and mouse cells exhibit similar surface antigens, transcription factors, and enzymatic activity (i.e., strong alkaline phosphatase activity), have a high nucleus to cytoplasm ratio, and require growth factors generated from other living cells in order to multiply indefinitely in culture. Even if they are little, the distinctions between human and mouse pluripotent cells are significant. Even while Oct3/4 and Sox2, the transcription factors that cause pluripotency in adult cells, are similar, the extracellular cues required to control them are different. Human embryonic stem cells require the signaling proteins Noggin and Wnt for prolonged pluripotency, whereas mouse embryonic stem cells require leukemia inhibitory factor and bone morphogenic proteins. The variations in the adhesion molecule SSEA (SSEA-1 in mice, SSEA-3 & 4 in humans) are another example of how surface markers used to identify pluripotent cells vary somewhat between the two species.16 Therefore, although studies on pluripotency in mouse cells are useful, there is little chance that they will directly relate to human treatment.Last but not least, the moral and ethical conundrums that surround the research are a significant distinction between mouse and human stem cells. Few people have ethical concerns with using mouse models, but others believe that working with human embryonic stem cells is unethical. But because human and mouse cells are biologically different, most scientists think that focusing solely on rodents will miss out on information that could be useful for human treatment.14,15
Multipotent
Multipotent stem cells are usually identified using cell surface markers as well. By removing cells that express markers of committed cell types—a process known as lineage negative enrichment—and then further separating the cells that express the sca-1 and c-Kit surface markers that identify mesenchymal stem cells, mesenchymal stem cells can be isolated from the entire bone marrow aspirate. Flow cytometry can be used for both the lineage negative enrichment step and the sca-1/c-Kit isolation, which is covered in more detail in the review that follows. The newly identified cardiac stem cells can also be distinguished from the rest of the myocardium using the c-Kit surface marker.16,17,18 Finding the markers that identify early multipotent cells that will develop into pre-cardiac myocytes has been the focus of a lot of current cardiovascular research. The progenitor cells of the cardiovascular system, such as contracting cardiac myocytes, endothelial cells, and vascular smooth muscle cells, are produced by cells containing the particular mesodermal marker Kdr. As a result, these cells are thought to be the first to be specifically oriented towards the cardiovascular lineage. Although cells at this early stage continue to divide easily, they are destined to become cardiovascular system cells, which makes them potentially very useful for treatment.
Functional division of stem cells
Development of the entire body
The development of the organism determines whether distinct stem cells are present during division. ESCs from somatic stem cells can be identified. While it is feasible to derive ESCs without separating from the TE, this combination has growth limitations. Co-culture of proliferating behaviors is typically avoided due to their limited scope.18,19 The blastocyst, a stage of the pre-implantation embryo that occurs about four days after fertilization, is the source of ESCs. These cells are then put in a culture dish that has been loaded with culture media.Passage is a common yet ineffective method of subculturing cells on different dishes. Since these cells can eventually differentiate into any form of cell in the body, they can be referred to as pluripotent. The medicinal application of ESCs in treatments has been subject to ethical limitations since the start of their research. The majority of embryonic stem cells are produced from in vitro fertilized eggs rather than in vivo fertilized eggs. After development, somatic or adult stem cells—which are undifferentiated—are present throughout the body among differentiated cells. These cells serve to facilitate the growth, repair, and replacement of cells that are lost on a daily basis. These cells can only differentiate in a limited number of ways.20
Numerous tissues include mesenchymal stem cells.
These cells primarily develop into bone, cartilage, and fat cells in bone marrow. They are unique among stem cells in that they can specialize in any germ layer’s cells and exhibit pluripotence. Nerve cells and the oligodendrocytes and astrocytes that support them are produced by neural cells.
– Red, white, and platelet blood cells are all produced by hematopoietic stem cells.
– Keratinocytes, which create the skin’s protective layer, are one type of skin stem cell.Compared to ESCs, somatic stem cells take longer to proliferate. Adult stem cells can be reprogrammed to become pluripotent again. This can be accomplished by fusing with the pluripotent cell or by moving the adult nucleus into the cytoplasm of an egg. The renowned Dolly sheep was cloned using the same method.21,22 The development of the entire body”Invo’ves hESCs. They have the ability to differentiate into totipotent, multipotent, pluripotent, and unipotent cells.If pluripotent cells can also create the embryo’s extraembryonic tissues, they are referred to as totipotent cells.The ability of multipotent cells to differentiate into specific cell types within a particular tissue is limited. The stem cells that give rise to a single lineage of cells in tissue are referred to as either oligo or unipotent.
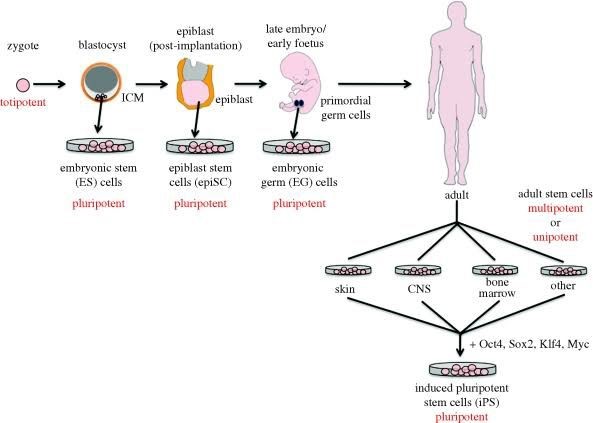
Fig.3: Functional division of stem cells
Current trends in stem cell therapy:
- The ectoderm, mesoderm, and endoderm are the three germ layers that develop from pluripotent ESCs. The ICM layer of the embryo's blastocyst is the source of these cells. Evans and Kaufman in the UK and Martin in the US initially isolated mouse ESC from the blastocyst's ICM (d2.5) in 1981. Thomson and associates isolated human ESCs (hESCs) from preimplantation blastocysts. For therapeutic purposes, hESCs have proven to be a great source of pluripotent cells.Pluripotent ESCs are typically derived from the ICM layer of the blastocyst using a standardized immunosurgery approach. In culture plates, the ESCs are separated and sown on feeder layers. Using particular antibodies to octamer-binding transcription factor ¾ (Oct3/4), stage-specific embryonic antigen 3 (SSEA-3), SSEA-4, TRA-1-60, and TRA-1-81, or by measuring alkaline phosphatase activity, the cells can be immunostained for pluripotency markers. ESCs have a strong telomerase activity and a normal karyotype Although ESC is a promising contender, there are serious issues about its use in regenerative medicine.The use of human embryos for hESC isolation raises serious ethical issues ESC research is governed by different laws in different nations. It is illegal to destroy human embryos for any kind of study in the United States. The rules allow for the use of hESC-lines obtained prior to August 9, 2001, for research purposes. There is limited progress in developing hESC therapeutics, and the majority of trials are conducted on animals.23,24 Research utilizing hESCs produced from abandoned embryos in in vitro fertilization clinics is permitted in the United Kingdom, while it is illegal in Italy.Analyzing the safety concerns related to ESCs in regenerative medicine is equally crucial. The ESCs have the ability to differentiate into any kind of bodily cell. However, this flexibility raises the possibility of teratomas and malignancies when these undifferentiated cells are implanted in vivo. An other technique involves transforming the undifferentiated ESCs into a particular cell type along a lineage in vitro, followed by the in vivo transplantation of the differentiated cell. Mice transplanted with hESC-derived cardiomyocytes did not develop teratomas.7,8 The transplanted progenitor cells do, however, occasionally continue to grow; for instance, nestin+ dopaminergic neurons produced from hESCs continue to grow in the striatum. Prior to transplantation, the undifferentiated cells may be screened using certain markers and then purified.In order to use ESCs for regenerative therapies, there are some documented ways to reduce the risk of cancer. A transmembrane protein called cluster of differentiation 133 (CD133) (prominin 1), which is typically expressed on cancer stem cells, has been found to be strongly expressed on hESCs. The ability to develop into the three embryonic germ layers in vivo was maintained by the CD133-deficient knock-out hESC strain. Nevertheless, there is a decreased proliferating potential, which leads to a decreased production of teratomas.14,15 Thus, ESCs may be sorted for transplantation using CD133.In regulated settings, hESC-derived progenitor cells remain intriguing prospects in regenerative medicine despite the safety concerns. 2009 saw the first approval of the hESC study for spinal cord damage, which included progenitor cells produced from hESC-derived oligodendrocytes. Clinical trials using hESC have been conducted to treat ischemic heart disease, diabetes mellitus, and macular degeneration with some encouraging outcomes in follow-up studies. The company ViaCyte has approved one clinical trial for Type 1 diabetes (ClinicalTrials.gov Identifier: NCT03163511, NCT02239354). These human pluripotent stem cell-derived pancreatic progenitor cells are generated in vitro and undergo in vivo differentiation into beta cells following implantation in an immunological isolation device. In Australia, hESC-based clinical trials for Parkinson's disease are underway.
Challenges in stem cell therapy:
- Under some circumstances, stem cells which are undifferentiated cells have the ability to self-renew and develop into specialized cells.5,6 Adult tissue stem cells, induced pluripotent stem cells (iPSC), epiblast-derived stem cells (Epi-SC), and embryonic stem cells (ESC) are the primary types of stem cells. It is commonly known that stem cells hold a great deal of promise for creating tissues with little chance of rejection or adverse effects.8,9 Nonetheless, there are a number of issues and disputes surrounding stem cell therapy that require attention.2,3
Advantages and Disadvantages of Stem Cell:
Advantages:
- It provides medical benefits in the fields of regenerative medicine and therapeutic cloning.
- Numerous illnesses, such as Parkinson's disease, Alzheimer's disease, spinal cord injuries, cancer, diabetes, etc., may benefit greatly from its ability to identify therapies and cures.
- Stem cells might be utilized in a lab to create limbs and organs that could be used in transplants or to treat illnesses.
- It will aid researchers in their understanding of cell growth and human development. Without using human or animal samples, scientists and physicians will be able to evaluate billions of potential medications and medicines. This calls for a procedure that mimics the impact the medication has on a specific cell population. This would reveal if the medication is helpful or problematic.
- Research on stem cells is especially useful for studying developmental phases that are not directly observable in a human embryo and are occasionally associated with serious clinical outcomes like infertility, birth abnormalities, and miscarriages. In the end, a more thorough comprehension of typical development will support the prevention or management of abnormal human development.
- One advantage of using adult stem cells to treat illness is that a patient may be treated using their own cells. Because patients' bodies wouldn't reject their own cells, the risks would be comparatively lower. Embryonic stem cells may be more adaptable than adult stem cells since they can differentiate into any type of cell in the body.
Disadvantages:
- The destruction of blastocysts derived from human eggs fertilized in a lab is part of the use of embryonic stem cells for research. According to those who think that life begins at conception, the blastocyst is a human life, and its abolition is wrong and unethical.
- The long-term consequences of such a meddling with nature are likewise completely unknown, just like with any other new technology.
- Not all illnesses may be cured by embryonic stem cells.
- According to a recent study, people with heart problems received stem cell therapy. It has been discovered to thin their coronary arteries.
- The fact that most adult stem cells are per-specialized—for instance, brain stem cells only produce brain cells, and blood stem cells only produce blood—is a disadvantage.
- Because they are derived from non-patient embryos, the patient's body may reject them. But today there is a remedy for this. The accessibility of stem therapy is another drawback; at the moment, very few medical facilities provide it.
CONCLUSION:
The increasing amount of research over the last ten years has made stem cell therapy a feasible possibility. The potential uses of stem cells have grown with each study, despite the numerous obstacles encountered. Stem cell research is currently showing great promise, with reports of clinical success in treating a variety of illnesses, including neurological diseases and macular degeneration, developing quickly. Due to the countless opportunities they present for employing patients' own cells to treat illnesses, iPSCs are sweeping the world of stem cell research. Using MSCs to regenerate dental and periodontal tissues has reached the clinic and will soon be a recognized treatment. Despite the difficulties that may appear overwhelming, stem cell research is progressing quickly, and cellular therapies will soon be useful. Fortunately, there are currently enormous efforts being made all over the world to establish standards and regulations that will guarantee patient safety. Human health will soon be greatly impacted by stem cell-based treatments.
REFERENCES
- Lapteva, L.; Vatsan, R.; Purohit-Sheth, T. Regenerative medicine therapies for rare diseases. Transl. Sci. Rare Dis. 2018, 3, 121–132. [CrossRef] [PubMed]
- Ulia, M.P.; Mantalaris, S. Stem cells bioprocessing: An important milestone to move regenerative medicine research into the clinical arena. Pediatric Res. 2008, 63, 6.
- Chen, F.-M.; Zhao, Y.-M.; Jin, Y.; Shi, S. Prospects for translational regenerative medicine. Biotechnol. Adv. 2012, 30, 658–672. [CrossRef] [PubMed]
- Rose, L.F.; Wolf, E.J.; Brindle, T.; Cernich, A.; Dean, W.K.; Dearth, C.L.; Grimm, M.; Kusiak, A.; Nitkin, R.; Potter, K.; et al. The convergence of regenerative medicine and rehabilitation: Federal perspectives. Npj Regen. Med. 2018, 3, 19. [CrossRef]
- Rajabzadeh, N.; Fathi, E.; Farahzadi, R. Stem cell-based regenerative medicine. Stem Cell Investig. 2019, 6, 19. [CrossRef]
- Rosenthal, N.; Badylak, S. Regenerative medicine: Today’s discoveries informing the future of medical practice. Npj Regen. Med. 2016, 1, 16007. [CrossRef]
- Sivandzade, F.; Cucullo, L. Regenerative stem cell therapy for neurodegenerative diseases: An overview. Int. J. Mol. Sci. 2021, 22, 2153. [CrossRef]
- Dehkordi, A.N.; Babaheydari, F.M.; Chehelgerdi, M.; Dehkordi, S.R. Skin tissue engineering: Wound healing based on stem-cellbased therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 111. [CrossRef]
- Granero-Moltó, F.; Weis, J.; Longobardi, L.; Spagnoli, A. Role of mesenchymal stem cells in regenerative medicine: Application to bone and cartilage repair. Expert Opin. Biol. Ther. 2008, 8, 255–268. [CrossRef]
- Ghasroldasht, M.M.; Matin, M.M.; Mehrjerdi, H.K.; Naderi-Meshkin, H.; Moradi, A.; Rajbaioun, M.; Alipour, F.; Ghasemi, S.; Zare, M.; Mirahmadi, M.; et al. Application of mesenchymal stem cells to enhance non-union bone fracture healing. J. Biomed. Mater. Res. Part A 2018, 107, 301–311. [CrossRef]
- Howard, D.; Buttery, L.; Shakesheff, K.M.; Roberts, S.J. Tissue engineering: Strategies, stem cells and scaffolds. J. Anat. 2008, 213, 66–72. [CrossRef] [PubMed]
- Willerth, S.M.; Sakiyama-Elbert, S.E. Combining stem cells and biomaterial scaffolds for constructing tissues and cell delivery. StemJournal 2019, 1, 1–25. [CrossRef]
- Boehler, R.M.; Graham, J.; Shea, L.D. Tissue engineering tools for modulation of the immune response. Biotechniques 2011, 51, 239–254. [CrossRef] [PubMed]
- Andorko, J.I.; Jewell, C.M. Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine. Bioeng. Transl. Med. 2017, 2, 139–155. [CrossRef] [PubMed]
- Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336(6200):688–90. https://doi.org/10.1038/ 336688a0.
- Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nature Biotechnol. 2001;19:971–4. https://doi.org/10.1038/nbt1001-971.
- Weathersbee PS, Pool TB, Ord T. Synthetic serum substitute (SSS): a globulin-enriched protein supplement for human embryo culture. J. Assist Reprod Genet. 1995;12:354–60.
- Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, SmugaOtto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, AntosiewiczBourget J, Teng JM, Thomson JA. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods. 2011;8:424–9.
- Sommer CA, Mostoslavsky G. Experimental approaches for the generation of induced pluripotent stem cells. Stem Cell Res Ther. 2010;1:26.
- Takahashi K, Yamanaka S. Induced pluripotent stem cells in medicine and biology. Development. 2013;140(12):2457–61 https://doi.org/10.1242/dev. 092551. 36. Shi D, Lu F, Wei Y, et al. Buffalos (Bubalus bubalis) cloned by nuclear transfer of somatic cells. Biol. Reprod. 2007;77:285–91. https://doi.org/10.1095/ biolreprod.107.060210.
- Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. Development. 1962;10:622–40 http:// dev.biologists.org/content/10/4/622.
- Kain K. The birth of cloning: an interview with John Gurdon. Dis Model Mech. 2009;2(1–2):9–10. https://doi.org/10.1242/dmm.002014.
- Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;24(51(6)):987–1000.
- Quinlan AR, Boland MJ, Leibowitz ML, et al. Genome sequencing of mouse induced pluripotent stem cells reveals retroelement stability and infrequent DNA rearrangement during reprogramming. Cell Stem Cell. 2011;9(4):366–73.


 Varpe Tanmay*
Varpe Tanmay*



 10.5281/zenodo.14866081
10.5281/zenodo.14866081