Abstract
Ailanthus altissima, also known as the "Tree of Heaven," is native to regions such as China, North Vietnam, and Kashmir, and belongs to the Simaroubaceae family and Ailanthus genus. This review explores the therapeutic potential of Ailanthus altissima, focusing on its bioactive compounds and their pharmacological effects. The plant contains a range of active constituents, including proteins, flavonoids, alkaloids, quassinoids, terpenylated coumarins, triterpenoids, fatty acids, and volatile oils. These compounds contribute to various pharmacological actions, such as antibacterial, antiviral, antioxidant, cytotoxic, anti-inflammatory, antipyretic, analgesic, antihistaminic, antiparasitic, insecticidal, and anti-progestogenic effects. Moreover, Ailanthus altissima has demonstrated potential for treating ailments like diarrhea and inflammation. The plant's rich variety of bioactive compounds indicates significant potential for further therapeutic applications, supporting the need for ongoing scientific investigation.
Keywords
Tree of heaven, Alianthone, Anti-inflammatory, Papermaking, Antimicrobial.
Introduction
The plant Ailanthus altissima, also referred to as the "tree of heaven," "smoke tree," "ghetto palm," "Ailanthus, varnish tree, copal tree, stinking sumac, Chinese sumac, paradise tree," or chouchun in Chinese, is a member of the genus Ailanthus and family Simarubaceae, which includes up to 15 species. In Kashmir, this is referred to locally as "Alamthar" or "Handoon." China, North Vietnam1, and the Indian portion of Kashmir are the native habitats of Ailanthus altissima.[1] Adults can grow up to 27 meters tall and have breasts that are 1 meter in circumference. [2] The bark of Ailanthus altissima is smooth and light grey, while the twigs are stout, smooth to lightly pubescent, and reddish or chestnut in color. The twigs feature lenticels and heart-shaped leaf scars with multiple bundle scars along the edges. The finely pubescent, dome-shaped buds are partially concealed by the petiole. The branches vary from light to dark grey, are smooth and lustrous, and have raised lenticels that develop into fissures with age. The branch tips often become pendulous. All parts of the plant emit a distinctive strong odor, often compared to peanuts, cashews, or rotting cashews. The leaves are large, pinnately compound (either odd- or even-pinnate), and alternately arranged on the stem. They measure 30 to 90 cm in length and consist of 10–41 leaflets arranged in pairs, with the largest leaves typically found on vigorous young sprouts. The leaflets are ovate-lanceolate with entire margins, slightly asymmetric, and sometimes not directly opposite. Each leaflet ranges from 5 to 18 cm in length and 2.5 to 5 cm in width. [3] The flowers are small and form large panicles up to 50 cm long, located at the ends of new shoots. Individual flowers are yellowish-green to reddish, with five petals and sepals. Flowering occurs from mid-April in southern regions to July in northern areas. Ailanthus altissima is a dioecious species, with male and female flowers produced on separate trees. [4]

Figure 1: Ailanthus altissima plant
In traditional Chinese medicine, the roots, leaves, and bark are used mostly as an astringent. As a host plant for the ailanthus silkmoth, a moth involved in the manufacturing of silk, the tree has been widely cultivated in China and outside. Among the numerous active substances found in Ailanthus altissima were proteins, flavonoids, alkaloids, quassinoids, terpenylated coumarins, tetracyclic triterpenoids, fatty acids, and volatile oils. Among its numerous additional pharmacological actions were antibacterial, antiviral, antioxidant, cytotoxic, antidiarrheal, anti-inflammatory, antipyretic, analgesic, antihistaminic, antiparasitic, insect repellent, and anti-progestogenic.This plant's many parts are used as medicines to treat a range of illnesses. [5,6]
Taxonomical classification:
Table 1: Taxonomical Classification of Ailanthus altissima
|
Sr. No
|
Taxonomy
|
Overview
|
|
1
|
Kingdom
|
Plantae
|
|
2
|
Phylum
|
Spermatophyta
|
|
3
|
Subphylum
|
Angiospermae
|
|
4
|
Class
|
Dicotyledonae
|
|
5
|
Order
|
Rutales
|
|
6
|
Family
|
Simaroubaceae
|
|
7
|
Genus
|
Ailanthus
|
|
8
|
Species
|
Ailanthus altissima
|
|
9
|
Botanical name
|
Ailanthus altissima
(Mill.) Swingle
|
Vernacular name:
Arabic: shajarat el- sama
Chinese: baichun, chouchun, chunshu
English: tree of heaven, ailanthus, Chinese sumac, stinking shumac
Germany: Götterbaum
French: ailante, ailanteglanduleux, arbre des dieux, arbre du ciel, faux vernis du Japon;
Italy: ailanto, albero del paradiso;
Russian: ajlantvyso?ajšij
South Africa: hemelboom
Sweden: gudatraed, himmelstraed.
Synonyms:
- Ailanthus cacodendron (Ehrh) Schinz and thell
- Ailanthus erythrocarpa Carriere
- Ailanthus giraldii Dode
- Ailanthus glandulosa Desf
- Ailanthus guangxiensis S L Mo
- Ailanthus japonica K Koch
- Ailanthus japonica Dippel
- Ailanthus peregrine (Buc’hoz) F A Barkley
- Ailanthus procera salisb
- Ailanthus pongelion J F Gmel
- Ailanthus rhodoptera F Muell
- Ailanthus sinensis Dum Cours. Nom. Illeg.
- Ailanthus sutchuensis Dode
- Ailanthus vilmoriniana Dode
- Albonia peregrine Buc’hoz
- Choerospondias auriculata D.Chandra
- Rhus cacodendron Ehrh
- Toxicodendron altissimum Mill.
Traditional use:
Ailanthus altissima was used in traditional medicine for treatment of dysentery, gonorrhea,
hemorrhoids and a remedy for cough, gastric and intestinal upsets.
History:
The history of the tree of heaven (Ailanthus altissima) is deeply rooted in Chinese culture, where it has long been recognized for its medicinal and practical uses. It has been mentioned in numerous ancient Chinese medical texts and even appears in the oldest surviving Chinese lexicon. In traditional Chinese medicine, the bark, leaves, and roots are primarily used for their astringent properties. The tree has also been widely cultivated as a host plant for the ailanthus silkmoth, which is significant in silk production. Beyond its native China, the tree has become a cultural symbol in the West, famously serving as a central metaphor in Betty Smith’s American novel A Tree Grows in Brooklyn.
The tree was introduced to Europe in the 1740s by Pierre Nicholas d’Incarville, a French Jesuit. D’Incarville sent seeds from Peking (Beijing) across Siberia to his friend Bernard de Jussieu, a botanist. However, a misunderstanding arose as the seeds were initially thought to belong to the economically important Chinese varnish tree (Toxicodendron vernicifluum), which d’Incarville had encountered in the lower Yangtze region. His accompanying note highlighted this resemblance, leading to significant taxonomic confusion in subsequent years. In 1751, Jussieu shared seeds he had cultivated in France with Philip Miller, the curator of the Chelsea Physic Garden, and Philip C. Webb, an exotic plant collector in Busbridge, England. The naming of the plant became contentious, with multiple taxonomists proposing different names. Linnaeus initially named it Rhus succedanea in Paris, while Miller referred to it as Toxicodendron altissima in London. Disputes between Miller and John Ellis during the 1750s further complicated the nomenclature. Ehrhart later proposed the name Rhus cacodendron in 1782. In 1788, Desfontaines clarified the taxonomy, naming the tree Ailanthus glandulosa and recognizing it as distinct from sumacs. The name “Ailanthus” translates to “heaven-tree,” reflecting its lofty stature, but the term was invalidated due to homonymy. Eventually, Walter T. Swingle rectified the issue by renaming the species Ailanthus altissima, meaning “tallest,” a name now universally accepted.
Morophology of Ailanthus altissima:
Table 2: Morophology of Ailianthus altissima
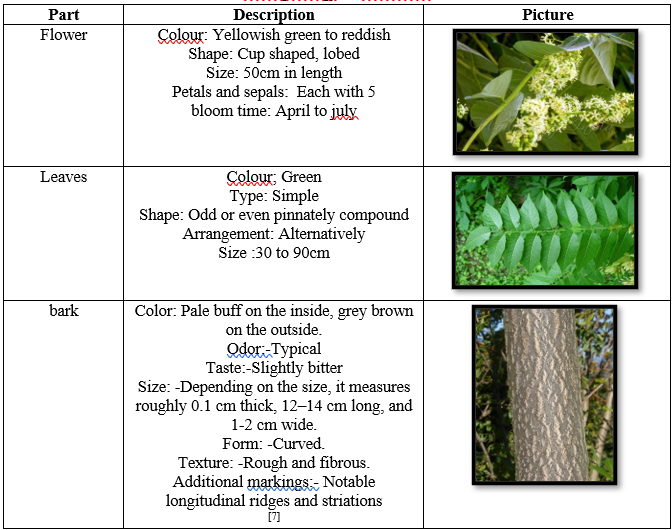
Microscopy of Ailanthus altissima:
Bark structure: Outer bark (periderm) with phellem (7-8 layers of tangentially compressed cells, rectangular, thickened cell walls, suberized; multicellular uniseriate lignified covering trichomes present even in transition), phellogen (3-5 layers of dead, cellulosic-walled, rectangular cells), stone cells (smaller in size compared to those in phelloderm, lignified, located below phellogen), phelloderm (multiple layers of polygonal parenchymatous cells; large sclereids in groups of 8-20 scattered throughout), pericyclicfibers (3-4 layers of lignified fibers at the phelloderm's end, above secondary phloem, between outer bark and inner bark). [8]

Figure2: Microscopy of Bark of Ailanthus altissima
Leaves Structure:
The microscopic characteristics of the leaves show that they have xylem and phloem vessels, covering trichomes, glandular trichomes, spongy mesophyll, palisade cells, and epidermal cells. Powder microscopy of leaves shows the presence of covering trichomes, calcium oxalate crystals , palisade cells, paracytic stomata, and epidermal cells. [7]
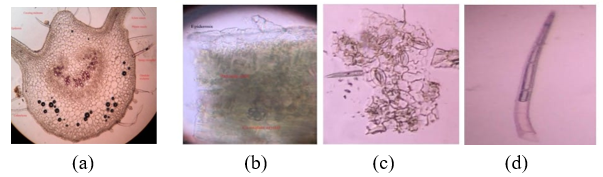
Figure3: (a): Transverse section, (b): Epidermis with palisade cells and Calcium oxalate crystals (c): Paracytic stomata (d): Covering trichomes
Flowers structure:
Several spherical pollen grains, approximately 30 µm in diameter, featuring three pores and a pitted exine; numerous single-celled linear covering trichomes, both short and long (up to 350 ?m in length), isolated and connected to the flower fragments; oxalate druses—separated from and/or contained inside perianthium pieces; pieces of the funiculus with long covering trichomes in the lower 1/3 to 1/2 part; pieces of the flower stalk with glandular trichomes with a multicellular head and a short two-celled stalk; pieces of the sepals with a hairy epidermis; pieces of the petals with a papillose epidermis; pieces of the anther with pollen grains. [9]

Figure 4: Microscopy of Flower of Ailanthus altissima
Geographical Distrubution:
The tree of heaven originated in central Asia, or China, and was brought to Europe around 260 years ago by Pierre d'Incaville, a French missionary, who sent seedlings from Nanking to Paris.The species has since spread to every continent with the exception of Antarctica, where it has established itself over a sizable portion of Europe. It is widespread throughout the Mediterranean region but is restricted by the cold weather in the north. Its growth has been aided by the global seed exchange that has occurred over the past 200 years as well as its capacity to flourish in deprived areas, cities, and fragmented landscapes.
Ailanthus altissima, or the tree of heaven, is originally native to China but has since spread to various parts of the world. Its geographical distribution includes:
1. Asia: Widely distributed throughout China, Taiwan, Korea, and Japan.
2. North America: Introduced in the 18th century, it has become established across the eastern United States and parts of Canada.
3. Europe: Common in southern and central Europe, particularly in urban areas and disturbed sites.
4. Africa: Found in several regions, primarily in urban environments.
5. Australia: Introduced and found mainly along the eastern coast. [10,11]
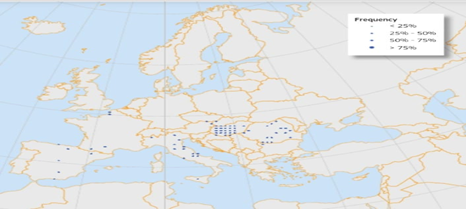
Figure5: World Map of Ailanthus altissima. The Blue Surface Symbol Map Shows The Distribution Area of Ailanthus altissima
Cultivation And Collection: -
Cultivation: -
1.Site Selection:
- Thrives in urban areas, disturbed soils, roadsides, and open fields.
- Tolerant of a wide range of soil types, including poor, rocky, and compacted soils.
- Prefers full sun but can also tolerate partial shade.
2. Propagation:
- Seeds: Ailanthus altissima produces large amounts of seeds (up to 325,000 seeds per tree annually), dispersed by wind.
- Root Suckers: The tree spreads aggressively through root suckers, allowing it to colonize large areas quickly.
3. Growth Rate:
- Extremely fast-growing, capable of reaching heights of 10-15 meters (30-50 feet) in just a few years.
- Can live up to 50 years under favorable conditions.
4. Pests and Diseases:
- Relatively free from pests and diseases in most regions outside its native range.
- Known to exude chemicals that inhibit the growth of other plants (allelopathy).
Management and Control:
1. Mechanical Control:
- Cutting: Effective only when paired with herbicide treatments, as cutting alone can stimulate root suckering.
- Girdling: Removing a strip of bark around the tree to kill it, followed by herbicide treatment, can reduce root suckering.
2. Chemical Control:
- Foliar Spraying: Herbicides like glyphosate and triclopyr are often used, but multiple applications may be necessary.
- Basal Bark Treatment: Applying herbicides directly to the bark during the tree's dormant period can be effective.
3. Biological Control:
- Research is ongoing into biological control methods, including the use of fungi or insects that target Ailanthus.
4. Prevention:
- Regular monitoring of disturbed sites and early removal of seedlings can help prevent large infestations.
Collection:
- Seed Collection
- Timing: Seeds are produced in late summer to early fall and can be collected once they mature, typically when they turn from green to a reddish-brown.
- Method: -Collect seeds directly from the tree using hand pruners or pole pruners. Seeds are held in clusters, making them easy to access.
- Drying and Storage: -Seeds should be thoroughly dried before storage to prevent mold. Store them in a cool, dry place, preferably in sealed containers. Under proper storage conditions, the seeds can remain viable for several years.
- Viability: Ailanthus altissima seeds have a high germination rate. For conservation or botanical studies, seeds can be germinated in controlled conditions or stored for long-term use in seed banks
2. Herbarium Specimen Collection
- Purpose: Herbarium collections are important for documenting plant distribution, morphology, and taxonomy.
- Parts Collected: Herbarium specimens typically include parts of the stem, leaves, flowers, and fruits (seeds) pressed and mounted on herbarium sheets.
- Collection Methods:
- Use pruning shears to cut small branches with leaves and fruit attached.
- Press and dry the plant material between layers of blotting paper or newspaper in a plant press.
Documentation: Each herbarium specimen should be documented with collection date, location (including GPS coordinates), habitat description, and any associated species. [12,13]

Figure 6: Collection of Ailthanus Altissima
Chemical constituents:
Ailanthus altissima contained proteins, flavonoids, alkaloids, quassinoids, terpenylated coumarins, tetracyclic triterpenoids, fatty acids, volatile oils and many other active compounds.
Chemical constituents in the Seeds:
Approximately 27.5–27.6g of protein and 55.5–59.1g of fat/100g were included in the seed, along with 6?-tigloyloxy chaparrinone, ailanthone, and quassiin. Ailantinols A-G, shinjulactone A-O, and altissinol A-H were among the several quassinoids found in the plant's various sections . The ethanolic extract of the root bark of Ailanthus altissima yielded three neolignan glycosides:(7,9,9’-trihydroxy-3,3’,5’-trimethoxy-8-O-4’-neolignan-4-O-?-D-glucopyranoside, sonchifolignan B and citrusin B).From Ailanthus altissima's stem bark, many terpenylated coumarins were identified, including (2'R,3'R)-7-(2',3'-dihydroxy-3',7'-dimethylocta-6'-nyloxy)6,8-dimethoxycoumarin,6,8-dimethoxy-7-(3’,7’-dimethylocta-2’,6’-dienuloxy)coumarin, (2’R,3’R,6’R)-7-(2’,3’-dihydroxy-6’,7’-epoxy-3’,7’-dimethylocataoxy)-6-8-dimethoxy coumarin, and (2’R,3’R,4’S,5’S)-6-8-dimethoxy-7-(3’,7’-dimethyl-4’,5’-epoxy-2’-hydroxyocta-6’-enyloxy)coumarin. [14,15,16]
Chemical constituents in the Leaves:
The alkaloid linuthine, 12% tannin, and isoquercetin and quercetin are present in the leaves The leaves' methanolic extracts had the highest level of total phenolic content, and the hydrodistilled residues' extracts had the highest level of total flavonoid content. By using HPLC-DAD–MS, the most common phenolic chemicals found were quercetin-3-galloyl hexoside, epicatechin, gallic acid, chlorogenic acid, HHDP-galloylglucose, and rutin . The ethanolic extract fraction had the largest level of total phenolic compounds (12.25%) when compared to the other extracts or fractions. Low and colleagues, on the other hand, extracted eight different chemicals from the plant's blossoms. These compounds included rutin, diethyl-2,2',3,3',4,4'-hexahydroxybiphenyl-6,6'-dicarboxylate, methyl brevifolin carboxylate, ellagic acid, and gallic and ethyl gallate. [17,18,19]
Chemical constituents in the Bark:
The bark of Ailanthus altissima was used to identify tetracyclic triterpenoids (altissimanins A–E) and a terpenylated coumarin (altissima coumarin G). [20]
Chemical constituents in the Fruit:
The fruits of Ailanthus altissima Swingle were also used to isolate cerebroside and three cycloartan triterpenes.Their structures were identified as 1-O-beta-Dglucopyranosyl-(2S,3R,4E,9E)-2-(2’Rhydroxyhexadecenoy)-4,9-octadecadiene-1,3-diol;9,19 -cyclolanost-23(Z)-ene-3beta, 25-diol; cycloart-25-ene3beta, 24R-diol; and cycloart-25-ene-3beta,24S-diol [21]
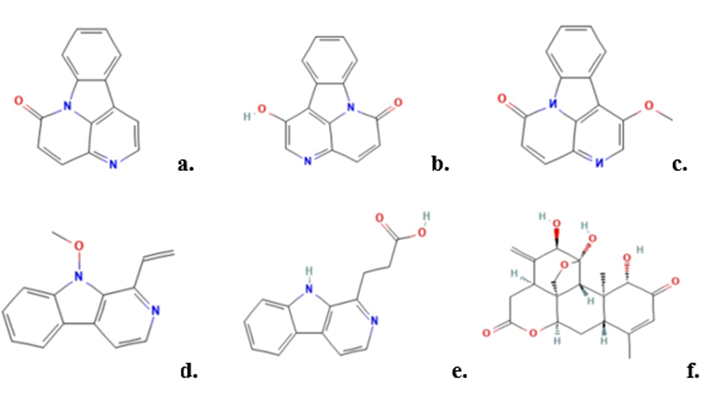
Figure7:chemical consistuents of Ailanthus altissima: (a) canthin-6-one; (b) 1-Hydroxycanthin-6-one; (c) 1-Methoxycanthin-6-one; (d) 4-Methoxy -1-vinyl ?-carboline; (e) ?-carboline-1-propionic acid; (f) Alianthone
Extraction method:
Methanolextraction:
1 kg dried and powdered plant material extracted with 70% methanol using a Soxhlet apparatus. The extract was concentrated under reduced pressure to yield a residue. The residue was dissolved in 500 ml distilled water and fractionated with petroleum ether, chloroform, ethyl acetate, and n-butanol (60–80°C). Each fraction was dried with anhydrous sodium sulfate and concentrated under reduced pressure to obtain 29 g petroleum ether, 14 g chloroform, 10.5 g ethyl acetate, and 32 g n-butanol fractions. [22]
Water extraction:
The Ailanthus tree's 1) leaf and 2) bark (about 10–20 cm above the tree's lower portion); 3) young stems; 4) roots; and 5) fruit were the limbs that were used. The limbs were divided into smaller pieces and let to dry for three days at 50°C in a separate container.Ten grams of the dried limbs were soaked in 1000 milliliters of distilled water in individual containers for three, six, nine, twelve, and fifteen days at room temperature in order to determine the ideal duration for soaking the tissues in water. [23]
Ethanol extraction:
They were then dried and ground into a powder, and the maceration method of extraction was performed for eight hours at 30° C using a solvents, including ethanol. A Whatman no. 1 filter paper was used to filter the extracted material in order to eliminate any undesirable or intractable substances.
Phytochemical analysis: [8,24,25]
Table 3: Phytochemical Analysis of Ailanthus altissima
|
Sr.no
|
Test
|
Ethanol
|
Ethyl acetate
|
chloroform
|
Hexane
|
Aqueous
|
|
1
|
Alkaloids
Drangondroff test
Mayer test
Wagner test
|
--
--
--
|
++
++
++
|
--
--
--
|
++
++
++
|
++
++
++
|
|
2
|
Carbohydrate
|
-
|
-
|
-
|
-
|
-
|
|
3
|
Saponins
|
-
|
++
|
++
|
-
|
++
|
|
4
|
Phenol
|
++
|
++
|
-
|
-
|
++
|
|
5
|
Terpenoids
|
++
|
--
|
++
|
--
|
--
|
|
6
|
Steroids
|
--
|
++
|
--
|
++
|
--
|
|
7
|
flavonoid
|
++
|
++
|
--
|
--
|
--
|
|
8
|
tannin
|
--
|
--
|
--
|
--
|
--
|
Physiochemical analysis:- [8,26]
Table 4: Physiochemical Properties of Ailanthus altissima
Ash value
|
Types of ash value
|
%W/W
|
|
Total ash
|
12.47+ 0.64
|
|
Acid insoluble ash
|
1.19+0.56
|
|
Water soluble ash
|
3.14 + 0.51
|
|
Sulfated ash
|
5.14+0.82
|
Extractive value
|
Types of extractive value
|
%W/W
|
|
Petroleum ether
|
2.12+0.12
|
|
Alcohol
|
6.57+0.94
|
|
Water
|
3.35+0.26
|
|
Foreign oraganic matter
|
1.59+0.33
|
|
Loss on drying
|
1.35+0.48
|
Pharmaceutical Uses:
Antimicrobial effect
As per the study conducted by Rahman et al., [27] The agar disk diffusion method can be used to assess the antibacterial efficacy against 11 different foodborne bacteria, six of which are gram positive and five of which are gram negative. The growth of six gram-positive bacteria (Listeria monocytogenes ATCC 19116, ATCC 19118, and ATCC 19166), two gram-negative bacteria (Pseudomonas aeruginosa KCTC 2004 and Escherichia coli ATCC 8739), and Staphylococcus aureus (ATCC 6538 and KCTC 1916) were all significantly inhibited by the methanol extract and its various polar subfractions. The 12.1–23.2 mm range was determined to contain the zones of inhibition of methanol extract and its various polar subfractions against the tested microorganisms. [28]
Methanolic extracts from the leaves of A. altissima were found to be more effective against Gram-positive bacteria, such as B. subtilis, S. aureus, and L. monocytogenes. Nevertheless, these extracts were ineffective against Gram-negative bacteria such S. enteritidis, E. coli, and S. typhimurium. Gallic acid, rutin, hyperoside, epicatechin, luteolin, and derivatives of apigenin are among the chemicals found in the leaf extracts that may be responsible for this plant's antibacterial activity. bactericidal properties of A. altissima's methanolic leaf extract, which is frequently utilized to effectively target secondary metabolites and phenolic substances. [29]
Antiviral effect
Rice stripe disease, caused by the rice stripe virus (RSV), is one of the most destructive plant viral diseases affecting rice, leading to significant production losses. RSV infects various Poaceae species under natural conditions. Ailanthone, a C20 quassinoid synthesized through the secondary metabolism of Ailanthus altissima, is a biologically active natural compound with promising potential as a lead structure for pesticide development. The antiviral properties of Ailanthone were explored using Nicotiana benthamiana plants and rice protoplasts, which allowed for systemic RSV infection and replication. The effects of Ailanthone on RSV-related gene expression were analyzed using reverse transcription polymerase chain reaction (RT-PCR) and Western blot techniques. Results demonstrated that Ailanthone exhibits a dose-dependent inhibitory effect on RSV NSvc3 expression in both virus-infected tobacco plants and rice protoplasts. Further investigation revealed that Ailanthone strongly suppresses the expression of seven RSV protein-encoding genes, with the most significant impact observed on NS3, NSvc3, NS4, and NSvc4. These findings enhance our understanding of the antiviral properties of Ailanthone and its potential as a natural agent for combating plant viruses. [30] The antitumor-promoting properties of ailantinol E, ailantinol F, ailantinol G, and related compounds isolated from Taiwan-grown Ailanthus altissima were assessed against Epstein-Barr virus early antigen activation induced by 12-O-tetradecanoylphorbol-13-acetate in Raji cells.
Anti plasmodium effect
Quassinoids interfere with the parasite's protein synthesis, hindering the production of essential proteins. They also impair mitochondrial function, disrupting energy production within the parasite. Additionally, quassinoids compromise the parasite's ability to metabolize hemoglobin, a critical process for its survival. Meanwhile, alkaloids bind to the parasite's DNA, disrupting replication and transcription. These compounds also induce oxidative stress, causing significant damage and ultimately leading to the parasite's death. [31,32,33,34]
Antidiarroheal effect
Flavonoids and Alkaloids reduce smooth muscle contractions by modulating the release of neurotransmitters such as acetylcholine, which is involved in increasing gut motility. Help relax the intestinal muscles, slowing the passage of stool and alleviating diarrhea. Tannins and Saponins exhibit antimicrobial effects against pathogens commonly associated with diarrhea, such as Escherichia coli and Salmonella species. Tannins can precipitate proteins on the intestinal mucosa, forming a protective layer that reduces irritation and microbial adherence. [35]
Anti inflammatory effect
Quassinoids and flavonoids inhibit the production of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-?), interleukin-1? (IL-1?), and interleukin-6 (IL-6). They also suppress the expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), thereby reducing the synthesis of prostaglandins and nitric oxide (NO), which are key mediators of inflammation. Polyphenols and alkaloids block the activation of nuclear factor kappa B (NF-?B), a transcription factor that regulates inflammatory gene expression. By preventing NF-?B translocation to the nucleus, they effectively halt the inflammatory cascade. Flavonoids and tannins neutralize reactive oxygen species (ROS), alleviating oxidative stress closely associated with inflammation. This action protects cellular components from oxidative damage, promoting the resolution of inflammation. [36]
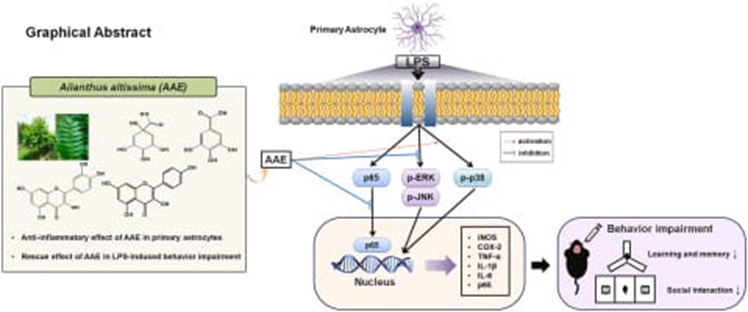
Figure 8:-Anti-inflammatory effect of Ailanthus altissima
Analgesic effect
Quassinoids and Alkaloids act on central and peripheral nervous system pathways to reduce pain signals. Inhibit neurotransmitter release, such as substance P and glutamate, which are involved in nociception (pain perception).Interact with opioid receptors, mimicking the effects of endogenous opioids to reduce pain sensation. [37]
Antihistaminic effect
Flavonoids and Polyphenols stabilize mast cells, preventing degranulation and the release of histamine and other allergic mediators. Reduce calcium influx into mast cells, a critical step in the release of histamine. Alkaloids act as competitive antagonists at histamine H1 receptors, blocking histamine binding and reducing allergic symptoms such as itching, swelling, and redness.Inhibit H1 receptor-mediated contraction of smooth muscles in the airways and gastrointestinal tract. [38]
Antioxidant effect
Flavonoids and Polyphenols act as free radical scavengers, directly neutralizing ROS, such as superoxide anion (O??), hydrogen peroxide (H?O?), and hydroxyl radicals (OH•), which contribute to oxidative stress and cellular damage. Flavonoids like quercetin and kaempferol are known for their potent antioxidant activities, reducing oxidative damage at the cellular level. Polyphenols and Alkaloids of Ailanthus altissima promotes the activity of endogenous antioxidant enzymes such as superoxide dismutase (SOD), catalase, and glutathione peroxidase. These enzymes help neutralize ROS and reduce oxidative damage. The plant extract helps increase the levels of reduced glutathione (GSH), a key antioxidant that supports cellular detoxification processes. Quassinoids and Alkaloids help modulate signaling pathways involved in the production of ROS, such as NF-?B (nuclear factor-kappa B) and MAPK (mitogen-activated protein kinase) pathways, effectively reducing the production of inflammatory mediators and oxidative stress. [39]
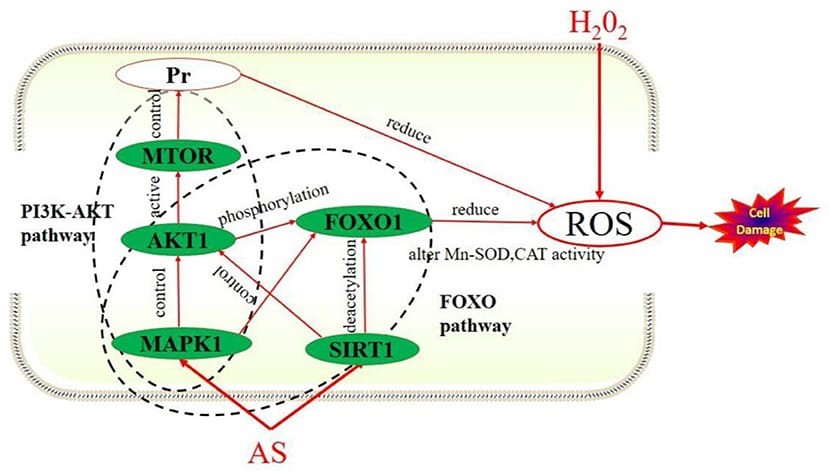
Figure 9:-Anti-oxidant effect of Ailanthus altissima
Anti –progestogenic effect
It was determined whether Ailanthus altissima had progestogenic or anti-progestogenic qualities. Progesterone response element-driven luciferase reporter gene bioassay was used to analyze plant extracts. It was discovered that Ailanthus altissima possesses properties similar to those of an anti-progestogen. [40]
Anticancer effect
Certain compounds in Ailanthus altissima may induce programmed cell death (apoptosis) in cancer cells. For example, a study highlighted the potential of a compound called "ailanthone" to activate apoptosis in tumor cells.Ailanthus altissima contains antioxidant compounds, which can help reduce oxidative stress—a key factor in cancer progression. By neutralizing free radicals, these compounds may help protect cells from DNA damage that could lead to cancer.Plant extracts from Ailanthus altissima may inhibit the proliferation of cancer cells. The inhibition may occur through interference with cell cycle progression, affecting the growth and division of cancer cells.Chronic inflammation is a major contributing factor to cancer development. Ailanthus altissima may have anti-inflammatory properties that could play a role in preventing or slowing down cancer progression by modulating inflammatory pathways.Some studies suggest that Ailanthus altissima can modulate the expression of genes involved in cell survival, apoptosis, and metastasis. By influencing these pathways, Ailanthus altissima extracts could reduce the likelihood of cancer cell spread. [41]
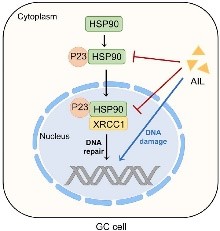
Figure 10:-Anti-cancer effect of Ailanthus altissima
An alternative fiber source for papermaking
Ailanthus altissima is a fast-growing, invasive tree species found in various parts of the world. Its rapid growth enables quick harvesting, offering a renewable source of fiber. Given its invasive nature in many regions, utilizing Ailanthus altissima for papermaking could help control its spread while supporting sustainable fiber sourcing.
The wood of Ailanthus altissima, particularly its bark and branches, contains fibrous material that can be processed into paper pulp. The fiber composition of the tree is similar to that of other hardwood species, providing a balance of strength and softness in the final paper. Paper produced from Ailanthus altissima fibers exhibits good durability and strength, comparable to papers made from other hardwoods. The quality of the paper can be tailored for different uses, such as newsprint, packaging, or writing paper. Additionally, Ailanthus altissima paper may have desirable attributes like smoothness, strength, and a relatively high level of whiteness. [42]
Toxic Effect:
1. Human Health Effects:
a. Dermatitis: Contact with the sap of Ailanthus altissima can cause allergic contact dermatitis in sensitive individuals. The plant’s leaves and bark contain compounds that can lead to skin irritation and rashes.
b. Respiratory Irritation: The pollen of Ailanthus altissima is known to cause allergic reactions in people, including sneezing, coughing, and other respiratory symptoms. Some sensitive individuals may develop allergic rhinitis or asthmatic reactions after inhaling its pollen.
c. Cardiotoxicity: Prolonged exposure to Ailanthus altissima has been linked to cases of myocarditis and coronary vasospasm in humans, leading to symptoms such as chest pain, shortness of breath, and palpitations.
2. Ecological and Environmental Effects
a. Invasive Nature: Ailanthus altissima is highly invasive, often outcompeting native plant species due to its fast growth and ability to release allelochemicals. These chemicals inhibit the growth of other plants, reducing biodiversity and altering ecosystems.
b. Soil Degradation: The allelochemicals released by Ailanthus altissima, particularly ailanthone, disrupt the nutrient balance in the soil, making it harder for native plants to re-establish even after the removal of Ailanthus.
3. Toxicity in Animals
a. Gastrointestinal Issues: There are documented cases where ingestion of Ailanthus altissima leaves, bark, or seeds by livestock (horses and cattle) resulted in gastrointestinal irritation. Symptoms include vomiting, diarrhea, and in severe cases, death. [43,44]
CONCLUSION:
In conclusion, Ailanthus altissima is a valuable source of bioactive compounds, including flavonoids, alkaloids, quassinoids, and terpenoids, which contribute to its wide range of pharmacological effects. The plant’s antibacterial, antiviral, antioxidant, anti-inflammatory, analgesic, and other therapeutic properties highlight its potential for various health-related applications. While its preclinical evidence is promising, further research, especially clinical trials, is needed to fully confirm its therapeutic effectiveness, understand its mechanisms of action, and ensure its safety for broader use. Given its diverse bioactive properties, Ailanthus altissima shows considerable promise for the development of new natural health products and therapeutic agents, making it a promising candidate for further exploration in the fields of medicine and pharmacology.
REFERENCES
- Kowarik I, Säumel I. Biological flora of central Europe: Ailanthus altissima (Mill.) swingle. Perspectives in Plant Ecology, Evolution and Systematics. 2007 Jul 27;8(4):207-37.
- Miller J. Ailanthus altissima (Mill.) swingle. ailanthus. Silvics of north america. 1949;2:101-4.
- Bashir A, Farooq S, Bhat ZA. Pharmacognostic standardization and phytochemical evaluation of Ailanthus altissima (Mill.) Swingle leaves. Journal of Drug Delivery and Therapeutics. 2019 Apr 15;9(2-s):179-83.
- Miller JH. Nonnative invasive plants of southern forests: a field guide for identification and control. US Department of Agriculture, Forest Service, Southern Research Station; 2006.
- Al-Snafi AE. The pharmacological importance of Ailanthus altissima-A review. International Journal of Pharmacy Review and Research. 2015;5(2):121-9.
- Mulanda ES, Awori RM, Chuhila Y, Adero MO, Amugune NO, Akunda E, Kinyamario JI. A DNA-barcode for Melia volkensii Gürke (Meliaceae) and its phylogenetic relationship with some economically important relatives.
- Bashir A, Farooq S, Bhat ZA. Pharmacognostic standardization and phytochemical evaluation of Ailanthus altissima (Mill.) Swingle leaves. Journal of Drug Delivery and Therapeutics. 2019 Apr 15;9(2-s):179-83.
- Kalaskar MG, Sapkal PR, Tatiya AU, Jain PD, Surana SJ. Morphoanatomical and physicochemical studies on Ailanthus excelsa roxb. stem bark: a tree of heaven. Journal of Drug Delivery and Therapeutics. 2019 Jan 15;9(1):128-31.
- Andonova T, Muhovski Y, Slavov I, Vrancheva R, Georgiev V, Apostolova E, Naimov S, Mladenov R, Pavlov A, Dimitrova-Dyulgerova I. Phenolic profile, antioxidant and DNA-protective capacity, and microscopic characters of Ailanthus altissima aerial substances. Plants. 2023 Feb 17;12(4):920.
- Caudullo G, Tinner W, De Rigo D. Picea abies in Europe: distribution, habitat, usage and threats.
- Delivering Alien Invasive Species. Handbook of alien species in Europe. Springer Science & Business Media; 2008 Nov 14.
- Heisey RM. Identification of an allelopathic compound from Ailanthus altissima (Simaroubaceae) and characterization of its herbicidal activity. American Journal of Botany. 1996 Feb;83(2):192-200.
- Murillo R, Elvira S, Munoz D, Williams T, Caballero P. Genetic and phenotypic variability in Spodoptera exigua nucleopolyhedrovirus isolates from greenhouse soils in southern Spain. Biological Control. 2006 Aug 1;38(2):157-65.
- Wang Y, Wang WJ, Su C, Zhang DM, Xu LP, He RR, Wang L, Zhang J, Zhang XQ, Ye WC. Cytotoxic quassinoids from Ailanthus altissima. Bioorganic & medicinal chemistry letters. 2013 Feb 1;23(3):654-7.
- Tamura S, Fukamiya N, Okano M, Koyama J, Koike K. A new quassinoid, ailantinol H, from Ailanthus altissima. Natural product research. 2006 Nov 1;20(13):1211-5.
- Dao TT, Tran TL, Kim J, Nguyen PH, Lee EH, Park J, Jang IS, Oh WK. Terpenylated coumarins as SIRT1 activators isolated from Ailanthus altissima. Journal of natural products. 2012 Jul 27;75(7):1332-8.
- Momin RK, Kadam VB. Determination of lipid and alkaloid content in some medicinal plants of genus Sesbania. Journal of Ecobiotechnology. 2011 May 3;3(3).
- List PH, Hörhammer L. Hagers Handbuch der Pharmazeutischen Praxis. (No Title). 1979.
- Lou KQ, Tang WZ, Wang XJ. Study on chemical constituents from flowers of Ailanthus altissima. Zhong yao cai= Zhongyaocai= Journal of Chinese Medicinal Materials. 2012 Oct 1;35(10):1605-7.
- Hong ZL, Xiong J, Wu SB, Zhu JJ, Hong JL, Zhao Y, Xia G, Hu JF. Tetracyclic triterpenoids and terpenylated coumarins from the bark of Ailanthus altissima (“Tree of Heaven”). Phytochemistry. 2013 Feb 1;86:159-67.
- Zhao CC, Shao JH, Wang N, Li X, Wang JH. A new cerebroside from fruits of Ailanthus altissima Swingle. Natural Product Research. 2006 Nov 1;20(13):1187-91.
- Bayçu G, Said A, Farag A, Rashed K. Protein patterns and chemical constituents of Ailanthus altissima (Miller) Swingle and Ailanthus excelsa Roxb. European Journal of Biology. 2008;67(1):81-8.
- Bagheri F, Cici SZ. Study on inhibitory effects of Ailanthus altissima on the growth of weeds and agricultural plants.
- Ali ZM, Yadav AK, Pazhamalai V, Romauld SI. PHYTOCHEMICAL AND FTIR ANALYSIS OF AILANTHUS TRIPHYSA LEAVES.
- Mani M, Rathore DS. Pharmacological Evaluation of Anti-Fertility Effect of Stem Bark of Ailanthus altissima in Wistar Albino Rats. Research Journal of Pharmacy and Technology. 2016;9(5):497-500.
- Pramila Ghumare, D. B. Jirekar, Mazahar Farooqui And S. D. Naikwade Physico-Chemical, Phytochemical Screening Andantimicrobialactivity Of Ailanthus Excelsa Leaves Int. J. Chem.
- Rahman A, Kim EL, Kang SC. Antibacterial and antioxidant properties of Ailanthus altissima Swingle leave extract to reduce foodborne pathogens and spoiling bacteria. Journal of food safety. 2009 Nov;29(4):499-510.
- Zhao CC, Shao JH, Li X, Xu J, Zhang P. Antimicrobial constituents from fruits of Ailanthus altissima SWINGLE. Archives of pharmacal research. 2005 Oct;28:1147-51.
- Boukhibar H, Laouani A, Touzout SN, Alenazy R, Alqasmi M, Bokhari Y, Saguem K, Ben-Attia M, El-Bok S, Merghni A. Chemical Composition of Ailanthus altissima (Mill.) Swingle Methanolic Leaf Extracts and Assessment of Their Antibacterial Activity through Oxidative Stress Induction. Antibiotics. 2023 Jul 29;12(8):1253.
- Tan Q, Zhu J, Ju Y, Chi X, Cao T, Zheng L, Chen Q. Antiviral Activity of Ailanthone from Ailanthus altissima on the Rice Stripe Virus. Viruses. 2023 Dec 31;16(1):73.
- Tamura S, Fukamiya N, Okano M, Koyama J, Koike K. A new quassinoid, ailantinol H, from Ailanthus altissima. Natural product research. 2006 Nov 1;20(13):1211-5.
- Chang YS, Woo ER. Korean medicinal plants inhibiting to Human Immunode?ciency Virus type 1 (HIV?1) fusion. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2003 Apr;17(4):426-9.
- Okunade AL, Bikoff RE, Casper SJ, Oksman A, Goldberg DE, Lewis WH. Antiplasmodial activity of extracts and quassinoids isolated from seedlings of Ailanthus altissima (Simaroubaceae). Phytotherapy Research. 2003 Jun;17(6):675-7.
- Bray DH, Boardman P, O'neill MJ, Chan KL, Phillipson JD, Warhurst DC, Suffness M. Plants as a source of antimalarial drugs 5. Activities of Ailanthus altissima stem constituents and of some related quassinoids. Phytotherapy Research. 1987 Mar;1(1):22-4.
- Mani M, Sachan N, Tandon A, Wahi AK. Anti-diarrhoeal activity of methanolic extract of root bark of Ailanthus altissima Swingle (Family: Simaroubaceae) on experimental animals. Int J Pharm Sci Res. 2010;1:197-202.
- Rashed K, Slowing K, Said A, Cueto M. Analgesic, antipyretic and antiulcer activities of Ailanthus altissima (Mill.) Swingle. Phytopharmacology. 2012;3(2):341-50.
- Kim SR, Park Y, Li M, Kim YK, Lee S, Son SY, Lee S, Lee JS, Lee CH, Park HH, Lee JY. Anti-inflammatory effect of Ailanthus altissima (Mill.) Swingle leaves in lipopolysaccharide-stimulated astrocytes. Journal of Ethnopharmacology. 2022 Mar 25;286:114258.
- Rahman A, Kim EL, Kang SC. Antibacterial and antioxidant properties of Ailanthus altissima Swingle leave extract to reduce foodborne pathogens and spoiling bacteria. Journal of food safety. 2009 Nov;29(4):499-510.
- Mo YN, Cheng F, Yang Z, Shang XF, Liang JP, Shang RF, Hao BC, Wang XH, Zhang HJ, Wali A, Lu CF. Antioxidant activity and the potential mechanism of the fruit from Ailanthus altissima swingle. Frontiers in Veterinary Science. 2021 Dec 13;8:784898.
- Ahmed HM, Yeh JY, Tang YC, Cheng WT, Ou BR. Molecular screening of Chinese medicinal plants for progestogenic and anti-progestogenic activity. Journal of biosciences. 2014 Jun;39:453-61.
- Wang CM, Li HF, Wang XK, Li WG, Su Q, Xiao X, Hao TF, Chen W, Zhang YW, Zhang HY, Wu W. Ailanthus altissima-derived ailanthone enhances gastric cancer cell apoptosis by inducing the repression of base excision repair by downregulating p23 expression. International Journal of Biological Sciences. 2021;17(11):2811.
- Baptista P, Costa AP, Simões R, Amaral ME. Ailanthus altissima: An alternative fiber source for papermaking. Industrial Crops and Products. 2014 Jan 1;52:32-7.
- Alshahrani TS. Interactions of allelopathy and competition affecting Ziziphus spina-christi and Prosopis juliflora seedlings. West Virginia University; 2004.
- Heisey RM. Allelopathic and herbicidal effects of extracts from tree of heaven (Ailanthus altissima). American Journal of Botany. 1990 May;77(5):662-70.


 Dr. Priyanka Ganesh Kale *
Dr. Priyanka Ganesh Kale *
 S. R. Dound
S. R. Dound











 10.5281/zenodo.14799841
10.5281/zenodo.14799841