Abstract
The Aim of this work is to identify the Critical process parameters and optimized then to achieve robust process. Objective of work is to ensure high quality and stable manufacturing of developed and manufacturing products, it provide framework for capturing the technical information need for product manufactured and compliance with regulatory guidelines. In this article we focused on Technology transfer, there goals, scope of technology transfer and optimization of critical process parameters in manufacturing of Levofloxacin tablet dosage form. In optimization parameters dry mixing, wet mixing, %LOD, Compression parameters of machine are optimized and keeping it fixed in Exhibit batch and successfully transferred the Process from R&D to manufacturing site.
Keywords
Technology transfer, Critical Process parameters, Optimization batches, Levofloxacin
Introduction
In order to develop new products and make them available to the public, such as medicines, educational tools, electronic devices, safety equipment, and health services, technology transfer is the process of moving scientific discoveries from one organization to another .The relationship between the fields of business, science, engineering, law, and government is technology transfer.[1-2] Technology transfer plays a crucial role in the process of finding new drugs and developing new medical products. This process is important for to elucidate necessary information for technology transfer from R & D (Research &Development) to PDL (process development laboratory).Technology transfer aids in the development of dosage forms in a number of ways, including process efficiency, product quality maintenance, and the achievement of standardized procedures, all of which contribute to timely and economical production.[3-4]
GOALS OF TECHNOLOGY TRANSFER: [5]
According to ICH Q10 guidelines
- Is an important stage in the process of development that leads to successful commercial manufacturing?
- To employ all of the information acquired as the foundation for the manufacturing control strategy, the process qualification methodology, and ongoing continuous improvement.
- The transfer of knowledge about products, processes, and analytical methods between development and manufacturing locations.
- To guarantee that process and parameter variability are adequately managed and robust enough to withstand the demands of a commercial production setting. To confirm that the parameters set during development remain within the designated design space and/or are modified during scale-up
STEPS INVOLVES IN TECHNOLOGY TRANSFER PROCESS [6]
During development of a formulation, it is important to understand the procedure of operations used, critical and non-critical parameters of each operation, production environment, equipment and excipient availability should be taken into account during the early phases of development of formulation.
A. Development of technology by R&D. (Research Phase)
- Design of procedure and selection of excipients by R&D – Selection of materials anddesign of procedures is developed by R&D on the basis of innovator product characteristics. (b) Identification of specifications and quality by R&D – Quality of product should meet the specifications of an innovator product.
B. Technology transfer from R&D to production (development phase):
R&D gives the product development laboratory a technology transfer dossier (TTD) document that includes all of the following formulation and drug product information:
- Master Formula Card (MFC) – Includes product name along with its strength, generic name, MFC number, page number, effective date, shelf life and market.
- Master Packing Card – Gives information about packaging type, material used for packaging, stability Profile and shelf life of packaging.
- Master Formula – Describes formulation order and manufacturing instructions. (Process order and environment conditions.
- Specifications and Standard Test Procedures (STP’S) – Helps to know active ingredients and
- excipients profile, in-process parameters, product release specifications and finished product details.
OPTIMIZATION AND PRODUCTION
Validation studies
It is carried out to confirm that the process can stabilize the product using the transferred manufacturing formula before production is put into action. Validation studies are carried out to confirm that the process can stabilize the product using the transferred manufacturing formula before production is put into action.
Scale up for production
During the small-scale development of the product and processes, scale up entails the transfer of technology. When developing a process, it is imperative to take the production system and environment into account. For the formulation of a solid dosage form, various procedures are used, such as dispensing, sifting, blending, compaction/dry granulation/wet granulation, compression, and coating. Pharmaceutical experts can easily become engrossed in the specific step of the manufacturing process that they are directly responsible for, from blending to film coating. Operators concentrate on keeping their segment of the production process running smoothly. But the whole manufacturing line can be improved, even before production begins, if technology transfer is implemented thoughtfully. Effective technology transfer helps to provide process efficiency and control and maintain product quality
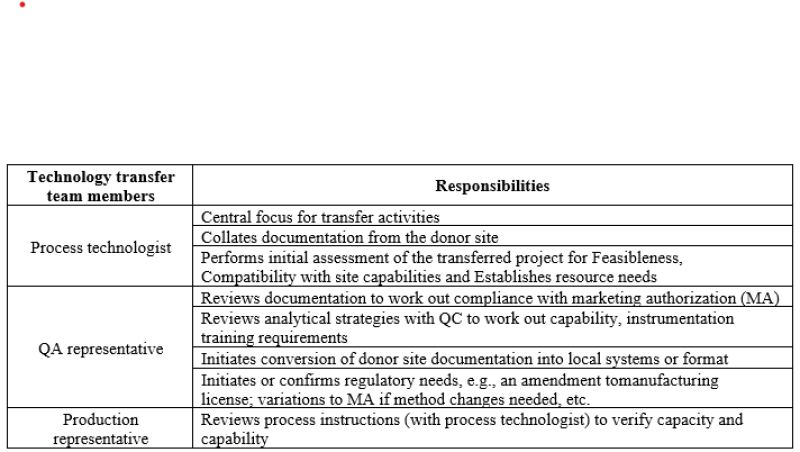
Technology transfer team Members and Their responsibilities.[7]
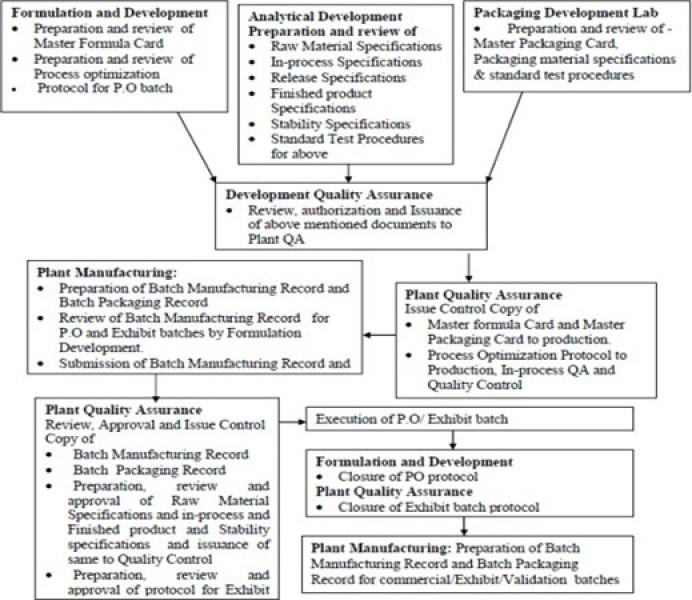
Fig 1: Representing Responsible persons in TT
Flowchart for technology transfer for process Optimization and Exhibit batches. [7]
Description of Drug and material
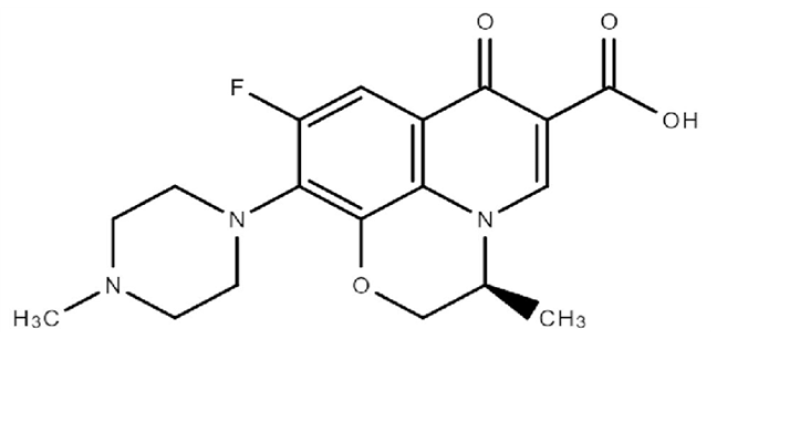
Figure 3: Representing Structure of Levofloxacin
Chemically speaking, Levofloxacin is known as (S)-9-fluro-2,3-dihydro-3-methyl-10-(4-methyl piperazine-1-yl)-7-oxo-7H-pyrodo[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid. Its molecular weight is 361.368 and its molecular formula is C18H20FN3O4. (1), Its freely soluble in water, organic solvent ethanol, butanol, acetic acid and ethyl acetate. [17-19] Pharmacology and MOA: Levofloxacin is a broad-spectrum antibiotic that is active against both gram positive and gram-negative bacteria. Its function by inhibiting DNA gyrase and thereby inhibiting cell division.
Polymer profile:
- Croscarmellose sodium: Its Synonym is Crossed linked carboxymethylcellulose sodium and Chemical name Cellulose, Carboxymethyl ether. Its functional category is tablet and capsule disintegrant.
- Hypromellose: Its Synonym is Benceel MHPC and Chemical name Cellulose Hydroxypropyl methyl ether. Its functional category is Bioadhesive material.
MATERIAL AND METHOD:
In manufacturing of Optimization batches, the active drug with raw material in specific grade are taken .It includes Levofloxacin hemihydrates, Lactose monohydrate, croscarmellose cellulose, hypermellose, microcrystalline cellulose, etc and the equipment’s used for manufacturing of tablets.
List of equipment’s used for process as per specified by GMP.
Table no 1: Representing equipment used in Process
Process flow diagram and Critical process parameters which have to be optimized
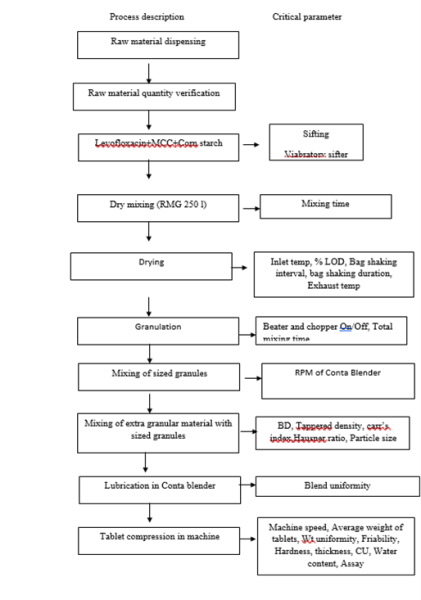
Figure 4: Representing Critical process parameters and manufacturing steps
In manufacturing of Levofloxacin, initially the selection of vendor for raw material was done by R &D unit of industry Kandewali, Mumbai. They synthesized the kilo size batch in the supervision of TT Person.All the list of raw material and list of critical process parameter are collected in documentary form, the major documents are as follows
- MMD (Master manufacturing documents): This document contains the list of raw material and manufacturing method.BMR was prepared by using MMD given by R&D.
- DPAD (Drug product analytical Data): This document gives information about the analytical data like absorbance, Dissolution chromatographic data. Manufactured of Optimization batches of Levofloxacin tablets 250 mg, 500 mg and 750mg.
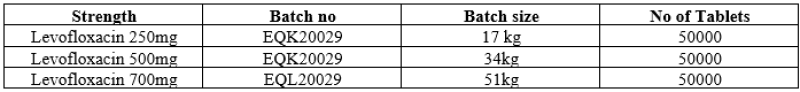
Common blend was prepared and bifurcated into three strength i.e 250,500 and 750mg respectively.102Kg common bled prepared of Batch No EQJ20019,This was bifurcated into three strength as follows
Table no 2: Representing First Optimization batch details
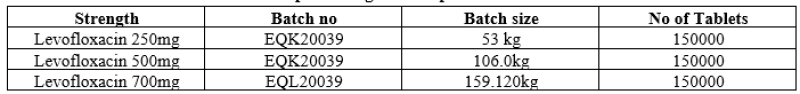
Similarly, Second batch EQJ20029 with batch size 102.00 kg.This was bifurcated into three strength as follows
Table no 3: Representing Second Optimization batch details
Third batch EQJ20039 having common blend batch size is 318.240 kg and it is bifurcated into three strength as follows
Table no 4: Representing Third Optimization batch details
Results obtain in optimization batches
- Dry mixing

Table no 5: Representing % Blend uniformity in Optimization batches.
After reviewing above data it was concluded that dry mixing to be done for 10 min in the exhibit batch.
- Wet mixing:
Wet mixing is done in RMG Granulator and recorded the ammeter reading of impeller at the end of granulation point.
Table no 6: Representing Granulator parameters in Optimization batches.
After reviewing above data it was concluded that WET mixing to be done by keeping Beater in slow, fast and slow speed with chopper remain off in throughout process, it gives optimum granules.
- Drying
Drying of optimization batches was carried out in FBD and optimized the different parameters like inlet temperature, exhaust temperature, bag shaking interval duration, and % LOD.
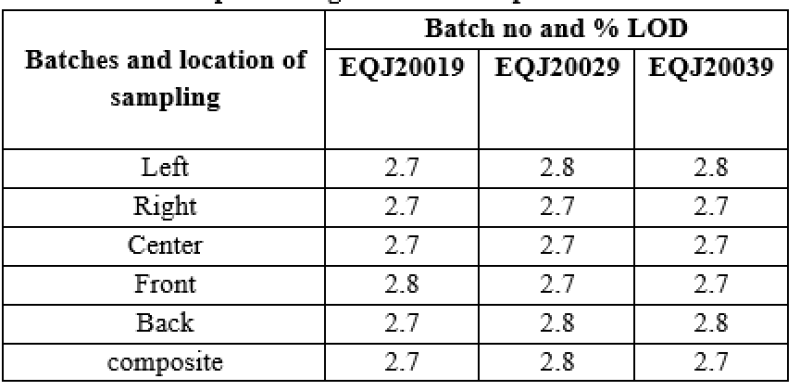
Table no 7: Representing % LOD in Optimization batches.
After reviewing above data it was concluded that %LOD was found NMT 1.2% .It was recommended for the exhibit batch.
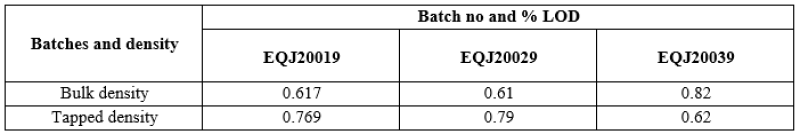
- Sizing:
The dried granule were milled through 1.2mm screen using combo mill with co mill screen at speed 550 rpm in first optimization batch and 250-350RPM in second and third optimization batch.
Table no 8: Representing % LOD in Optimization batches.
It was found suitable to mill the dried granules through 1.2mm screen using Co-mill. The same was recommended for milling purpose in the Exhibit batch.
- Prelubrication:
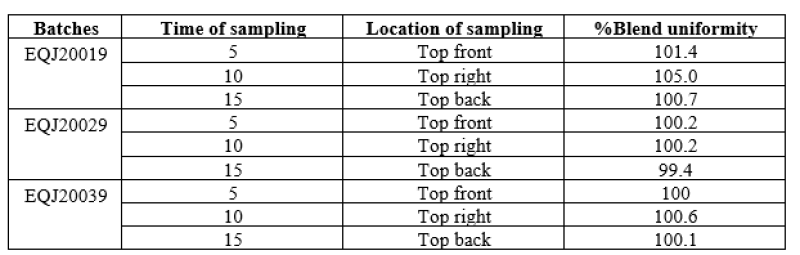
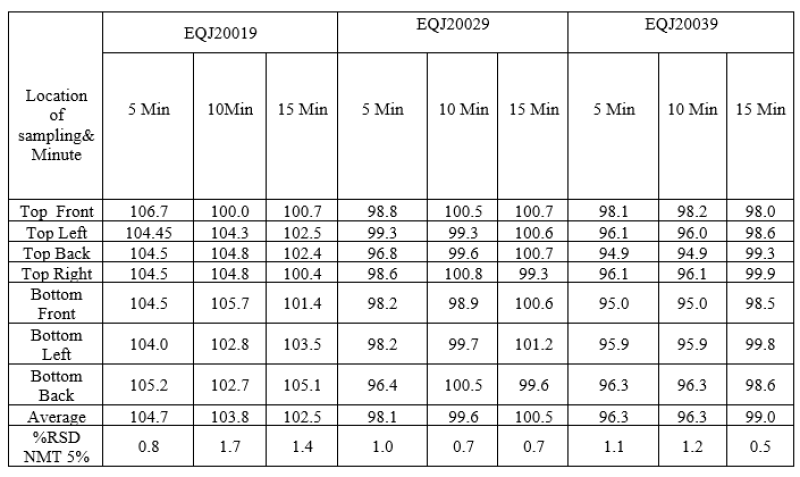
Prelubrication was done in V blender at 10+ 1rpm for mixing of extra granular material with sized granules. In that Microcrystalline cellulose, crospovidone, colloidal silicon dioxide was sifted through sieve through #40 then mix in the optimization batches. In order to optimize the mixing time, mixing was carried out for 5, 10, 15 minutes.Following table indicate % of blend uniformity of collected from different sampling location in RMG.
Table no 9: Representing % Assay of Blend uniformity in Optimization batches.
After reviewing above data it was decided to carry out Prelubrication for 10 minutes in the Exhibit Batch.
- Lubrication:
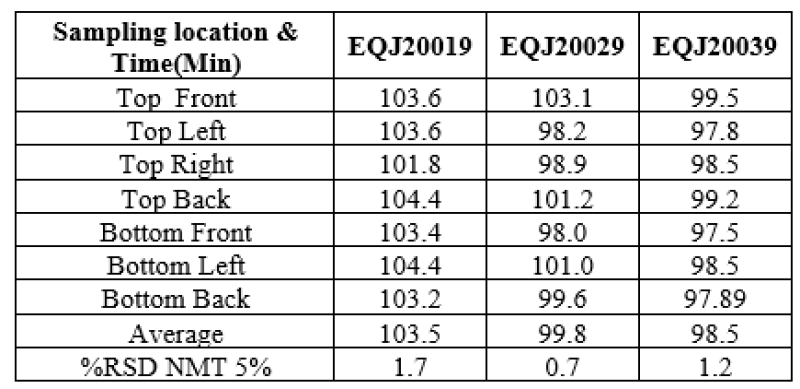
Pre lubricated blend was lubricated with magnesium sterate for 2 minutes in V Blender at 10+1 rpm.% Blend uniformity(% asay ) was found within specification and satisfactory. Following table represent the data.
Table no 10: Representing % % Assay Blend uniformity in Optimization batches.
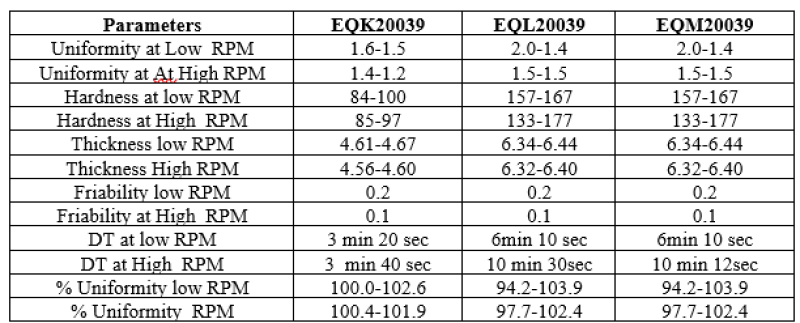
All the da6ta mentioned above, it was decided to carry out lubrication for 2 min in the exhibit batch.
- Compression:
Compression was performed on double rotary compression machine as per specification. Following challenges were carried out during compression in optimization batches.
• Machine speed challenge test
• Hardness challenges test
In it three optimization batches were compressed and carried out the machine challenge and hardness test. At initially in first and second optimization batches sticking and picking defects is observed due to the moisture in the blend .After keeping LOD NMT1.2% the problem was solved and fixed the machine RPM and Hardness in range. Following table represent the optimized parameters obtained in third optimization batches of Levofloxacin tablets.
Table no 11: Representing Machine challenge and Hardness challenge test in Optimization batches.
- Coating:
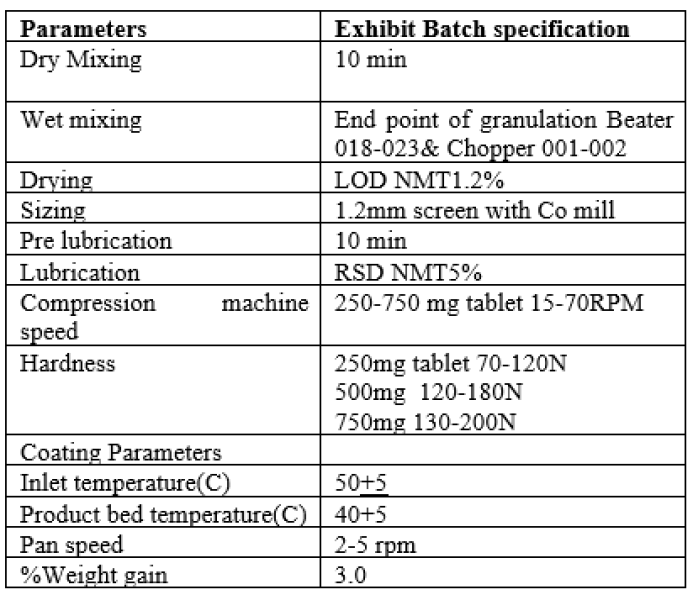
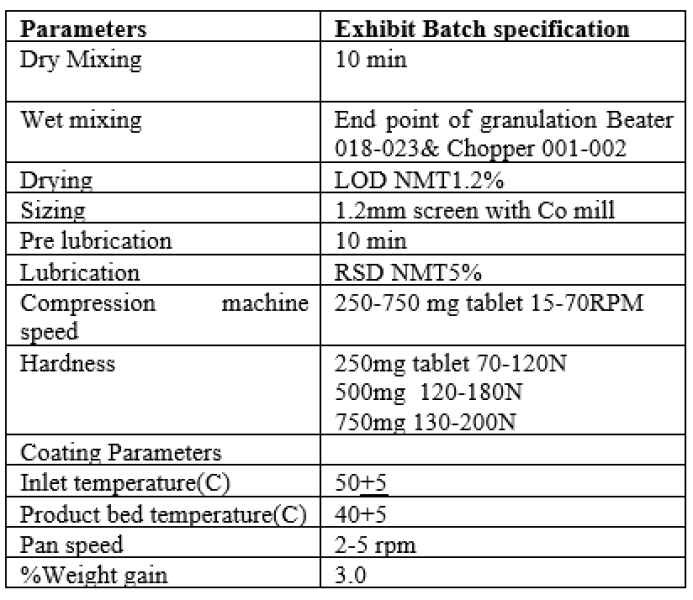
Coating of Levofloxacin tablets was carried out in SFC-100 Coating machine. It was processed in three stages, it include Preheating, coating and drying.Diffrent coating parameters like inlet temperature, Product bed temperature, Pan speed, spray rate, gun to bed distance and finally % eight gain was studied and finalized in exhibit batch of Levofloxacin.
Summary of Parameters optimized and final for exhibit batch of Levofloxacin tablets
Table no 12: Representing Summary of Parameters Optimized for Exhibit batch.
CONCLUSION
Levofloxacin 250mg, 500mg and 750 mg film coated tablets was prepared using FBE Granulation technology on lab scale size. This study was carried out through a systematic plan, critical process parameters were optimized in three optimization batches and parameters fixed in commercialization batch to produce stable and robust manufacturing process. The overall successful three consecutive batches of Levofloxacin film coated tablets verify the successful technology transfer from R&D to Manufacturing site.
REFERENCES
- Domb E, Mlodozeniec A. Using TRIZ to Accelerate Technology Transfer in the Pharmaceutical Industry. InEuropean TRIZ Association TRIZ Futures Conference, Florence Italy 2004 Nov 3.
- http://www.research.cornell.edu/cotab
- Reamer A. Technology transfer and commercialization: their role in economic development. www. eda. gov/PDF/eda_ttc. pdf. 2003.
- Dumpala R, Patil C. REVIEW ON TECHNOLOGY TRANSFER IN PHARMACEUTICAL INDUSTRY; FACTS AND STEPS INVOLVED.
- Millili G. Scale-up & technology transfer as a part of pharmaceutical quality systems. In Pharmaceutical Quality System (ICH Q10) Conference 2011 (pp. 14-16).
- Singh A, Aggarwal G. Technology transfer in pharmaceutical industry: a discussion. Law and government. 2010;1(2).
- Savale SK. TECHNOLOGY TRANSFER IN PHARMACEUTICAL INDUSTRY: PROCESS TRANSFER FROM DEVELOPMENT TO COMMERCIALIZATION..


 Nilesh I Patil*
Nilesh I Patil*
 Parag R. Patil
Parag R. Patil















 10.5281/zenodo.10798162
10.5281/zenodo.10798162