Abstract
Managing the multi-stage biological process of wound healing can be challenging, especially in cases that are chronic. Metal nanoparticles have emerged as promising agents for improving wound healing via novel drug delivery systems as nanotechnology advances. This review examines the current trends and applications of nano-based drug delivery systems, with a particular emphasis on silver and gold nanoparticles. Silver nanoparticles (AgNPs) are known for their strong antimicrobial properties, which speed up wound healing by reducing bacterial colonization and promoting tissue regeneration. Gold nanoparticles (AuNPs) have significant advantages due to their anti-inflammatory and antioxidant properties, making them useful in chronic wound management. We investigate these nanoparticles' mechanisms of action, production methods, and promise to transform wound care, along with the difficulties encountered in implementing them in clinical settings. The analysis ends with a summary of potential future developments, with a focus on the use of nano-drug systems to personalized and precision medicine for the treatment of wound care.
Keywords
Wound healing, Nanoparticles, Metal nanoparticles, Silver nanoparticles (AgNPs), Gold nanoparticles (AuNPs), Nano drug delivery systems, chronic wounds, tissue regeneration, nanotechnology in medicine.
Introduction
A wound is defined as any injury to the body and typically damage to the epidermis of skin that disturbs its normal anatomy and function. Wounds are categorized as open or closed wounds based on the underlying cause of wound formation and acute or chronic wounds based on wound healing physiology [2]. There are two types of wounds, mainly chronic wounds, and acute wounds.
Acute wounds are classified as surgical wounds and accidental wounds. Surgical wounds include incisions, excisions, and surgically debrided wounds, and accidental wounds include burn wounds, electrical, chemical, and thermal wounds and injuries. These wounds normally undergo the processes of inflammation, tissue proliferation, and remodeling.
Chronic wounds take a long time to heal and recover, due to failure in normal progression of the healing stages and/or local infection. These wounds are commonly caused by chronic diseases such as diabetes mellitus, autoimmune diseases, hypoxia, trauma, and inadequate care at the early stages of wounding.
Since chronic wounds are often exposed to bacteria due to delayed wound healing, they enter the stage of infection. The primary cause of chronic wound infections is the growth of common bacteria.
Physiology of wound and wound healing:
The most wide spread organ in the human body is the skin. It protects the internal tissues from mechanical damage, infection, extreme temperature, and harmful sun rays. However, it also is more prone to injuries and damage[11]. When the skin is wounded, different cells come together to bring about healing.
The primary response to a wound is blood vessel constriction and the activation of platelets to form a fibrin clot. This clot slows or ceases the blood flow and allows the flow of inflammatory cells like neutrophils, which is the first line of defense against microbial infection.
After the inflammatory phase, angiogenesis begins, which involves endothelial cell proliferation, migration, and branching to form new blood vessels. Apart from the local cells, other cells from the bone marrow also support new blood vessel formation during wound healing[10,11].
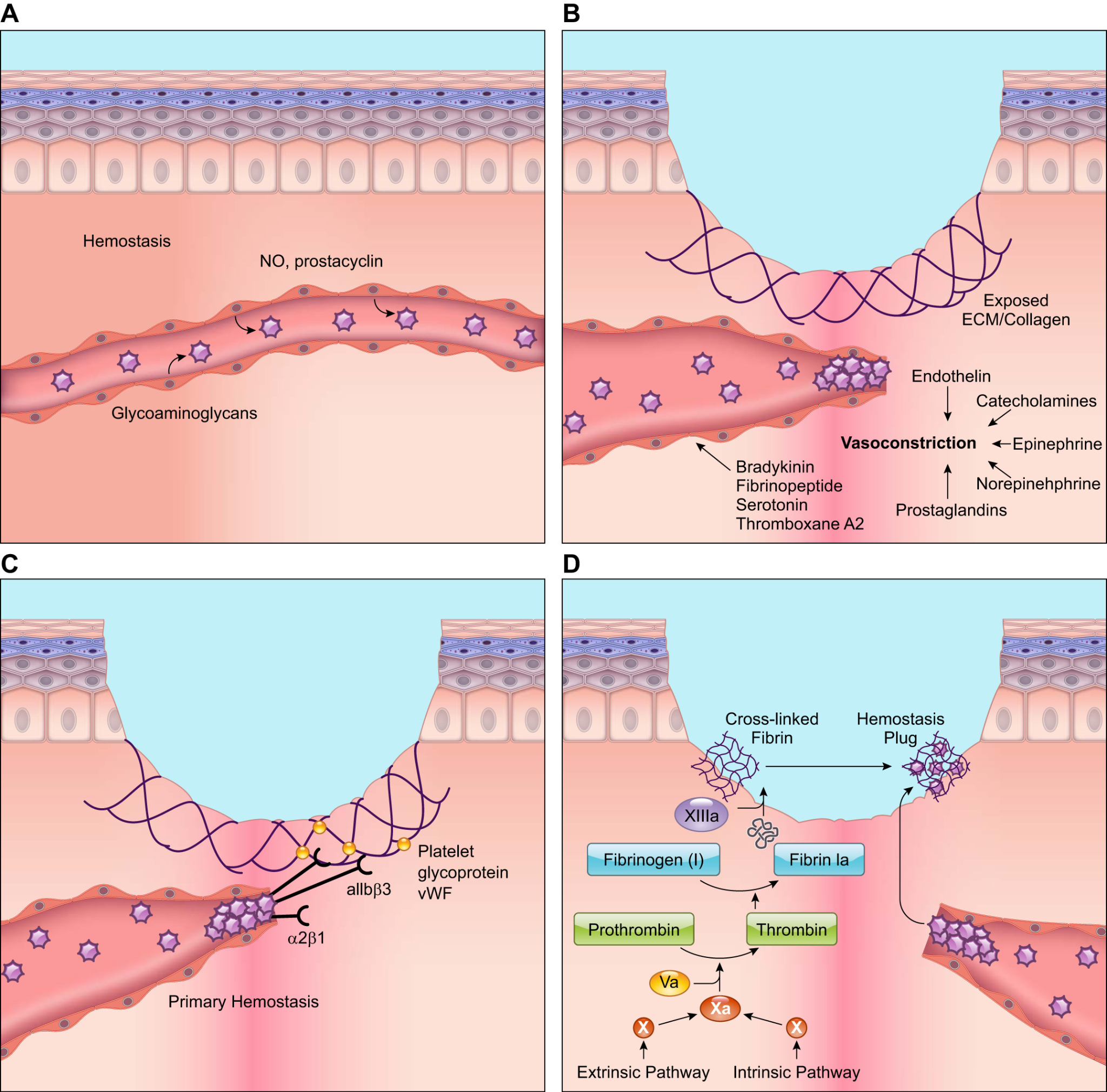
Figure 1: Physiology of wound healing[11]
A: Platelets move in close proximity to the vessel wall during hemostasis. However, endothelial cells emit anti-thrombotic substances including prostacyclin and nitric oxide (NO), which stop platelets from adhering to the endothelium lining and from aggregating. B: Wounding triggers the quick release of vasoconstrictors from wounded cells, which results in reflexive smooth muscle contraction and a brief halt to bleeding. C: The subendothelial matrix is exposed when blood vessels burst during wound healing. G protein-coupled receptors, integrins, and glycoproteins on the surface of platelets allow them to attach to this subendothelial matrix and to one another. Additionally, platelets release von Willebrand factor (vWF), which adheres to the subendothelial matrix. Through their surface receptors, platelets bind extracellular vWF, strengthening the platelet plug. D: Both intrinsic and extrinsic mechanisms cause Factor X to become activated, which in turn causes fibrinogen to cleave into fibrin. The aggregated platelet plug is bound by cross-linked fibrin to create the thrombus, which halts blood flow and offers a temporary healing matrix. Based on what is currently known, the illustration is a simplified depiction[66].
Cellular responses during wound healing
Wound healing in normal conditions happens in four phases[4]. The first of which is hemostatis which lasts for seconds to hours. In this stage vasoconstriction, platelet aggregation, complement activation, and thrombus formation takes place. The second stage is called the inflammatory phase which can last from hours to days and in this phase phagocytosis, macrophage activation, neutrophil infiltration, and lymphocyte infiltration takes place. The third stage of wound healing is the proliferation phase which lasts up to a few days or maybe even a week, in this stage fibroplasia, angiogenesis, reepithelialization, and matrix deposition happens. The final stage is the remodeling phase which may take a few weeks to a month, in this phase ECM (extracellular matrix) remodeling, epithelialization, increase of tensile strength, and scar formation takes place. After these four stages, the tissue is completely healed.
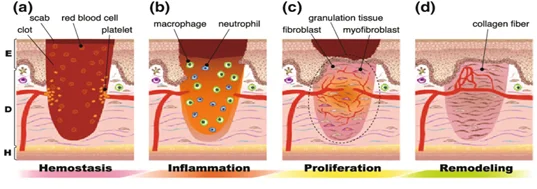
Figure 2: Natural wound healing phases[88]
WOUND HEALING MANAGEMENT:
Traditional treatment of wounds:
Dressings
Treating a wound depends on different factors such as the severity of the wound, the healing process, the patient's condition (such as diabetes), the social setting, and the availability of wound dressing. Regardless of these factors, a wound should be treated as soon as possible to avoid microbial infection or scarring on the skin.
Dressings are usually made of a soft cloth-like material, which is meant to hold the skin taut and prevent the entry of an irritant, and help the wound heal faster[7,8]. Occlusive dressings were introduced in the late 20th century. These dressings would help in collagen synthesis, faster reepithelialization, and reducing the wound's pH. All of these would contribute to the faster healing process of the wound.
Modern wound treatment:
The first modern wound dressing was created in the mid-1980s, which provided more moisture and had absorbing fluids (iodine-containing gels, polyurethane foams, and hydrocolloids). Modern wound dressing is made to heal the wound rather than just cover the wound. These keep the wound from dehydration and promote healing[8].
Semi-permeable foam dressings
These are made of hydrophobic and hydrophilic foam, sometimes with adhesive borders. The foam has a capability of absorbing the wound drainage depending upon the thickness of the wound. However, the disadvantage of semi-permeable foam dressing is that frequent dressing is required and it is not suitable for low-exudating wounds, scars, and dry wounds.
Semi-permeable film dressings
These are composed of transparent and adherent polyurethane, which allows the transmission of water vapor and gasses like oxygen and carbon dioxide from the wound. These dressings are elastic and so conform to any shape, without requiring any additional tapping.
Hydrocolloid dressing
Among all dressings, these are the most widely used interactive dressings and consist of an inner and outer layer, of which the inner is the colloidal layer and the outer is the water-impermeable layer. These dressings are used on light to moderately exudating wounds, providing a moist environment for the wound to heal. Since they do not cause any pain on removal, it may be recommended for pediatric wound care management. A disadvantage of Hydrocolloid dressings is that they may not be indicated for neuropathic ulcers or extremely exudating wounds.
Alginate dressing
Derived from marine brown algae (seaweed), alginate dressings are absorbent and biodegradable in nature. They help in maintaining a moist environment and minimize bacterial contamination. Alginate dressings are made from sodium and calcium salts consisting of mannuronic and guluronic acid units. Ions present in the alginate are exchanged with blood in the wound to form a protective film[8,9].
Topical pharmaceutical agents
Topical agents in the form of solutions, ointments, and creams, have shown that they play a more effective role in the treatment of wound healing[6]. These agents aid in the removal of the necrotic tissue, antimicrobial agents treat the infection, and help with the regeneration of tissue.
Factors affecting the wound healing process:
Although there is a variety of dressings available, there may be some internal and external factors that may affect the process and rate of wound healing. Local factors include hypothermia, pain, infection, radiation, and tissue oxygen tension whereas systemic factors are the overall health or disease state of the individual that affect the individual's ability to heal[8]. Other factors also include age, poor nutrition, and nutrition deficiency.
The availability, type, and organization of collagen, which is a structural protein present in the skin, plays an active role in the natural healing process. It initiates fibroblast formation, thus speeding up the migration of endothelial upon contact with wound tissue. Collagen remodeling (synthesis of new collagen to heal the skin) continues for months after wound closure[8,10].
Another important component is Hyaluronic acid (HA), which is a glycosaminoglycan component. Although these traditional dressing treatment methods are easy to use and cost-effective, they also have several disadvantages, which includes lack of controlling moisture and even patient comfort.
Nano-formulations:
Introduction and significance over traditional formulations
Nano-formulations are defined as a formulation or combination of drugs that utilize nanotechnology to enhance their therapeutic efficacy[12]. They are specifically designed to improve the delivery and performance of existing drugs by reducing toxicity, improving solubility, and increasing bioavailability. A nano formulation can be made up of various nano-sized materials such as liposomes, polymeric nanoparticles, solid lipid nanoparticles, and dendrimers[13,14]. Nano formulations are designed to improve drug stability and solubility in the blood. The drug is delivered to the tissue and is absorbed more quickly and efficiently. The smaller size of the drug particles allows for better penetration into tissues and cells, increasing their therapeutic effectiveness[15]. Nano formulations have multiple benefits over traditional drug delivery such as improved drug stability, reduced toxicity, and increased efficacy. They have the potential to increase drug efficacy and reduce side effects, leading to better patient outcomes. They have shown great promise in the treatment of various cancers, infectious diseases, and inflammatory conditions[16].
Types of Nano Formulations:
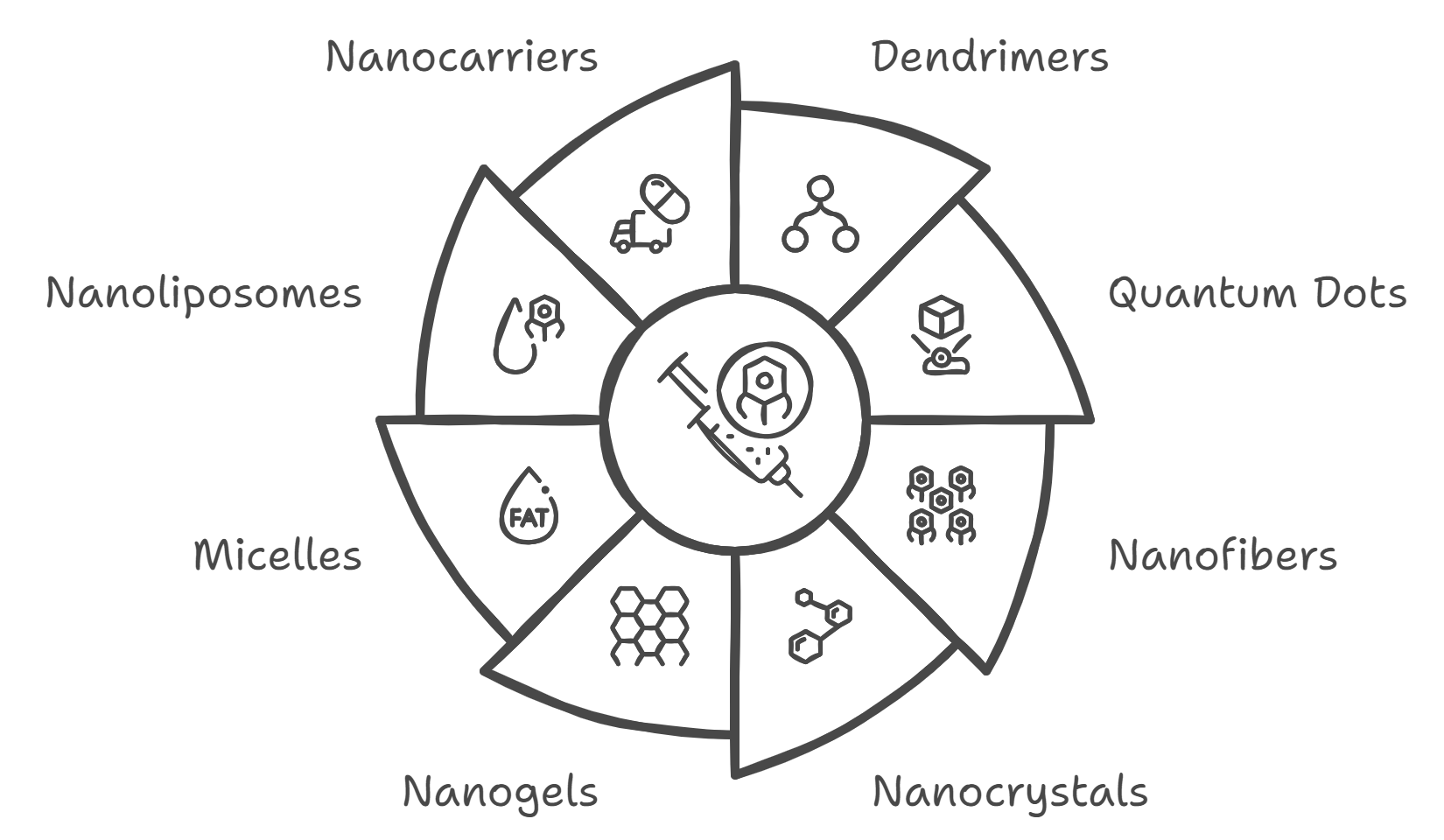
Figure 3: types of nano formulations
- Nanoparticles: Tiny particles (1-100 nm) that can improve the solubility, stability, and bioavailability of poorly water-soluble drugs. They can be made from metals, polymers, lipids, or ceramics[17]. Examples include: Polymeric nanoparticles: Made from biodegradable polymers like PLGA (poly(lactic-co-glycolic acid)), used for controlled drug release. Solid lipid nanoparticles (SLNs): Consist of a solid lipid core, used to enhance drug stability and control release.
- Nanocapsules: These are nanoparticles with a core-shell structure, where the core encapsulates the drug. They offer controlled release and targeted delivery. Examples include:[18] Liposomes: Bilayer vesicles that can encapsulate hydrophilic and hydrophobic drugs. They improve drug stability and reduce toxicity by targeting specific tissues or cells.
Nanospheres: Spherical nanoparticles that can deliver drugs more efficiently and improve the pharmacokinetics of the drug.
- Dendrimers: Highly branched, star-shaped macromolecules with multiple functional groups. They can carry drugs, genes, or imaging agents and offer precise control over drug delivery and release[19].
- Quantum Dots: semiconductor material at nanoscale with unique optical properties. They are used for imaging and tracking drug delivery in real time[20].
- Nanofibers: Nanometer-sized fibers used in drug delivery systems, such as wound dressings or scaffolds in tissue engineering, to release drugs over time[21].
- Nanocrystals: Nanoscale crystals of active pharmaceutical ingredients (APIs) that improve the dissolution rate and bioavailability of poorly soluble drugs. They are often used to enhance the oral bioavailability of such drugs[22].
- Nanogels: Cross-linked polymeric networks that can swell in the presence of fluids and release drugs in response to environmental stimuli. They are used for controlled and targeted drug delivery[23].
- Micelles: Self-assembled structures of surfactants or block copolymers with a hydrophobic core and hydrophilic shell. They are used to improve the solubility of hydrophobic drugs and for targeted drug delivery[24].
- Nanoliposomes: Lipid-based nanoparticles that can encapsulate both hydrophobic and hydrophilic drugs. They enhance drug delivery by modifying the pharmacokinetics and targeting specific cells or tissues[25].
- Nanocarriers: General term for nano-sized delivery systems, including nanoparticles, nanocapsules, and micelles, designed to transport drugs to specific sites in the body with high precision[26].
Applications:
- Targeted Drug Delivery: Nanoformulations can be engineered to target specific cells or tissues, minimizing side effects and improving therapeutic efficacy.
- Improved Solubility and Bioavailability: Many drugs suffer from poor solubility and bioavailability. Nanoformulations can enhance these properties, making drugs more effective at lower doses.
- Controlled Release: Nanoformulations can provide controlled and sustained release of drugs, reducing the frequency of dosing and improving patient compliance.
- Enhanced Stability: Nanoparticles can protect drugs from degradation due to environmental factors such as light, heat, and oxygen[31,27,32].
Overall, nano formulations offer many benefits over traditional drug delivery methods, including improved solubility, stability, bioavailability, and targeted delivery[30,33,34].
Advances and Innovations:
Personalized Medicine
Nano-formulations are at the forefront of personalized medicine, where treatments are tailored to individual genetic profiles. By using nanoparticles that target specific molecular markers associated with a patient’s disease, therapies can be customized for greater efficacy and reduced side effects[35].
Combination Therapies
Nano-formulations can be designed to deliver multiple drugs simultaneously. This approach can be particularly beneficial in treating complex diseases that require combination therapies, such as cancer, where different drugs work synergistically to overcome resistance mechanisms and enhance therapeutic outcomes[36].
Immunotherapy
In the realm of cancer treatment, nano-formulations are being developed to enhance the effectiveness of immunotherapies. For example, nanoparticles can be used to deliver immune checkpoint inhibitors directly to tumor sites or to activate the immune system more effectively[37].
Diagnostic Applications
Beyond drug delivery, nanoparticles are being explored for diagnostic purposes. Nano-formulations can be used in imaging techniques such as MRI, CT scans, and optical imaging to provide high-resolution images of tissues and organs. They can also be used for the detection of biomarkers associated with specific diseases[38].
Gene Therapy
Nanoparticles are being used to deliver genetic material, such as DNA, RNA, or gene-editing tools like CRISPR/Cas9, to specific cells. This can potentially correct genetic mutations at the source and offer therapeutic solutions for genetic disorders[39].
NANOPARTICLES
Nanoparticles are incredibly tiny particles with dimensions between 1 and 100 nanometers (nm), where 1 nanometer is one-billionth of a meter. Their small size gives them unique physical and chemical properties compared to bulk materials[44].
Types: Nanoparticles come in various forms, including metal nanoparticles (like Au, Ag Cu, ZnO, Silica, TiO2, SPIONs), semiconductor nanoparticles (quantum dots), and carbon-based nanoparticles (such as fullerenes and carbon nanotubes)[45].
Figure 4: (A) metal NPs; (B) quantum dots, and (C) silica-based NPs.
Properties: Due to their small size, nanoparticles can exhibit unique optical, electrical, and magnetic properties. For example, gold nanoparticles can appear red or purple depending on their size and shape due to their surface plasmon resonance[46].
Applications: Nanoparticles have diverse applications. In medicine, they are used for targeted drug delivery and imaging. In electronics, they are integral to the development of smaller and more efficient devices. They also have roles in environmental protection, such as in water purification, and in manufacturing, where they can be used to create stronger and lighter materials[47].
Nanoparticles have significant potential that can enhance drug delivery, improve therapeutic efficacy, and reduce side effects.
Advantages of Nano-Drug Delivery Systems:[48]
1. Targeted Delivery: Nanoparticles can be engineered to deliver drugs specifically to targeted cells or tissues, reducing the impact on healthy cells. This is particularly useful in cancer therapy, where nanoparticles can be designed to target tumor cells while sparing surrounding healthy tissue.
2. Controlled Release: Nanoparticles can provide controlled and sustained release of drugs over time, improving the efficacy and reducing the frequency of dosage. This is useful for medications that require long-term administration.
3. Improved Solubility and Bioavailability
Many drugs have poor solubility in water, which limits their effectiveness. Nanoparticles can increase the solubility of such drugs, thereby improving their bioavailability (the extent and rate at which the active ingredient or active moiety is absorbed and becomes available at the site of action).
4. Enhanced Stability
Nanoparticles can protect sensitive drugs from degradation due to environmental factors such as light, heat, and oxygen. This can enhance the shelf life and stability of pharmaceutical formulations.
5. Diagnostic Applications
Imaging: Nanoparticles, such as quantum dots and magnetic nanoparticles, can be used as contrast agents in imaging techniques like MRI, CT scans, and fluorescence microscopy. This improves the ability to visualize and diagnose diseases at an early stage.
Biosensors: Nanoparticles are used in the development of biosensors for detecting biomarkers associated with various diseases, including cancer, cardiovascular diseases, and infectious diseases.
6. Gene Delivery
Nanoparticles can be used to deliver genes or nucleic acids into cells for gene therapy. This can be used to correct genetic defects or to introduce therapeutic genes to treat diseases.
7. Vaccines
Nanoparticles can be utilized to develop more effective vaccines. For example, they can serve as adjuvants (substances that enhance the body's immune response to an antigen) or deliver antigens in a more effective manner.
8. Personalized Medicine
Nanoparticles can be tailored to meet individual patient needs, allowing for more personalized treatment strategies. This includes customizing the size, surface properties, and release profiles of nanoparticles to match specific patient profiles or disease states.
Challenges and Considerations:
Safety and Toxicity: Ensuring the safety of nanoparticles is crucial. They must be thoroughly evaluated for potential toxicity, especially when used in humans.
Regulation: The development and approval of nanoparticle-based pharmaceuticals involve rigorous regulatory scrutiny to ensure efficacy and safety.
Manufacturing: Scaling up the production of nanoparticles to meet pharmaceutical standards can be complex and costly[50].
Characterization of Nanoparticles:[52]
1. Microscopy Techniques
Transmission Electron Microscopy (TEM): Provides detailed images of nanoparticles at atomic resolution.
Scanning Electron Microscopy (SEM): Offers high-resolution images of the surface morphology and structure.
Atomic Force Microscopy (AFM): Measures the surface topography and mechanical properties of nanoparticles.
2. Spectroscopy Techniques
Dynamic Light Scattering (DLS): Measures the size distribution of nanoparticles in suspension based on the scattering of light.
X-ray Diffraction (XRD): Determines the crystalline structure and phase of nanoparticles.
UV-Visible Spectroscopy: Analyzes the optical properties and confirms the presence of nanoparticles based on their absorption and scattering of light.
3. Other Techniques
Nuclear Magnetic Resonance (NMR): Used for studying the chemical environment and interactions of nanoparticles.
Fourier Transform Infrared Spectroscopy (FTIR): Identifies functional groups and chemical bonding in nanoparticles.
Types of Nanoparticles:
1. Metal Nanoparticles
Gold Nanoparticles: Known for their optical and electronic properties, used in imaging, drug delivery, and diagnostics.
Silver Nanoparticles: Have antimicrobial properties, used in coatings, wound dressings, and medical devices[53].
2. Semiconductor Nanoparticles
Quantum Dots: Nanoscale semiconductor particles with size-tunable optical properties, used in imaging and electronic devices.
Titanium Dioxide (TiO2) Nanoparticles: Used in photocatalysis, sunscreens, and as pigments[54].
3. Carbon-Based Nanoparticles
Fullerenes: Molecules of carbon in the form of a hollow sphere, used in drug delivery and material science.
Carbon Nanotubes: Cylindrical nanostructures with high strength and electrical conductivity, used in electronics and nanocomposites[55].
4. Polymeric Nanoparticles
Nanocapsules: Encapsulate drugs in a polymeric shell for controlled release.
Nanospheres: Solid polymeric nanoparticles used for drug delivery and as carriers[56].
5. Liposomes
Liposomes: Spherical vesicles with a lipid bilayer, used for delivering drugs, genes, and vaccines[57].
Advantages of metal nanoparticles over conventional treatment for wound healing
It is clear that the application of metallic nanoparticles can create a novel therapeutic approach for wound care, showing strong results in lowering microbial infections, delaying the healing process, and lessening the harm brought on by the chronic inflammatory process. One of the main advantages of using NPs is that, in contrast to traditional drug delivery techniques, they can deliver medications, genes, and peptides in higher concentrations with fewer side effects, boosting the effectiveness of the treatment[87].
Recently, copper and silver nanoparticle-containing wound dressings have been created, showing promising preclinical results. Notably, compared to employing silver alone, the combination of nano-sized silver and copper showed an eight-fold reduction in the number of bacteria in an in vitro model of wound infection.[88].
Synthesis of metal nanoparticles (NP):[62]
These nanoparticles are synthesized using nanotechnology, thereby reducing the metal to its nuclear size. Its synthesis can be carried out by biological, chemical or physical methods, each with its own pros and cons.
1. Physical synthesis: Although these approaches avoid the contamination of the NPs with solvents, the process requires a high amount of energy for condensation and evaporation, which could also raise the cost of synthesis.
Physical Vapour Deposition (PVD): Involves evaporating a material in a vacuum and then condensing it onto a substrate to form nanoparticles.
Milling: Mechanical grinding of bulk materials into nanoparticles using high-energy mills.
2. Chemical synthesis: In this process, reducing and protecting agents are utilized to prevent agglomeration, and thus producing NPs with high purity and stability. But, the contamination can be caused by the use of strong chemicals.
Chemical Vapour Deposition (CVD): Involves the chemical reaction of gaseous precursors to form nanoparticles on a substrate.
Sol-Gel Process: A chemical technique where a liquid sol transitions to a solid gel phase, which can then be processed into nanoparticles.
Hydrothermal and Solvothermal Synthesis: Uses high-pressure and high-temperature conditions in aqueous or organic solvents to synthesize nanoparticles.
3. Biological method of synthesis: It offers an eco-friendly and a perfect alternative to the other two methods. Biological techniques use microorganisms like bacteria and fungi or plants.
Plants used in biological synthesis act as biofactories, making this method the most cost-effective and environmentally friendly. Scale up is easy since these plants are abundant, easily available and can be grown in various environmental conditions. Biological techniques also eliminate the need for toxic, strong chemicals typically used in the physical and chemical methods, therefore making it a safer and more sustainable option. With all the advantages, there are some disadvantages, like variability in the shape and size of the NPs produced due to the different species of plant, different weather conditions and harvesting times.
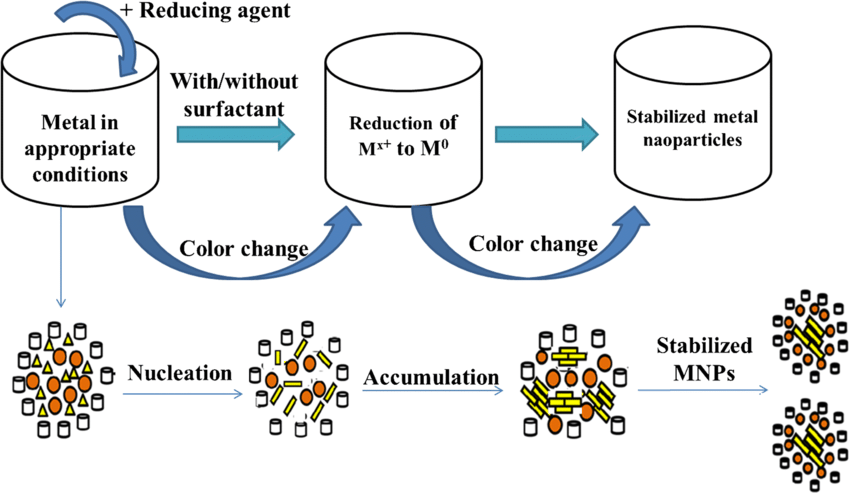
Figure 5: Simplified scheme for preparation of metal nanoparticles[73]
The general synthesis follows two approaches, bottom-up and top-down. The bottom-down approach is called a constructive process that synthesizes the NPs from atoms to clusters then to NPs[63]. Generally includes processes like sol-gel, spinning and biosynthesis. Top-down approach destroys the initial structures to synthesize NPs[64].
Most commonly used metals are silver, gold, iron, cobalt and nickel and their respective oxides.
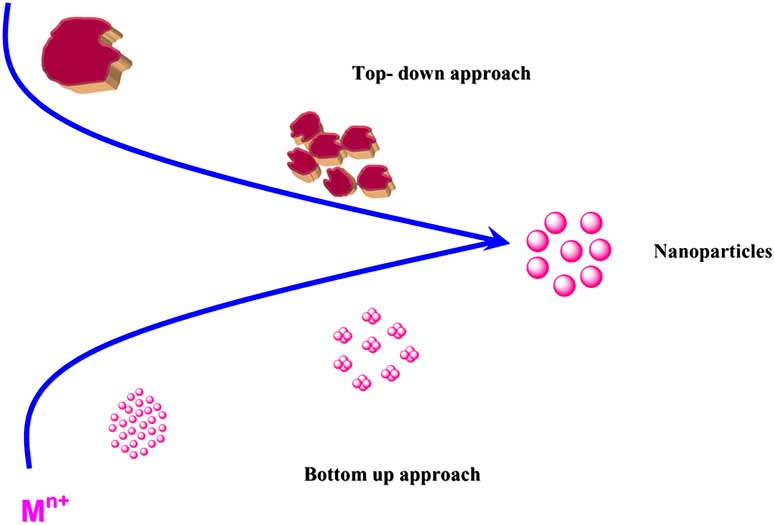
Figure 6: General route for the synthesis of metal nanoparticles[72]
SILVER NANOPARTICLES:
Silver compounds and ions have been extensively used for both hygienic and healing purposes, due to their strong bactericidal effects, as well as a broad spectrum antimicrobial activity. Taking advantage of its bactericidal properties, various silver-containing preparations have been used for the treatment of chronic wounds.
Silver is applied to burns, either in the form of impregnated bandages or as a cream containing silver sulfadiazine as the active agent, a product that is still considered the benchmark silver product. Silver-based formulations exhibit fast and broad spectrum antibacterial activity against both gram-positive and negative bacteria. In recent years, the mechanism of action of silver has been investigated: it seems that silver shows a multilevel antibacterial effect, due to blockage of respiratory enzyme pathways, as well as alteration of microbial DNA and the cell wall. Silver NPs (Ag NPs) have been shown to possess unusual physical, chemical and biological properties . The effectiveness of Ag NP-containing dressings has been widely tested in vitro, and much research work has been published[58].
Biopolymers are abundantly available from natural sources and are used in numerous applications in pharmaceutical sciences and medicine. Silver nanoparticles and biopolymer-based biomaterials (AgNP-BMs) are non-cytotoxic and safe for patients in wound care management. The unique intrinsic features of AgNP-BMs promote wound healing and effectively control the growth of microorganisms at the wound site, and this strategy plays an important role in the treatment of both acute and chronic wounds[60].
Silver nanoparticles enhance wound healing by promoting collagen expression and growth factors, leading to re-epithelialization, vasculogenesis, and collagen fiber deposition. They also induce fibroblast differentiation, promoting wound contraction and faster remodeling. Silver nanoparticles have antibacterial properties, reducing bacteria resistance and increasing effectiveness against multidrug-resistant microorganisms. The wound dressing industry has patented and commercialized silver-based dressings, which reduce wound area, increase collagen deposition, and modulate pro-inflammatory cytokines, reducing scar occurrence and displaying antibacterial properties against Pseudomonas aeruginosa and fibroblasts.
Microbial concentration in the wound milieu has crucial aspects concerning healing properties and avoiding incremental costs on antibiotic use. Supervising bacterial outgrowth in the surgical wound becomes fundamental for effective wound renovation. Reduction of the bacterial growth with a dressing composed of nanoparticles, hastens the wound healing course. Most in vivo investigations reveal proper wound healing features due to intrinsic antibacterial, anti-inflammatory, and hemostatic characteristics. Furthermore, except for antimicrobial properties, silver surgical textiles demonstrate a boost in healing features, as a result, silver exploitation has a positive effect on cell migration and proliferation quality[68].
It is explained in the literature that silver nanoparticles can modulate anti-inflammatory cytokine release and promote closure of the wound immediately without the growing scar[61].
Synthesis of silver nanoparticles:[65]
1. Chemical method for synthesis of silver nanoparticles
This method is beneficial because the equipment is more simple and easy to use than the biological methods. In brief, the silver ions receive electrons from the reducing agent and convert into the metallic form, therefore aggregate to form silver nanoparticles. Among the widely used silver salts, most commonly used is AgNO3, due its low cost.
It usually incorporates three main components: metal precursors, reducing agents and coating or stabilizing agents (Table 1). Basically, the reduction of silver salts involves nucleation and subsequent growth. Even though the yield is higher than the physical method, the overall method is very expensive and the ingredients used are toxic and unsafe, mostly the reducing agents used are toxic.
|
Reducing agent
|
Precursor agent
|
Capping agent
|
Experimental conditions
|
|
Trisodium citrate
|
Silver nitrate
|
Trisodium citrate
|
Diameter ? 10–80?nm; temperature ? boiling point
|
|
Ascorbic acid
|
Silver nitrate
|
Daxad 19
|
Diameter ? 15–26?nm; temperature ? boiling point
|
Table 1: components in synthesis of silver nanoparticles[65]
2. Physical method used in the silver nanoparticle synthesis
These methods include evaporation and condensation and laser ablation. However, a huge amount of energy is required, and a long time to complete the whole process. Usually, it has a gas-phase route that lets a tube furnace produce nanospheres at atmospheric pressure. At the center of this tube, there's a vessel with the base metal, which is evaporated into the carrier gas and thereby enables the final synthesis.
Laser ablation is another technique used in physical synthesis. A bulk metal source in a liquid medium can be used to produce silver nanoparticles. Following the irradiation with a pulsed laser, the medium contains only the NPs.
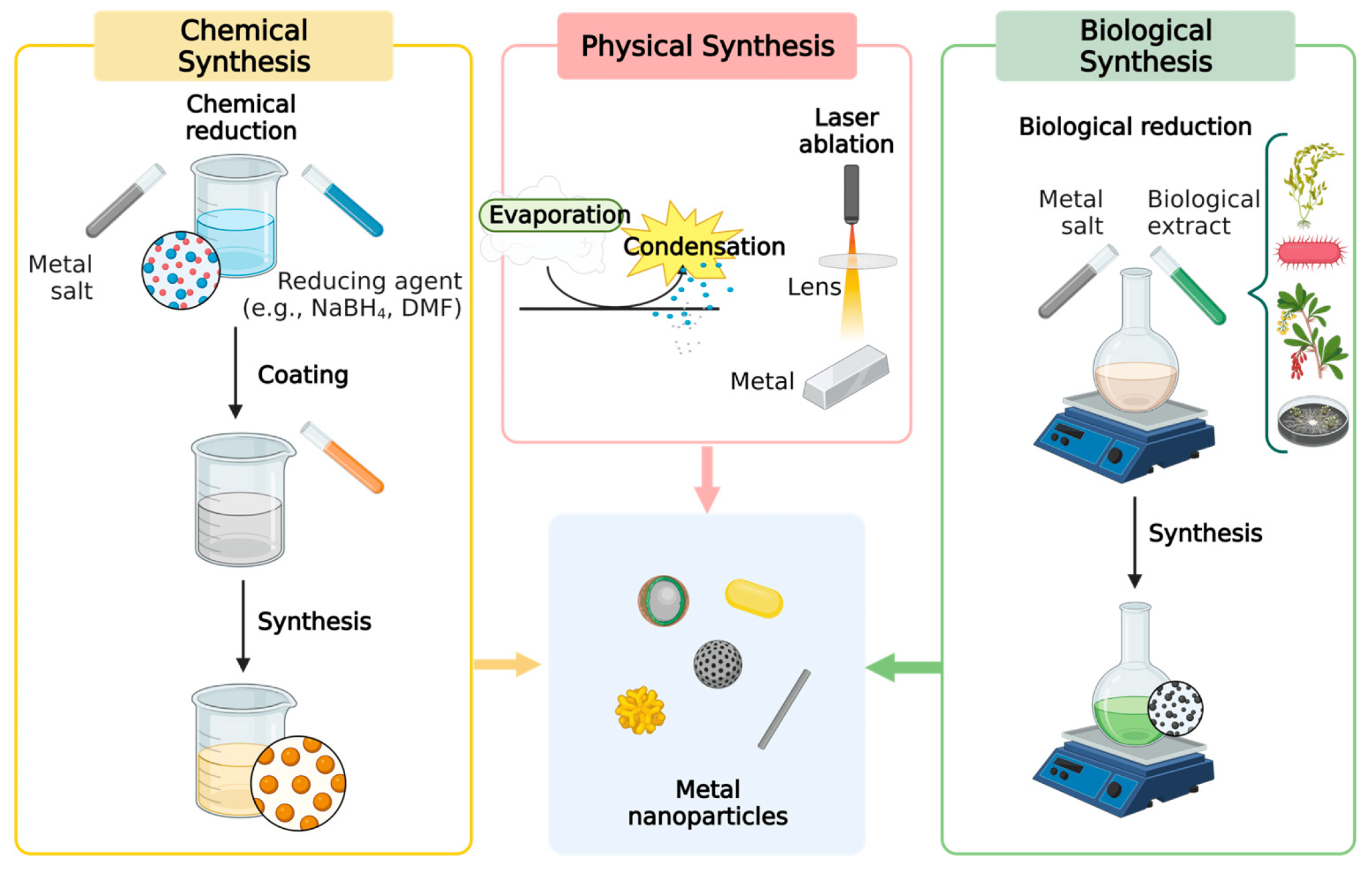
Figure 7: Common synthesis techniques employed for metal NPs[61]
3. Biological methods of preparation of silver nanoparticles
Biological methods are the alternative for limiting the chemical methods, harmful effects of the chemicals and to reduce the cost of production. Using biological methods of preparation includes the use of bacteria, fungi, plant extracts not only for silver nanoparticles but for several other NPs. This method gives a higher yield with minimal effect on the environment. Therefore selection of appropriate plant extracts or sustainable materials is very important to enhance the production.
Biological synthesis utilizes a solvent, reducing agent and a non-toxic material. It also has a presence of amino acids, proteins and secondary metabolites, which prevents the particle aggregation. Major advantage is that it provides a controlled and uniform particle shape and size.
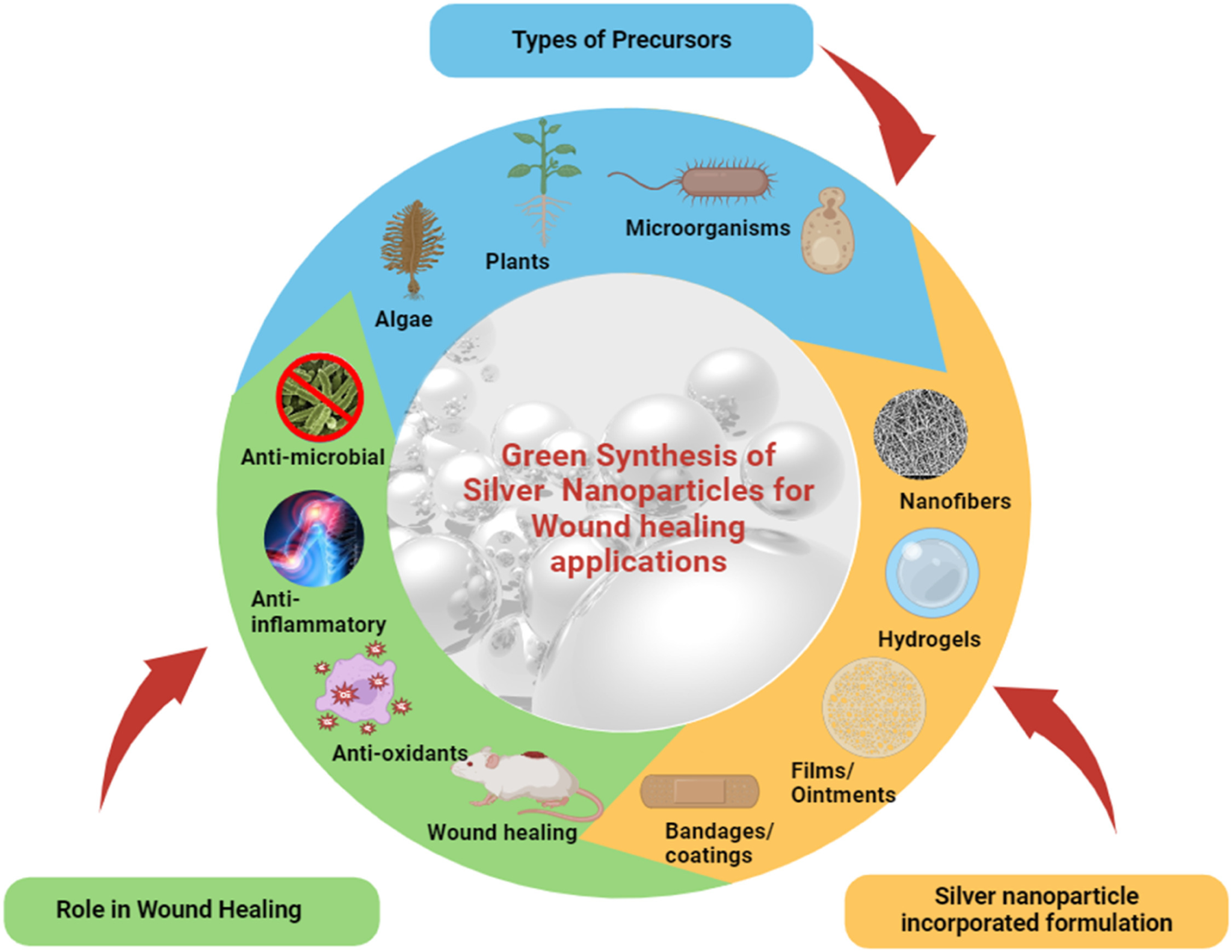
Figure 8: Outline of Green synthesized AgNPs for wound healing applications[67]
Antimicrobial activity of silver nanoparticles:[65]
Ag nanoparticles are the most often utilized inorganic nanoparticles as antibacterial agents, according to literature.
The numerous injection-molded plastic items, textiles, and coating-based applications all greatly benefit from the antibacterial application of Ag additions. Ag nanoparticles are also useful in a variety of biological contexts. Ag nanoparticles have demonstrated a high level of antibacterial activity that is similar to that of its ionic form.
Ag nanoparticles have also been shown to have antibacterial properties against bacteria that are resistant to drugs. According to published research, Ag nanoparticles' antibacterial activity stems from bacterial outer membrane destruction.
Ag was also said to be less harmful than a lot of other disinfectants. reviewed the Ag nanoparticles' antibacterial mechanisms and any possible effects on the environment and public health. In order to create Ag compounds, composites, and alloys with the least amount of toxicity and the greatest amount of antibacterial action, we believe that further study needs to be done.
Mechanism of action of silver nanoparticles:
AgNPs are crucial for wound healing because of their broad-spectrum resistance to numerous infections, high conductivity, chemical stability, and catalytic activity. It has long been recognized that silver has strong antibacterial properties[69] and demonstrates remarkable effectiveness against bacteria that produce biofilms and multidrug-resistant pathogens that are commonly found in chronic wounds. Antibacterial action is affected by size, shape, dose, and stabilizer. AgNPs can prevent bacterial development by destroying the bacterial cell membrane and producing free radicals, which cause the bacterial cell to leak membrane proteins and lipopolysaccharides, ultimately leading to cell death[71]. Oxygen and hydrogen atoms of thiol groups combine with silver ions to generate disulfide bonds, which hinder bacterial growth and DNA replication, ultimately leading to cell death. By controlling the expression of genes like p53, AgNPs can also cause cell death. Therefore, AgNPs are a good choice for using cutting-edge biological processes such as bone regeneration, wound healing, dental application, and catheter modification[66].
GOLD NANOPARTICLES:
Because of their targeted delivery, enhanced absorption, and safety, gold nanoparticles are widely used to provide a variety of bioactive substances, improving medication efficacy. They are known to be effective transporters, and their therapeutic impact increases with their level of dissemination.
These nanoparticles are used to treat a variety of conditions, including tissue healing, because of their anti-inflammatory and antioxidant qualities. Depending on the surface, they have strong antioxidant qualities in squelching free radicals such OH (hydroxyl), H2O2 (hydrogen peroxide), and NO (nitric oxide). Additionally, due to their large surface area, spherical gold nanoparticles are highly likely to absorb electrons and interact with reactive oxygen species (ROS) to eliminate or deactivate them[72].
AuNPs improve antibacterial activity and accelerate healing when added to hydrogel or polymer-based dressings.
Gels and creams for topical use:
AuNP-containing formulations are given topically to wounds to heal them locally and stop infections.
Biomaterials Integrated into Nanoparticles:
AuNPs can be incorporated into scaffolds for tissue engineering, promoting tissue regeneration and cell proliferation.
Intelligent Bandages:
AuNPs can be incorporated into these bandages to monitor wound conditions in real time, such as temperature and pH variations, which can reveal infection or the status of healing[73].
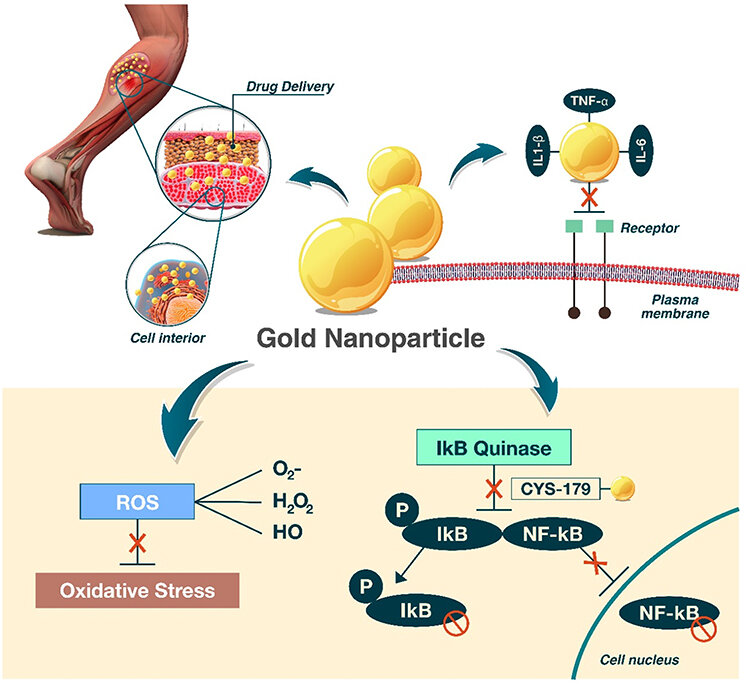
Figure 9: wound healing by gold nanoparticles[74]
Pressure sores and diabetic ulcers are examples of chronic wounds that are infamously hard to heal. Due to their several functions, gold nanoparticles have demonstrated significant promise in these situations: lowering the amount of bacteria, reducing inflammatory response, encouraging the production of collagen and angiogenesis[74].
Synthesis of gold nanoparticles:[66]
- Chemical method of preparation of gold nanoparticles
This method involves the reduction of gold salt, followed by stabilization of NPs. The reducing agent used is trisodium citrate, which reduces hydrogen tetrachloroaurate (III). This is the most common way to synthesize, but it uses expensive and toxic chemicals.
- Physical method of preparation of gold nanoparticles
AuNPs are produced by a self-assembly process by physical or even chemical method. This process is cost-effective and gives more control over the final product’s shape and size.
- Biological method of preparation of gold nanoparticles
Recently, this method has been preferred over the other methods because of its advantages of being environmentally friendly, which uses microorganisms, enzymes and plants. Like biological synthesis of AgNPs, biological method of synthesis AuNPs also eliminates the need for any harmful and toxic chemicals.
Mechanism of Action of gold nanoparticles:[66]
Gold nanoparticles are used for tissue repair given their anti-inflammatory and antioxidant properties. They quench free radicals like hydroxyl, hydrogen peroxide, and nitric oxide. Additionally, because spherical gold nanoparticles have a large surface area, they are highly likely to absorb electrons and interact with reactive oxygen species (ROS) to eliminate or deactivate them. As a result, they become a powerful antioxidant and are essential for wound healing. In addition to having potent catalytic activity in activities that scavenge free radicals, they can raise NRF2 levels, which triggers the activation of antioxidant genes. GNPs affect the thiol linkages on Keap1, changing its conformation and allowing NRF2 to carry on cytoprotective gene transcription. In rats, topical administration of GNPs has been shown to dramatically speed up the healing process by greatly increasing the expression of collagen and VEGF, as well as cytokines (IL10 and IL4) and growth factors (FGF and TGF?). four times quicker than in the other groups for wound closure.
Antimicrobial activity of gold nanoparticles:[70]
Because of their nontoxicity, strong functionalization capacity, polyvalent effects, simplicity of detection, and photothermal activity, Au nanoparticles are thought to be extremely beneficial in the creation of antibacterial drugs.
Tiwari et al. examined the antibacterial and antifungal properties of Au nanoparticles functionalized with 5-fluorouracil against the following microorganisms: Aspergillus fumigates (A. fumigates), S. aureus, P. aeruginosa, E. coli, and Aspergillus niger (A. niger). Due to their better internalization into gram negative bacteria, the authors observed that these nanoparticles exhibited greater activity on gram negative bacteria than on gram positive bacteria.
Because of the ROS-independent mechanism underlying their antibacterial effect, Au nanoparticles appear to be less harmful to mammalian cells than other nanometals. These nanoparticles are also excellent candidates for use as targeted antibacterial agents due to their great functionalization capabilities.
General overview of other metal nanoparticles:
Cu, ZnO, Silica nanoparticles:
Copper Nanoparticles:
Because of their organized structure, copper nanoparticles exhibit a more steady and controlled release of copper ions than copper salts, which results in less cytotoxicity than that caused by copper ions. According to some research, scaffolds with CuNPs demonstrated more stable copper ion release and degradation, which reduced cytotoxicity[85].
Zinc Oxide Nanoparticles
After iron, zinc oxide nanoparticles are the second most common metal oxide. They are cheap, safe, and simple to make (Kalpana et al., 2018). Zinc oxide nanoparticles' physical and chemical properties can be readily altered by altering their shape by the use of alternative synthesis methods, precursors, or materials (Bala et al., 2015)[84].
Silicon dioxide Nanoparticles
Silicon dioxide (SiO2) is used to make silica nanoparticles. Numerous techniques, such as sol-gel synthesis, hydrolysis, and chemical vapor deposition, are used to create them. High surface area and chemical stability silica nanoparticles can be employed in a variety of adsorption and catalysis processes, just like other nanoparticles. Silica nanoparticles have minimal toxicity and biocompatibility, which make them appropriate materials for use in biological and medical applications in addition to their physical and chemical characteristics[86].
Titanium dioxide Nanoparticles
In recent years, the construction industry has paid close attention to titanium dioxide nanoparticles in the form of anatase because of their potential to give infrastructures new capabilities, such as self-cleaning capabilities and the capacity to remove air pollutants through photocatalysis. Despite the fact that titanium dioxide nanoparticles (TiO2 NPs) enhance the mechanical and antibacterial qualities of traditional glass ionomer cements (GIC), there is ongoing debate regarding their biocompatibility.
SPIONs (Superparamagnetic Iron Oxide Nanoparticle)
The future of cancer theranostics, combinatorial cancer detection, and cancer treatment could be completely transformed by iron-oxide nanoparticles with superparamagnetic characteristics and small sizes. Superparamagnetic iron-oxide nanoparticles (SPIONs) exhibit exceptional tumor-targeting efficacy because of their special magnetic properties, which opens the door to successful customized cancer treatment. These magnetic particles are covered with biocompatible polymers like polyethylene glycol or dextran, which enhance the blood distribution profile of the therapeutic medicines and serve as chemical handles for their conjugation.
SIDE EFFECTS:
- Most studies reported that silver nanoparticles dressing has the potential to be used as an effective antibacterial agent for wound healing. However, clinical trials on the possible toxicity via dermal exposure are not sufficient enough, and thus more clinical studies are recommended on the potential toxicity via dermal exposure of silver nanoparticles[75].
- In the wound bed, metal nanoparticles can be harmful to healthy cells, especially when present in large concentrations. For example, oxidative stress caused by silver nanoparticles (AgNPs) might result in necrosis, apoptosis, or damage to cells.Even though they are less harmful, gold nanoparticles (AuNPs) can nonetheless stress cells if their concentration and size are not ideal[76].
- Hypersensitivity or allergic reactions to certain metal nanoparticles, especially silver. This can cause redness, itching, swelling, or other signs of inflammation at the wound site[77].
- Although metal nanoparticles at low concentrations can aid in wound healing, overuse of them can hinder the process. Excessive amounts of nanoparticles, particularly zinc and silver, may suppress fibroblast and keratinocyte activity, delaying wound healing and tissue regeneration[78].
- If metal nanoparticles are recognized as foreign items by the immune system, they may cause exaggerated inflammatory reactions. Excessive inflammation has the potential to postpone wound healing and extend the inflammatory period[79].
- Certain metal nanoparticles, like silver, have the ability to build up in tissues and may be harmful over time. Chronic inflammation may arise from persistent nanoparticle accumulation interfering with regular tissue healing processes.
- Reactive oxygen species (ROS) are produced by metal nanoparticles and are detrimental to cells. ROS can harm DNA, lipids, and proteins in cells, impairing their ability to function and delaying the healing of wounds. This is a prevalent worry regarding nanoparticles of silver[80].
- Although metal nanoparticles' antibacterial qualities help shield against infections, their broad-spectrum action can upset the skin's natural flora. This may lead to a state of dysbiosis, in which pathogenic bacteria proliferate, or impede the process of wound healing.
- Overuse or misuse of metal nanoparticles can cause aberrant collagen deposition and perhaps cause keloid formation or hypertrophic scarring, which can have a detrimental effect on tissue functions and cosmetic results[81].
- Metal nanoparticles work well against a variety of infections, but incorrect or extended use may cause germs to become resistant to them. This holds special importance for nanoparticles of silver[82].
Challenges and Future Directions:
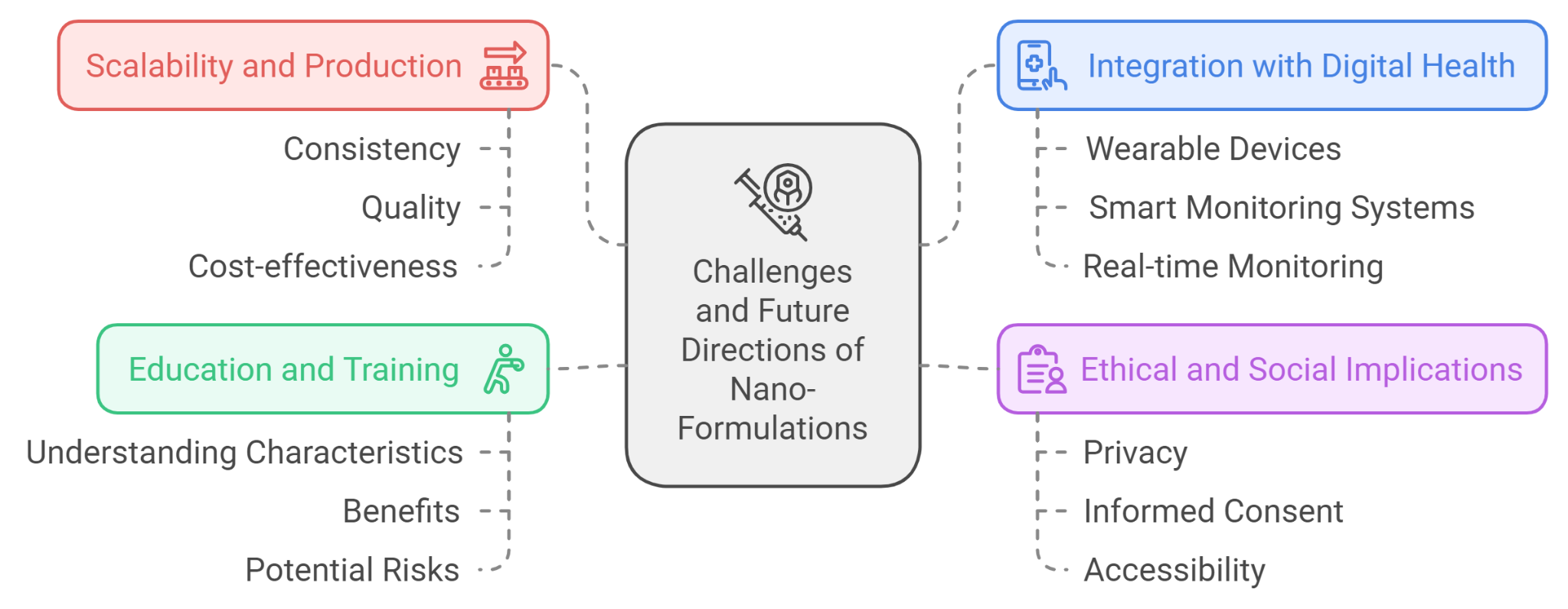
Figure 10: challenges and future directions of nano formulations
Scalability and Production:
One of the challenges in the field of nano-formulations is scaling up production from laboratory research to commercial manufacturing. Ensuring consistency, quality, and cost-effectiveness at a larger scale remains a significant hurdle[40].
Integration with Digital Health:
The integration of nano-formulations with digital health technologies, such as wearable devices and smart monitoring systems, is an emerging area. This integration could allow for real-time monitoring of drug delivery and therapeutic responses, leading to more adaptive and responsive treatment strategies[41].
Ethical and Social Implications:
The development and use of nano-formulations raise ethical and social questions, including concerns about privacy, informed consent, and the accessibility of advanced treatments. Addressing these concerns is crucial to ensure that the benefits of nanotechnology are equitably distributed and that patient rights are safeguarded[42].
Education and Training:
As nano-formulations become more prevalent, there is a need for education and training for healthcare professionals. Understanding the specific characteristics, benefits, and potential risks associated with nano-based therapies is essential for their effective implementation in clinical practice.
In summary, while nano-formulations offer transformative potential in drug delivery and beyond, ongoing research, regulatory advancements, and thoughtful consideration of ethical issues are essential for maximizing their benefits and minimizing potential risks[43].
CONCLUSION
The application of nanotechnology, particularly metal nanoparticles such as silver and gold, offers a transformative approach to wound healing management. Silver nanoparticles, with their robust antibacterial capabilities, have demonstrated exceptional promise in avoiding infections and increasing tissue regeneration, whereas gold nanoparticles provide anti-inflammatory and antioxidant advantages, making them excellent for healing chronic wounds. Despite the positive outcomes of preclinical investigations, the transition of nanoparticle-based therapeutics from research to clinical practice presents hurdles such as scalability, regulatory approval, and patient safety. Future research should prioritize refining nanoparticle formulations, increasing targeted distribution, and resolving concerns about toxicity and long-term impacts. With continuing improvements, nano drug delivery devices have the potential to transform wound care by providing more effective, tailored treatment choices for both acute and chronic wounds.
REFERENCES
- Alam, F., Sneed, K., & Pathak, Y. V. Recent Trends in Treating Wound Healing Using Nano Drugs Delivery Systems. J Pharma Res Dev, 2022 13(1): 1-9
- Patrick J. Pollock, Jim Schumacher, Chapter 23 - Principles of wound management, Editor(s): Tim S Mair, Sandy Love, Jim Schumacher, Roger KW Smith, Grant Frazer, Equine Medicine, Surgery and Reproduction (Second Edition), W.B. Saunders, 2012, Pages 469-487, ISBN 9780702028014
- Patrick J. Pollock, Jim Schumacher, Chapter 23 - Principles of wound management, Editor(s): Tim S Mair, Sandy Love, Jim Schumacher, Roger KW Smith, Grant Frazer, Equine Medicine, Surgery and Reproduction (Second Edition), W.B. Saunders, 2012, Pages 469-487, ISBN 9780702028014
- Naresh Kumar Rajendran, Sathish Sundar Dhilip Kumar, Nicolette Nadene Houreld, Heidi Abrahamse, A review on nanoparticle based treatment for wound healing, Journal of Drug Delivery Science and Technology, Volume 44, 2018, Pages 421-430, ISSN 1773-2247
- Murugesan Balamurugan, Sushil Kumar Singh, Jonathan C. Claussen, Chapter 13 - Electrochemical Immune Biosensors for Point-of-Care Detection of Hypoxia Biomarkers, Editor(s): Kshipra Misra, Priyanka Sharma, Anuja Bhardwaj, Management of High Altitude Pathophysiology, Academic Press, 2018, Pages 257-276, ISBN 9780128139998
- Toppo, Fedelic Ashish, and RAJESH SINGH Pawar. "Novel drug delivery strategies and approaches for wound healing managements." J Crit Rev 2, no. 2 (2015): 12À20.
- Rezvani Ghomi, E., Khalili, S., Nouri Khorasani, S. Esmaeely Neisiany, R., Ramakrishna, S. (2019), Wound dressings: Current advances and future directions. J. Appl. Polym. Sci., 136, 47738
- Dhivya, S., Padma, V. & Santhini, E. Wound dressings – a review. BioMed 5, 22 (2015)
- A. Agarwal, J.F. McAnulty, M.J. Schurr, C.J. Murphy, N.L. Abbott, 8 - Polymeric materials for chronic wound and burn dressings, Editor(s): David Farrar, In Woodhead Publishing Series in Biomaterials, Advanced Wound Repair Therapies, Woodhead Publishing, 2011, Pages 186-208, ISBN 9781845697006
- Mathew-Steiner, Shomita S., Sashwati Roy, and Chandan K. Sen. 2021. "Collagen in Wound Healing" Bioengineering 8, no. 5: 63.
- M. Rodrigues, N. Kosaric, C. Bonham, & G. Gurtner, "Wound healing: a cellular perspective", Physiological Reviews, vol. 99, no. 1, p. 665-706, 2019.
- Lopalco, A., Denora, N. (2018). Nanoformulations for Drug Delivery: Safety, Toxicity, and Efficacy. In: Nicolotti, O. (eds) Computational Toxicology. Methods in Molecular Biology, vol 1800. Humana Press, New York, NY.
- Sok Bee Lim, Amrita Banerjee, Hayat Önyüksel, Improvement of drug safety by the use of lipid-based nanocarriers, Journal of Controlled Release, Volume 163, Issue 1, 2012, Pages 34-45, ISSN 0168-3659
- Onoue S, Yamada S, Chan H. Nanodrugs: pharmacokinetics and safety. Int J Nanomedicine. 2014;9(1):1025-1037
- J.E. Kipp,The role of solid nanoparticle technology in the parenteral delivery of poorly water-soluble drugs,International Journal of Pharmaceutics,Volume 284, Issues 1–2,2004,Pages 109-122, ISSN 0378-5173
- Sezer, A. D. (Ed.). (2014). Application of Nanotechnology in Drug Delivery. InTech.
- Gautam, S., Das, D.K., Kaur, J. et al. Transition metal-based nanoparticles as potential antimicrobial agents: recent advancements, mechanistic, challenges, and future prospects. Discover Nano 18, 84 (2023).
- Dr. Xiao-Chao Yang, Dr. Bappaditya Samanta, Sarit S. Agasti, Youngdo Jeong, Zheng-Jiang Zhu, Subinoy Rana, Oscar R. Miranda, Prof. Vincent M. Rotello
- Abbasi, E., Aval, S.F., Akbarzadeh, A. et al. Dendrimers: synthesis, applications, and properties. Nanoscale Res Lett 9, 247 (2014).
- Drummen, Gregor PC. "Quantum dots—from synthesis to applications in biomedicine and life sciences." International Journal of Molecular Sciences 11, no. 1 (2010): 154-163.
- Kumar, Sarvan & VASHISTH, HEMANT. (2023). TYPES AND APPLICATION OF PHARMACEUTICAL NANOTECHNOLOGY: A REVIEW. International Journal of Current Pharmaceutical Research. 14-18. 10.22159/ijcpr.2023v15i3.3010.
- Goldberg, M., Langer, R., & Jia, X. (2007). Nanostructured materials for applications in drug delivery and tissue engineering. Journal of Biomaterials Science, Polymer Edition, 18(3), 241–268.
- Reuben T. Chacko, Judy Ventura, Jiaming Zhuang, S. Thayumanavan, Polymer nanogels: A versatile nanoscopic drug delivery platform,Advanced Drug Delivery Reviews,Volume 64, Issue 9,2012,Pages 836-851,ISSN 0169-409X
- Swati Biswas, Onkar S. Vaze, Sara Movassaghian, Vladimir P. Torchilin Book Editor(s):Dennis Douroumis, Alfred Fahr First published: 21 January 2013
- Josimar Oliveira Eloy, Marina Claro de Souza, Raquel Petrilli, Juliana Palma Abriata Barcellos, Robert J. Lee, Juliana Maldonado Marchetti,Liposomes as carriers of hydrophilic small molecule drugs: Strategies to enhance encapsulation and delivery,Colloids and Surfaces B: Biointerfaces,Volume 123,2014,Pages 345-363,ISSN 0927-7765
- Manju Rawat, Deependra Singh, S. Saraf, Swarnlata Saraf, Nanocarriers: Promising Vehicle for Bioactive Drugs, Biological and Pharmaceutical Bulletin, 2006, Volume 29, Issue 9, Pages 1790-1798, Released on J-STAGE September 01, 2006, Online ISSN 1347-5215, Print ISSN 0918-6158, https://doi.org/10.1248/bpb.29.1790
- Sharma, Sadhna, and Amandeep Singh. ‘Nanotechnology Based Targeted Drug Delivery: Current Status and Future Prospects for Drug Development’. Drug Discovery and Development - Present and Future, InTech, 16 Dec. 2011. Crossref, doi:10.5772/28902.
- Singh, A.P., Biswas, A., Shukla, A. et al. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Sig Transduct Target Ther 4, 33 (2019).
- Marek T. Wlodarczyk, Sylwia A. Dragulska, Ying Chen, Mina Poursharifi, Maxier Acosta Santiago, John A. Martignetti, Aneta J. Mieszawska, Pt(II)-PLGA Hybrid in a pH-Responsive Nanoparticle System Targeting Ovarian Cancer, Pharmaceutics, 15, 2, (607), (2023).
- Bennet, Devasier, and Sanghyo Kim. ‘Polymer Nanoparticles for Smart Drug Delivery’. Application of Nanotechnology in Drug Delivery, InTech, 25 July 2014. Crossref, doi:10.5772/58422.
- Analía Simonazzi, Alicia G. Cid, Mercedes Villegas, Analía I. Romero, Santiago D. Palma, José M. Bermúdez,Chapter 3 - Nanotechnology applications in drug controlled release,Editor(s): Alexandru Mihai Grumezescu,Drug Targeting and Stimuli Sensitive Drug Delivery Systems,William Andrew Publishing,2018,Pages 81-116,ISBN 9780128136898
- Raj Kumar, Sameer V. Dalvi, and Prem Felix Siril, ACS Applied Nano Materials 2020 3 (6), 4944-4961 DOI: 10.1021/acsanm.0c00606
- Sridhar, R., & Ramakrishna, S. (2013). Electrosprayed nanoparticles for drug delivery and pharmaceutical applications. Biomatter, 3(3).
- Mohammed MA, Syeda JTM, Wasan KM, Wasan EK. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics. 2017; 9(4):53.
- Fornaguera C, García-Celma MJ. Personalized Nanomedicine: A Revolution at the Nanoscale. Journal of Personalized Medicine. 2017; 7(4):12.
- Dual-drug delivery based charge-conversional polymeric micelles for enhanced cellular uptake and combination therapy Jianhong Liao, Yajing Song, Can Liu, Dan Li, Hua Zheng and Bo Lu Polym. Chem., 2019, 10, 5879 DOI: 10.1039/C9PY01105F
- Li, W., Wei, H., Li, H., Gao, J., Feng, S. S., & Guo, Y. (2014). Cancer Nanoimmunotherapy Using Advanced Pharmaceutical Nanotechnology. Nanomedicine, 9(16), 2587–2605.
- J.M. Provenzale and G.A. Silva American Journal of Neuroradiology August 2009, 30 (7) 1293-1301; DOI:10.3174/ajnr.A15903
- Franiak-Pietryga, B. Ziemba, B. Messmer, and D. Skowronska-Krawczyk, ‘Dendrimers as Drug Nanocarriers: The Future of Gene Therapy and Targeted Therapies in Cancer’, Dendrimers - Fundamentals and Applications. InTech, Apr. 25, 2018. doi: 10.5772/intechopen.75774.
- Muthu, M. S., & Wilson, B. (2012). Challenges Posed by The Scale-Up of Nanomedicines. Nanomedicine, 7(3), 307–309.
- You Han Bae, Kinam Park, Advanced drug delivery 2020 and beyond: Perspectives on the future, Advanced Drug Delivery Reviews, Volume 158, 2020, Pages 4-16, ISSN 0169-409X
- Kristin Bakke Lysdahl, Wija Oortwijn, Gert Jan van der Wilt, Pietro Refolo, Dario Sacchini, Kati Mozygemba, Ansgar Gerhardus, Louise Brereton, Bjørn Hofmann, Ethical analysis in HTA of complex health interventions, BMC Medical Ethics, 10.1186/s12910-016-0099-z, 17, 1, (2016).
- Vividha Dhapte, Varsha Pokharkar, Chapter 13 - Nanosystems for drug delivery: Design, engineering, and applications, Editor(s): Ashutosh Kumar Shukla, Siavash Iravani, In Micro and Nano Technologies, Green Synthesis, Characterization and Applications of Nanoparticles,Elsevier, 2019, Pages 321-345,ISBN 9780081025796
- Horikoshi, S. and Serpone, N. (2013). Introduction to Nanoparticles. In Microwaves in Nanoparticle Synthesis (eds S. Horikoshi and N. Serpone).
- Ullah Khan S, Saleh TA, Wahab A, Khan MHU, Khan D, Ullah Khan W, Rahim A, Kamal S, Ullah Khan F, Fahad S. Nanosilver: new ageless and versatile biomedical therapeutic scaffold. Int J Nanomedicine. 2018;13:733-762
- Ibrahim Khan, Khalid Saeed, Idrees Khan,Nanoparticles: Properties, applications and toxicities,Arabian Journal of Chemistry,Volume 12, Issue 7,2019,Pages 908-931,ISSN 1878-5352
- Bhuiyan, M. T. H., M. N. Chowdhury, and Mst Shahanaz Parvin. "Potential nanomaterials and their applications in modern medicine: an overview." ARC Journal of Cancer Science 2, no. 2 (2016): 25-33.
- Zhang, Lisha, F. X. Gu, J. M. Chan, A. Z. Wang, R. S. Langer, and O. C. Farokhzad. "Nanoparticles in medicine: therapeutic applications and developments." Clinical pharmacology & therapeutics 83, no. 5 (2008): 761-769.
- Muddineti, Omkara Swami, Balaram Ghosh, and Swati Biswas. "Current trends in using polymer coated gold nanoparticles for cancer therapy." International journal of pharmaceutics 484, no. 1-2 (2015): 252-267.
- Desai, Neil. "Challenges in development of nanoparticle-based therapeutics." The AAPS journal 14, no. 2 (2012): 282-295.
- Agustina et al 2020 IOP Conf. Ser.: Mater. Sci. Eng. 980 012005 DOI: 10.1088/1757-899X/980/1/012005
- A. K. Pradhan, K. Zhang, M. Bahoura, J. Pradhan, P. Ravichandran, R. Gopikrishnan and G. T. Ramesh Submitted: 23 April 2010 Published: 08 January 2011 DOI: 10.5772/13243
- Amir Reza Sadrolhosseini, Mohd Adzir Mahdi, Farideh Alizadeh and Suraya Abdul Rashid Reviewed: 18 July 2018 Published: 20 December 2018 DOI: 10.5772/intechopen.80374
- SARVAN, and HEMANT VASHISTH. 2023. “TYPES AND APPLICATION OF PHARMACEUTICAL NANOTECHNOLOGY: A REVIEW”. International Journal of Current Pharmaceutical Research 15 (3):14-18.
- Eatemadi, A., Daraee, H., Karimkhanloo, H. et al. Carbon nanotubes: properties, synthesis, purification, and medical applications. Nanoscale Res Lett 9, 393 (2014).
- Tong, Rong, Li Tang, Nathan P. Gabrielson, Qian Yin, and Jianjun Cheng. "Polymer–Drug Nanoconjugates." Pharmaceutical Sciences Encyclopedia: Drug Discovery, Development, and Manufacturing (2010): 1-32.
- M.K.; Iqbal, M.; Muharram, M.M.; Mohammad, M.; Ezzeldin, E.; Aldawsari, M.F.; Alalaiwe, A.; Imam, F. Development of Lipomer Nanoparticles for the Enhancement of Drug Release, Anti-Microbial Activity and Bioavailability of Delafloxacin. Pharmaceutics 2020, 12, 252.
- Park, Chang Min, Dengjun Wang, and Chunming Su. "Recent developments in engineered nanomaterials for water treatment and environmental remediation." Handbook of nanomaterials for industrial applications (2018): 849-882.
- Chen, Zhi, Dantong Zhou, X. Dong, W. Shen, Li Man-Rong, Cristina Della Pina, and Ermelinda Falletta. "Advanced nanomaterials for energy and environmental applications." Journal of Nanomaterials (2015): 1-2.
- Prasad, Ram, Atanu Bhattacharyya, and Quang D. Nguyen. "Nanotechnology in sustainable agriculture: recent developments, challenges, and perspectives." Frontiers in microbiology 8 (2017): 1014.
- Guerra, Fernanda D., Mohamed F. Attia, Daniel C. Whitehead, and Frank Alexis. "Nanotechnology for environmental remediation: materials and applications." Molecules 23, no. 7 (2018): 1760.
- Burlec, Ana Flavia, Andreia Corciova, Monica Boev, Denisa Batir-Marin, Cornelia Mircea, Oana Cioanca, Gabriela Danila, Marius Danila, Anca Florentina Bucur, and Monica Hancianu. "Current overview of metal nanoparticles’ synthesis, characterization, and biomedical applications, with a focus on silver and gold nanoparticles." Pharmaceuticals 16, no. 10 (2023): 1410.
- Krishnia, Lucky, Preeti Thakur, and Atul Thakur. "Synthesis of nanoparticles by physical route." In Synthesis and applications of nanoparticles, pp. 45-59. Singapore: Springer Nature Singapore, 2022.
- Abid, Namra, Aqib Muhammad Khan, Sara Shujait, Kainat Chaudhary, Muhammad Ikram, Muhammad Imran, Junaid Haider, Maaz Khan, Qasim Khan, and Muhammad Maqbool. "Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review." Advances in Colloid and Interface Science 300 (2022): 102597.
- Almatroudi, Ahmad. "Silver nanoparticles: Synthesis, characterisation and biomedical applications." Open life sciences 15, no. 1 (2020): 819-839.
- Mendes, Carolini, Anand Thirupathi, Maria EAB Corrêa, Yaodong Gu, and Paulo CL Silveira. "The use of metallic nanoparticles in wound healing: new perspectives." International Journal of Molecular Sciences 23, no. 23 (2022): 15376.
- Nandhini, J., E. Karthikeyan, E. Elizabeth Rani, V. S. Karthikha, D. Sakthi Sanjana, H. Jeevitha, S. Rajeshkumar, Vijayan Venugopal, and A. Priyadharshan. "Advancing engineered approaches for sustainable wound regeneration and repair: Harnessing the potential of green synthesized silver nanoparticles." Engineered Regeneration 5, no. 3 (2024): 306-325.
- Toczek, Jakub, Marcin Sad?ocha, Katarzyna Major, and Rafa? Stojko. "Benefit of silver and gold nanoparticles in wound healing process after endometrial cancer protocol." Biomedicines 10, no. 3 (2022): 679.
- Jeong, Yoon, Dong Woo Lim, and Jonghoon Choi. "Assessment of size?dependent antimicrobial and cytotoxic properties of silver nanoparticles." Advances in Materials Science and Engineering 2014, no. 1 (2014): 763807.
- Tiwari, Pooja M., Komal Vig, Vida A. Dennis, and Shree R. Singh. "Functionalized gold nanoparticles and their biomedical applications." Nanomaterials 1, no. 1 (2011): 31-63.
- Wang, Linlin, Chen Hu, and Longquan Shao. "The antimicrobial activity of nanoparticles: present situation and prospects for the future." International journal of nanomedicine (2017): 1227-1249.
- Jena, Bikash K., Sourov Ghosh, Rajkumar Bera, Ramendra S. Dey, Ashok K. Das, and C. Retna Raj. "Bioanalytical applications of Au nanoparticles." Recent Patents on Nanotechnology 4, no. 1 (2010): 41-52.
- el kurdi, Riham & Patra, Digambara. (2019). Gold and silver nanoparticles in resonance Rayleigh scattering techniques for chemical sensing and biosensing: a review. Microchimica Acta. 186. 10.1007/s00604-019-3755-4.
- A. Ravindran Girija, S. Balasubramanian, R. Bright, A. J. Cowin, N. Goswami, K. Vasilev, ChemNanoMat 2019, 5, 1176.
- A. Ravindran Girija, S. Balasubramanian, R. Bright, A. J. Cowin, N. Goswami, K. Vasilev, ChemNanoMat 2019, 5, 1176.
- Pan, Yu, Annika Leifert, David Ruau, Sabine Neuss, Jörg Bornemann, Günter Schmid, Wolfgang Brandau, Ulrich Simon, and Willi Jahnen?Dechent. "Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage." small 5, no. 18 (2009): 2067-2076.
- Natalia B. Golovina, Leonid M. Kustov,Toxicity of metal nanoparticles with a focus on silver,Mendeleev Communications,Volume 23, Issue 2,2013,Pages 59-65,ISSN 0959-9436.
- Heunis, T. D. J., and L. M. T. Dicks. "Nanofibers offer alternative ways to the treatment of skin infections." BioMed Research International 2010, no. 1 (2010): 510682.
- Luo, Yueh-Hsia, Louis W. Chang, and Pinpin Lin. "Metal?based nanoparticles and the immune system: activation, inflammation, and potential applications." BioMed research international 2015, no. 1 (2015): 143720.
- Luo, Yueh-Hsia, Louis W. Chang, and Pinpin Lin. "Metal?based nanoparticles and the immune system: activation, inflammation, and potential applications." BioMed research international 2015, no. 1 (2015): 143720.
- Liuya Wei, Jingran Lu, Huizhong Xu, Atish Patel, Zhe-Sheng Chen, Guofang Chen,Silver nanoparticles: synthesis, properties, and therapeutic applications,Drug Discovery Today,Volume 20, Issue 5,2015,Pages 595-601,ISSN 1359-6446.
- Mironava, T., Hadjiargyrou, M., Simon, M., Jurukovski, V., & Rafailovich, M. H. (2010). Gold nanoparticles cellular toxicity and recovery: Effect of size, concentration and exposure time. Nanotoxicology, 4(1), 120–137.
- Khurana C, Chudasama B. Nanoantibiotics: strategic assets in the fight against drug- resistant superbugs. Int J Nanomedicine. 2018;13(T-NANO 2014 Abstracts):3-6
- Thangavel Lakshmipriya, Subash C.B. Gopinath, 1 - Introduction to nanoparticles and analytical devices, Editor(s): Subash C.B. Gopinath, Fang Gang,
- Nanoparticles in Analytical and Medical Devices, Elsevier, 2021, Pages 1-29, ISBN 9780128211632.
- Yufeng Wang, Wei Zhang, Qingqiang Yao, Copper-based biomaterials for bone and cartilage tissue engineering, Journal of Orthopaedic Translation, Volume 29, 2021, Pages 60-71.
- Hichem Moulahoum, Faezeh Ghorbanizamani, Tutku Beduk, Duygu Beduk, Ozge Ozufuklar, Emine Guler Celik, Suna Timur, Emerging trends in nanomaterial design for the development of point-of-care platforms and practical applications, Journal of Pharmaceutical and Biomedical Analysis, Volume 235, 2023, 115623.
- Mendes C, Thirupathi A, Corrêa MEAB, Gu Y, Silveira PCL. The Use of Metallic Nanoparticles in Wound Healing: New Perspectives. Int J Mol Sci. 2022 Dec 6;23(23):15376. doi: 10.3390/ijms232315376. PMID: 36499707; PMCID: PMC9740811.
- Mendes, Carolini, Anand Thirupathi, Maria EAB Corrêa, Yaodong Gu, and Paulo CL Silveira. "The use of metallic nanoparticles in wound healing: new perspectives." International Journal of Molecular Sciences 23, no. 23 (2022): 15376.
- Aminzai, Mohammad & Patan, Abubaker. (2024). Recent Applications and Evaluation of Metal Nanoparticle–Polymer Hybrids as Chronic Wound Dressings. Journal of Nanomaterials. 2024.


 Swardhuni Pawar*
Swardhuni Pawar*










 10.5281/zenodo.14019257
10.5281/zenodo.14019257