Abstract
Transdermal drug delivery systems are intended to make systemic treatment easier by applying medicine topically to healthy skin. These systems offer advantages such increased patient compliance, safety, and efficacy. They are made up of discrete, self-contained dosage forms that release medications into the bloodstream at controlled rates. One of the easiest organs to reach is the skin which offers benefits over conventional administration systems, such as less variation in the plasma drug level and no chance of gastrointestinal problems. Still, a major obstacle to efficient medication delivery is the stratum corneum's restricted permeability. The potential of vesicular systems has been brought to light by recent developments, especially ethosomes, which are soft lipid vesicles made of phospholipids, water, and high ethanol concentrations. Ethosomes are intended to improve skin penetration by the disrupting the stratum corneum's lipid composition. In this review, ethosomes are categorized as classical, binary and transethosomes including their contents and mechanisms along with the effects of ethanol and ethosomes on drug absorption are being described. Ethosome preparation and characterisation methods are presented, as well as their uses in a range of therapeutic contexts, including inflammatory diseases, acne and androgenic alopecia etc. Although ethosomes provide benefits including increased patient compliance and drug permeability, issues like formulation costs and possible skin irritation must be resolved. overall, ethosomes represent a new and exciting approach to transdermal drug administration, and further research is being conducted to increase their efficiency and expand their therapeutic applications.
Keywords
Ethosomes, transdermal, penetration, novel drug delivery.
Introduction
Recently, transdermal drug delivery systems have been created with the goal of achieving systemic treatment through topical application to the intact skin surface. A transdermal treatment system is a set of discrete, self-contained dosage forms that are applied to the skin and release the drug into the systemic circulation at a controlled rate. Enhanced efficacy, increased safety, and improved patient compliance are just a few benefits that transdermal administration can offer.1 One of the largest and easiest organs in the human body to access is the skin. When using the skin to deliver drugs, it has several benefits over more conventional methods, such as reduced variations in plasma drug levels, prevention of gastrointestinal issues, drug’s first-pass metabolism and increased patient compliance. The limited permeability of skin restricts the number of medications that may be administered trans-dermally, which is one of the biggest drawbacks of this method of drug delivery. Except for lipophilic and low molecular weight medications, the stratum corneum is the strongest barrier preventing the passage of most pharmaceuticals, making the skin a great barrier to molecular transport.2 In the current period of global research, the application of vesicular systems has drawn significant consideration. The exterior part of these aqueous vesicular carriers is made up of lipid bilayers. The lipid bilayer of the vesicular carrier traps the lipophilic medications, while the hydrophilic pharmaceuticals are contained in the interior aqueous environment. The medication contained within these carriers travels through the skin by disrupting the lipid arrangement of the stratum corneum, hence adjusting the delivery rate.3
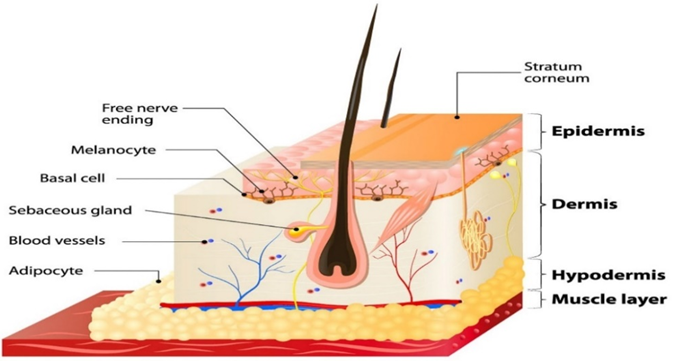
Figure 1: Diagrammatic view of skin
Ethosomes are malleable, soft lipid vesicles that are mostly made up of water, ethanol (or isopropyl alcohol) in relatively high concentrations (20–45%), and phospholipids. Touitou and her associates created ethosomes for the first time in 1997. Because of its great deformability, this carrier exhibits unique characteristics that are associated with its capacity to pass through human skin intact. These vesicular phospholipids can function as the vesicle-forming element of the ethosomal system due to the physicochemical properties of ethosomes. Phospholipids are employed in concentrations ranging from 0.5 to 10%. Examples of phospholipids with different chemical structures include phosphatidyl choline (PC), hydrogenated PC, and phosphatidyl ethanolamine (PE).4

Figure 2: Structure of ethosome
Categories Of Ethosomes5
Following are some major classifications of ethosomes.
1.Classical ethosomes
These modified classical liposomes exhibit significantly improved skin penetration. These consist of phospholipids, water and ethanol at a significantly higher concentration of up to 45% w/v. For transdermal drug delivery, the classical ethosomes are thought to be superior to liposomes due to their smaller vesicular size, negative zeta potential, and higher entrapment efficiency.
2.Binary ethosomes
These were made by mixing a different type of alcohol with the conventional ethosomes. The two most often used alcohols are propylene glycol and isopropyl alcohol.
3.Transethosomes
This is recognized as the newest and most sophisticated ethosomal system. It consists of the basic components of traditional ethosomes plus an additional component that functions as a surfactant or an edge activator to improve penetration.5

Figure 3: Categorization of ethosomes
Composition of ethosomes6
Ethosomes typically contain phospholipids with a range of different chemical structures, such as hydrogenated PC, phosphatidic acid (PA), phosphatidylcholine (PC), phosphatidylserine (PS), phosphatidylethanolamine (PE), phosphatidylglycerol (PPG), phosphatidylinositol (PI), hydrogenated PC, ethanol, water, and propylene glycol (or other glycols).6
Mechanism Of Drug Penetration7
There are two steps to the drug absorption mechanism from ethosomes.
- Ethanol effect
- Ethosome effect
1.Ethanol effect
Through the skin, ethanol improves permeation. It is commonly understood how the penetration increasing effect works. Ethanol seeps into the internal lipid sand and increases the permeability of the lipid in the cell membrane while decreasing the density of the lipid multilayer.
2.Ethosome effect
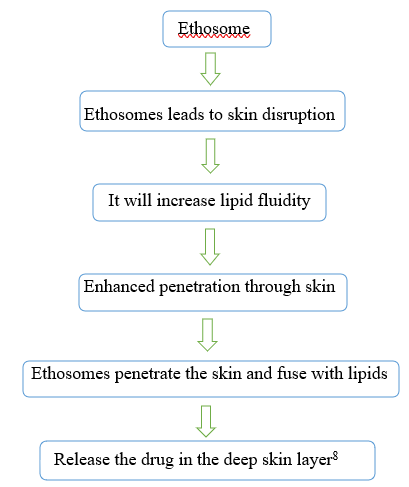
The amount of phospholipid and ethanol present affects the ethosomal size. It has been seen that as phospholipid concentration rises, vesicle size increases, while ethosome size falls when ethanol concentration rises.9
Table 1: Different additives involved in formulation of ethosomes10
|
Class
|
Example
|
Uses
|
|
Phospholipids
|
Soya phosphatidyl choline Egg phosphatidyl choline Dipalmityl phosphatidyl choline Distearyl phosphatidyl choline
|
It influences on the size, entrapment efficacy, zeta potential and penetration properties of the vesicles.
|
|
alcohol
|
Ethanol, Isopropyl alcohol
|
For providing the softness for vesicle membrane As a penetration enhancer
|
|
Polyglycol
|
propylene glycol, Transcutol RTM
|
As a skin penetration enhancer
|
|
cholesterol
|
cholesterol
|
For providing the stability to vesicle membrane
|
|
Edge activators
|
N-DMSO, Tween[22], Span
|
Enhances skin permeability
|
|
Dye
|
Rhodamine-123 Rhodamine red Fluorescene Isothiocynate(FITC) 6 – Carboxy fluorescence
|
For characterization study
|
|
Others
|
Dicetyl phosphate
|
Prevent aggregation of vesicles
|
|
vehicles
|
Carbopol D-934, HPMC
|
As a gel former
|
Advantages11
1. It is feasible to deliver big molecules, such as peptides and protein molecules.
2. The formulation uses non-toxic raw materials.
3. Improved medication permeability through skin for transdermal administration.
4. The ethosomal drug delivery technology has broad applications in the veterinary, cosmetic, and pharmaceutical industries.
5. Great patient compliance: Because the ethosomal medication is administered as a gel or cream, it is semisolid, which results in great patient compliance.
6. A straightforward approach to medication distribution that contrasts phonophoresis, iontophoresis, and other more intricate techniques
7. The Ethosomal system may be commercialized right away and is passive and non-invasive.
Disadvantages11
1. They need elevated blood pressure. Only strong molecules—those needing a daily dosage of 10 mg or less—are included.
2. It is usually intended to provide steady, sustained drug delivery rather than a way to produce quick bolus-type drug input.
3. Sufficient solubility of the medication in aqueous and lipophilic conditions to enter the systemic circulation and reach the cutaneous microcirculation.
4. The drug's molecular size should be appropriate for transdermal absorption. 5. Not every type of skin can cling to adhesive properly.
5. It might not be cost-effective.
6. Low yield.
7. Dermatitis or skin irritation brought on by enhancers and excipients used in medication delivery systems.
Methods Of Ethosome Preparation12,13
The following techniques are typically used to prepare ethosomes:
1.Hot method
2.Cold method
3.Classic mechanical dispersion method
1.Hot method:
The phospholipids are first heated to 40°C and dissolved in water in this process. Ethanol and propylene glycol are heated to the same temperature as the aqueous phase at the same time. Depending on the properties it has, the medication dissolves in ethanol or water. To reduce particle size, the organic phase is introduced to the aqueous phase and sonicated.
2.Cold method
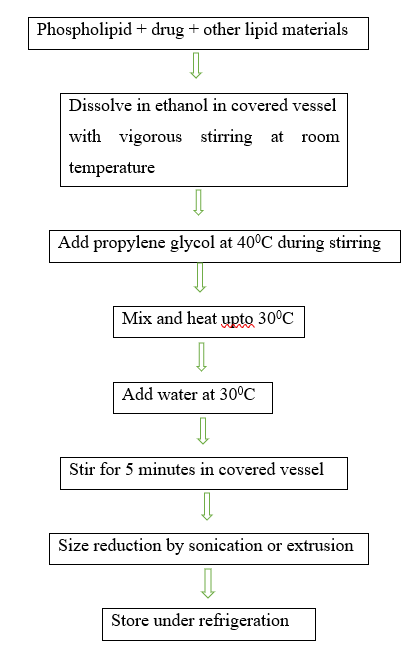
3.Classic mechanical dispersion method
Soya phosphotidyl choline is dissolved in a round bottom flask with a 3:1 ratio of methanol to chloroform. Using a rotating vacuum evaporator, the organic solvents are eliminated above the lipid transition temperature, forming a thin lipid film on the flask wall. Lastly, by vacuuming the contents for an entire night, any remaining solvent combination is extracted from the deposited lipid film. Rotating the flask at an appropriate temperature allows for the addition of various concentrations of drug-containing hydroethanolic mixture for hydration.
Characterizations Of Ethosomes:14,15,16
1. Visualization
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) can both be used to visualize ethosomes.14
2. Zeta potential and vesicle dimensions
Photon correlation spectroscopy (PCS) and dynamic light scattering (DLS) with an automated inspection system can be used to detect particle size and zeta potential.14
3.Skin permeation studies:
Confocal laser scanning microscopy (CLSM) can be employed to evaluate how well the ethosomal preparation penetrates the skin layers.14
4. Measurement of Surface Tension Activity
A Du Nouy ring tensiometer can be used to measure the surface tension activity of a medication in an aqueous solution using the ring method.14
5.Transition temperature:
Differential scanning calorimetry (DSC) can be used to find the vesicular lipid systems' transition temperature. The Mettler DSC 60 computerized with Mettler Toledo star software system is utilized. By heating the aluminum crucibles at a rate of 10 degrees per minute, the transition temperature was determined between 20 and 300 degrees Celsius.15
6.Degree of turbidity and deformability:
The ethosomal preparation's turbidity can be measured with a nephelometer, and the degree of deformability can be determined using the extrusion method.15
7.Efficiency of Entrapment (EE)
How thoroughly a drug is trapped in ethosomes can be ascertained using the ultracentrifugation method. Because a lipid that forms a bilayer structure effectively maintains the drug in place, the chemical makeup of the lipid has a substantial impact on the EE of the drug in the ethosomes. However, the drug may fit because of the lipid structure's flaws. The vesicles are separated in a high-speed cooling centrifuge that rotates at 20,000 rpm for ninety minutes at a temperature of 4°C.After lysing the vesicles in methanol and removing the liquid supernatant from the sediment, use the following formula to calculate the drug concentration in the sediment.
% Entrapment= Actual Content / Theoretical Content x 10016
8.Studies of Stability
The ability of ethosomal preparations to retain the drug (i.e., show drug-retentive behavior) can be evaluated when they are held for varying amounts of time at various temperatures, such as 25 °C (room temperature, RT), 37 °C, and 45 °C (1, 20, 40, 60, 80, and 120 days). After being nitrogen flushed, the ethosomal preparations were put into sealed vials with a capacity of 10 mL. The size and appearance of the vesicles were evaluated using DLS and TEM to ascertain the stability of the ethosomes.16
9.Drug Content
The amount of drug present is calculated using a UV spectrophotometer. The dosage of the medication can also be ascertained by employing an altered HPLC method.16
10. drug deposition and in vitro drug release
It is possible to utilize dialysis bag diffusion or Franz diffusion cells with synthetic or biological membranes for in vitro drug release investigations and drug deposition of ethosomal preparation.16
11.Phospholipid Interaction with Ethanol
Proton decoupled 31P-NMR and differential scanning calorimetry were employed to look at how phospholipids and ethanol interacted.16
Table 2: Compilation of reported works on Ethosome
|
Active ingredient
|
Formulation
|
Applications
|
|
Lamivudine and Stavudine (2019)17
|
Transdermal patches
|
Treatment of HIV
|
|
Paeonol(2018)18
|
Gel
|
Anti-inflammatory activity
|
|
Naproxen Sodium (2017)19
|
Gel
|
treatment of rheumatoid arthritis and ankylosing spondylitis
|
|
Azelaic acid (2016)20
|
Gel
|
Treatment of Acne
|
|
Cryptotanshinone (2016)21
|
Gel
|
Acne Treatment
|
|
Indomethacin (2016)22
|
Gel
|
rheumatoid arthritis and musculo-skeletal disorders
|
|
Tolterodine tartrate (2013)23
|
Gel
|
treatment of overactive bladder
|
|
Isotretinoin (2013)24
|
Gel
|
treatment of severe acne
|
|
Aceclofenac(2010)25
|
Gel
|
treatment of rheumatoid arthritis and osteoarthritis
|
|
Diclofanac potassium (2010)26
|
Gel
|
treatment of rheumatoid arthritis and osteoarthritis
|
|
Lamivudine (2007)27
|
Suspension
|
Treatment of HIV
|
Table 3: Applications of ethosomes as a drug carrier28,29,30,31
|
Drug
|
Applications
|
|
Finasteride
|
the treatment of androgenic alopecia and as surgical alternative for benign prostatic hyperplasia.28
|
|
DNA
|
Better expression of genes Selective targeting to dermal cells29
|
|
Bacitracin
|
Improved dermal deposition Improved intracellular delivery Increased bioavailability29
|
|
Ammonium glycyrrhizinate
|
Improved dermal deposition exhibiting sustained release Improved biological anti-inflammatory activity 29
|
|
Trihexyphenidyl hydrochloride
|
Improved transdermal flux Provide controlled release Improved patient compliance Biologically active at dose several times lower than the currently used formulation29
|
|
NSAIDS (Diclofenac) (Aceclofenac)
|
Selective and prolong delivery of drug to desired site. Superior to the marketed gel for the topical administration30
|
|
Pilosebaceous (Minoxidil)
|
High penetration into deep layers of the skin. Targeting30
|
|
Propanolol
|
Better skin permeation30
|
|
Methotrexate
|
Enhanced transdermal flux, lower lag time, higher entrapment efficiency and better stability profile30
|
|
Antibiotic (Erythromycin) (Cannabidol)
|
Complete inhibition of infection, prolonged drug action. Improved skin deposition and biological activity30
|
|
Triptolide
|
Treatment of skin inflammation31
|
|
Benzocaine
|
Topical anaesthesia31
|
CONCLUSION
Ethosomes are a promising advancement for transdermal drug delivery systems, providing a number of benefits over conventional techniques. Because of their distinct lipid content, which enables superior drug stability, controlled release, and increased skin penetration, they are especially useful for administering a variety of medicinal medicines. Ethosomes have a great deal of potential for clinical use in the future as research into their formulation and delivery systems remains ongoing. This will open up the possibility to more efficient and patient-friendly treatment alternatives. To fully realize the advantages of ethosomal technology in the pharmaceutical sector, future research should concentrate on addressing existing obstacles such as portability and safety concerns.
REFERENCES
- Kumar N, Dubey A, Mishra A, Tiwari P. Ethosomes: A Novel Approach in Transdermal Drug Delivery System. International journal of pharmacy & life sciences. 2020 May 1;11(5).
- Kesharwani R, Patel DK, Sachan A, Kumar V, Mazumdar B. Ethosomes: A novel approach for transdermal and topical drug delivery. Research Journal of Topical and Cosmetic Sciences. 2015;6(1):15-20.
- Akhtar N, Varma A, Pathak K. Ethosomes as vesicles for effective transdermal delivery: from bench to clinical implementation. Current clinical pharmacology. 2016 Aug 1;11(3):168-90.
- Nandure HP, Puranik P, Giram P, Lone V. Ethosome: A Novel Drug Carrier. International journal of pharmaceutical research & allied sciences. 2013 Jul 1;2(3).
- Waqar MA, Zaman M, Hameed H, Munir M. Ethosomes: A novel approach for the delivery of drug. International Journal of Pharmacy & Integrated Health Sciences. 2023 Jul 31;4(2):31-46.
- Udapurkar P P, Kamble S R, Biyani K R. Ethosomes- Novel vesicular carriers for enhancing transdermal drug delivery. International journal of pharmaceutical and chemical science.2015 March;vol(4)1.
- Sujatha V, Vishnuvaravidyadhar T, Parvathi M, Srauryapkash Reddy A. A review on transdermal drug delivery system by ethosomes. Pharmatutor. 2014 Nov 1;2(2):50-5.
- Jaiswal PK, Kesharwani S, Kesharwani R, Patel DK. Ethosome: A new technology used as topical & transdermal delivery system. Journal of Drug Delivery and Therapeutics. 2016 May 15;6(3):7-17.
- Sheel S, Biswas P, Karmakar V, Khanam S. Ethosome as a potential transdermal drug delivery system. Journal of Pharmaceutical and Biological Sciences. 2022;10(2):72-8.
- Zahid SR, Upmanyu N, Dangi S, Ray SK, Jain P, Parkhe G. Ethosome: a novel vesicular carrier for transdermal drug delivery. Journal of drug delivery and therapeutics. 2018 Nov 15;8(6):318-26.
- Dibyalochan M, A.Monika,Bakhsi V, M.Akiful,Chinmaya K S.Ethosomes:A novel approach for transdermal drug delivery.International journal of chem tech research.2018,11(8):219-226.
- Hariharanb S, Justinc A. Topical delivery of drugs using ethosomes: A review. Indian drugs. 2019 Sep;56(08):7.
- Verma P, Pathak K. Therapeutic and cosmeceutical potential of ethosomes: An overview. Journal of advanced pharmaceutical technology & research. 2010 Jul;1(3):274.
- Rajendra M,Chandrashekhar K B,Srinivas A.Ethosome as novel drug delivery carriers-A Review.IAJPBB.2016;3(12);1639-1643.
- Shelke S, Shahi S, Kale S, Patil V, Deshpande D. Ethosomes: a novel deformable carrier. World journal of pharmaceutical sciences. 2015 Sep 17:1830-9.
- Ojha M,Chaudhary V,Tiwari P.Ethosomes-A modernistic approach fot drug delivery.International journal of pharmaceutical research and applications.2023 june,vol 8(3),2566-2577
- Gupta R,Bhardwaj A,Gupta S,Jain Alok P.Formulation and evaluation of transdermal patch from lamivudine and stavudine-loaded ethosomes.JETIR June 2019,vol6(6).
- Ma H, Guo D, Fan Y, Wang J, Cheng J, Zhang X. Paeonol-loaded ethosomes as transdermal delivery carriers: design, preparation and evaluation. Molecules. 2018 Jul 17;23(7):1756.
- Sultana SS, Sailaja AK. Preparation and evaluation of naproxen sodium loaded liposomes, ethosomes and transferosomes. Journal of Bionanoscience. 2017 Aug 1;11(4):284-91.
- Mistry A, Ravikumar P. Development and evaluation of azelaic acid based ethosomes for topical delivery for the treatment of acne. Indian J. Pharm. Educ. Res. 2016 Jul 1;50(3s):S232-43.
- Yu Z, Lv H, Han G, Ma K. Ethosomes loaded with cryptotanshinone for acne treatment through topical gel formulation. PLoS One. 2016 Jul 21;11(7):e0159967.
- Malla B, Malla K, Sah AK, Koirala A, Karki S, Prajuli DR, Thapa B. Preparation and Characterization of Liposomes and Ethosomes Bearing Indomethacin for Topical Drug Delivery. International Journal of Medicine and Biomedical Sciences. 2016 Sep 30;1(3):6-11.
- Lakshmi PK. Statistically optimised ethosomes for transdermal delivery of tolterodine tartrate. Pakistan journal of pharmaceutical sciences. 2013 Nov 1;26(6).
- David SR, Hui MS, Pin CF, Ci FY, Rajabalaya R. Formulation and in vitro evaluation of ethosomes as vesicular carrier for enhanced topical delivery of isotretinoin. International Journal of drug delivery. 2013 Jan 1;5(1):28.
- Barupal AK, Gupta V, Ramteke S. Preparation and characterization of ethosomes for topical delivery of aceclofenac. Indian journal of pharmaceutical sciences. 2010 Sep;72(5):582.
- Vijayakumar R. Formulation and evaluation of diclofenac potassium ethosomes (Doctoral dissertation, Madurai Medical College, Madurai).
- Jain S, Tiwary AK, Sapra B, Jain NK. Formulation and evaluation of ethosomes for transdermal delivery of lamivudine. Aaps Pharmscitech. 2007 Oct;8:249-57.
- Sujatha S, Sowmya G, Chaitanya M, Reddy VK, Monica M, Kumar KK, Sujatha S. Preparation, characterization and evaluation of finasteride ethosomes. Int J Drug Delivery. 2016;8:01-11.
- D Patel,P Bhargava.Ethosomes-A phytodrug delivery system.Advance research in pharmaceutical and biologics.2012,vol2(1).
- Pratima NA, Shailee T. Ethosomes: A novel tool for transdermal drug delivery. International Journal of Research in Pharmacy & Science. 2012 Jan 1;2(1).
- Nandure HP, Puranik P, Giram P, Lone V. Ethosome: A Novel Drug Carrier. International journal of pharmaceutical research & allied sciences. 2013 Jul 1;2(3).


 Ravikiran Kadolkar*
Ravikiran Kadolkar*
 Snehal Ogale
Snehal Ogale
 Dr. Nagesh C
Dr. Nagesh C





 10.5281/zenodo.14762118
10.5281/zenodo.14762118