Abstract
Over the past three decades, formulation technology has significantly advanced, particularly in drug delivery systems. Innovations include novel dosage forms and new uses for existing drugs, offering benefits like improved patient compliance, sustained drug concentration, reduced dosing frequency, targeted delivery, and minimized side effects. Transdermal drug delivery systems (TDDS) are key developments, allowing controlled, continuous medication administration through the skin, bypassing gastrointestinal degradation and hepatic first-pass metabolism, and enhancing bioavailability and patient compliance. The FDA approves roughly one transdermal product every 2.2 years, with the first patch approved four decades ago. This research examines the skin's role as a barrier, clinical trials, patents, commercialization, and the benefits and limitations of TDDS. Various TDDS methods are reviewed, highlighting their advantages, disadvantages, and potential applications. Recent advancements demonstrate TDDS's effectiveness and potential across diverse sectors, emphasizing their transformative impact on drug delivery and therapeutic practices.
Keywords
Hypolipidemic, Hyperlipidermic, Rats, Wistar, Fat, Diet
Introduction
Hypolipidemic activity refers to the ability of a substance or drug to lower the lipid levels in the body. Lipids are fatty substance like cholesterol and triglycerides that are important for various body functions. However, high levels of lipids in the blood can lead to the health problems like heart diseases. When a substance exhibits hypolipidemic activity, it helps to reduce the levels of lipids in the blood, promoting better cardiovascular health. This can be achieved through different mechanisms such as inhibiting the production of lipids, increasing their breakdown, or enhancing their elimination from the body. There are several natural and synthetic compounds that have been found to possess hypolipidemic activity. For example certain plant extracts, such as garlic and fenugreek, have been shown to have lipid-lowering effects. These extracts contain bioactive compounds that can regulate lipid metabolism and improve lipid profiles. In addition to natural compounds, pharmaceutical drugs like statins are commonly prescribed for their hypolipidemic condition. Statins work by inhibiting an enzyme involved in cholesterol synthesis, there by reducing cholesterol levels in the blood. Regular physical activity and a healthy diet also play a crucial role in maintaining optimal lipid levels. Engaging in aerobic exercises like jogging, swimming, or cycling can help to increase high density lipoprotein (HDL) cholesterol, also known as “good” cholesterol, while reducing low density lipoprotein (LDL) cholesterol, or “bad” cholesterol. When it comes to diet, incorporating foods rich in omega-3 fatty acids, such as fatty fish( like salmon and mackerel ), walnuts, and flax seeds, can have positive impact on lipid levels. These healthy fats help to lower the triglycerides and increase HDL cholesterol. Its important to note that hypolipidemic condition always be approached under the guidance of a healthcare professional. They can assess your lipid profile, recommend appropriate interventions, and monitor your progress. PIPER BETEL, commonly known as evergreen vine that belongs to the Piperaceae family. It has heart shaped leaves with a glossy appearance and can grow upto 5-6 feet in height. The plant thrives in warm and humid climates, which makes it perfect for regions like Southeast Asia. In addition to its traditional use in medicine and a stimulant, the leaves of the Piper Betel plant also have cultural significance. They are often used in religious ceremonies, as well as in culinary preparations like wrapping food or adding flavor to dishes.
MATERIALS AND METHODS
PLANT COLLECTION

Fig no 1 Piper Betel plant
The piper betel plant was identified and the leaves are collected from the Narsapur regional area.
PREPARATION OF PLANT EXTRACT :
The leaves of piper betel were washed with tap water and shade dried. The leaves were taken and powdered coarsely and stored in a container for further use. The air dried and powdered piper betel leaves are taken and weighed (50gm) were extracted with ethanol solvent by using Soxhlet apparatus for 24 hours, at a temperature not exceeding the boiling point. Then the extract was collected from the Soxhlet apparatus is a glass beaker (500ml) and kept the extract in heating mantle for air drying and the residues were collected and stored in a room temperature.





Fig no 2 Soxhlet extraction process
EXPERIMENTAL ANIMAL :
Were six female wistar rats (250 – 270 g) were selected and divided into three groups each group has two animals. The animals were maintained in a well ventilated room in polypropylene cages and the rats were fed on pellets and vanaspathi dalda and tap water. All the experiments were carried out according to the guidelines recommended by the committee for the purpose of control and supervision of experiments on animals ( CPCSEA), Government of india.
INDUCTION OF HYPERLIPIDEMIA :
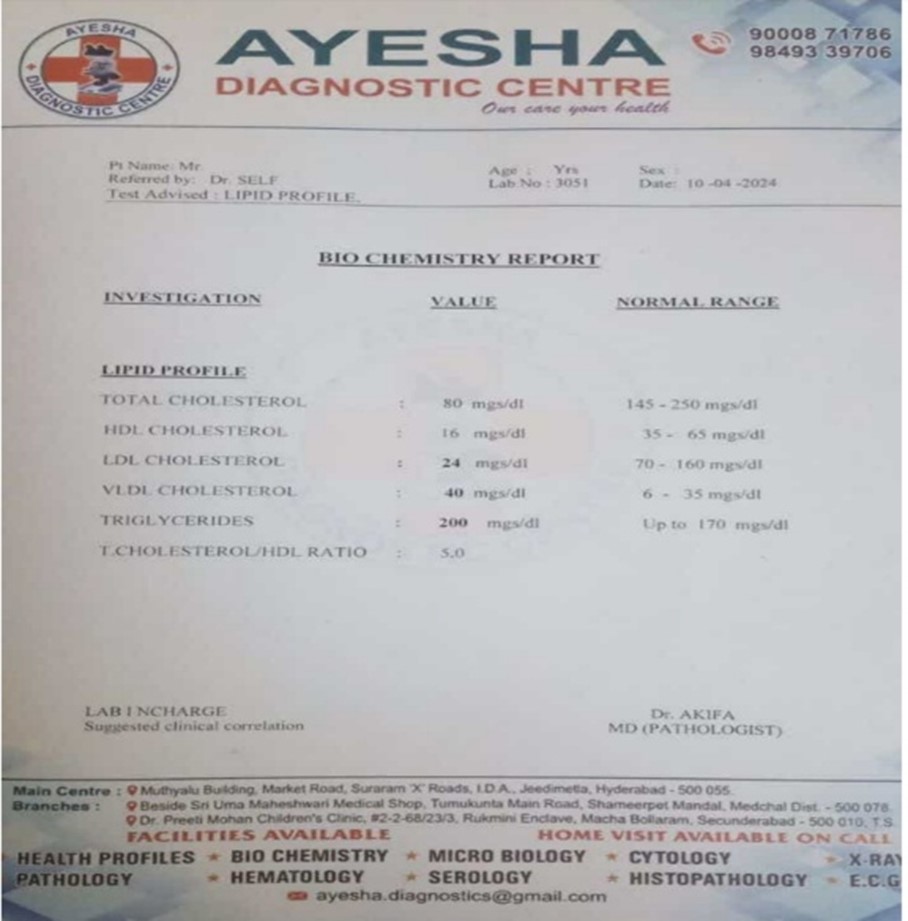
Induction of hyperlipidemia was done by high fat food, using vanaspathi dalda and pellets foe two weeks (14 days). Then blood samples was collected and sent to near by diagnostic center ( Ayesha diagnostic center ) and the lipid profile shows the hyperlipidemic results.
EXPERIMENTAL DESIGN :
Induction of hyperlipidemia in female wistar rats by inducing high fat diet, after two weeks the blood samples were taken and checks the lipid profile of high fat diet induced female wistar rats.
The rats are divided into three groups, each group contains two rats.
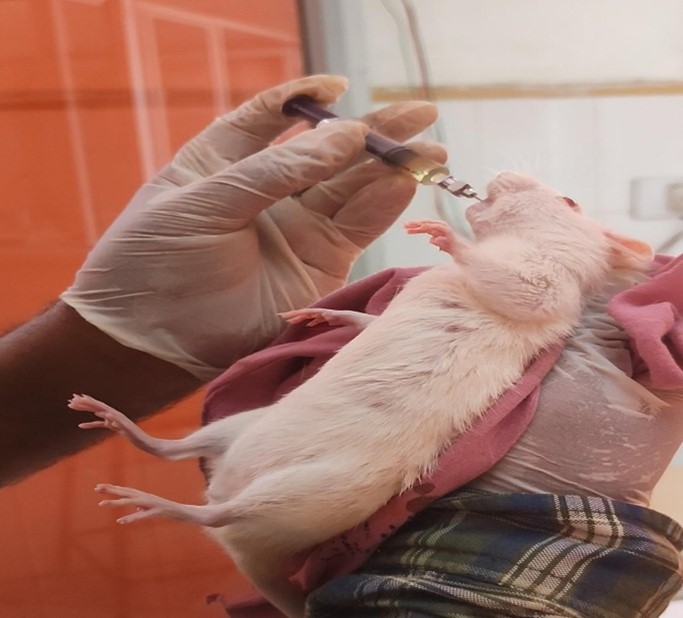
Fig no 3 Oral route of administration.

Fig no 4 Intraperitoneal route of administration
GROUP 1 :
control group rats are administered with distilled water.
GROUP 2 :
High fat diet induced rats are administered orally by ethanolic extract of piper Betel leaves ( Dose 30 mg/kg ) to seven days.
GROUP 3 :
Hyperlipidemia induced rats are administered intraperitoneal route by prepared Extract of piper betel ( Dose 20 mg/kg ) to seven days.
GROUP 4 :
Standard group animals are administered orally by ATARVOSTATIN drug 10 mg/kg.
BLOOD COLLECTION :

Fig no 5 Tail vein blood collection
Fig no 6 1ml of collected blood sample
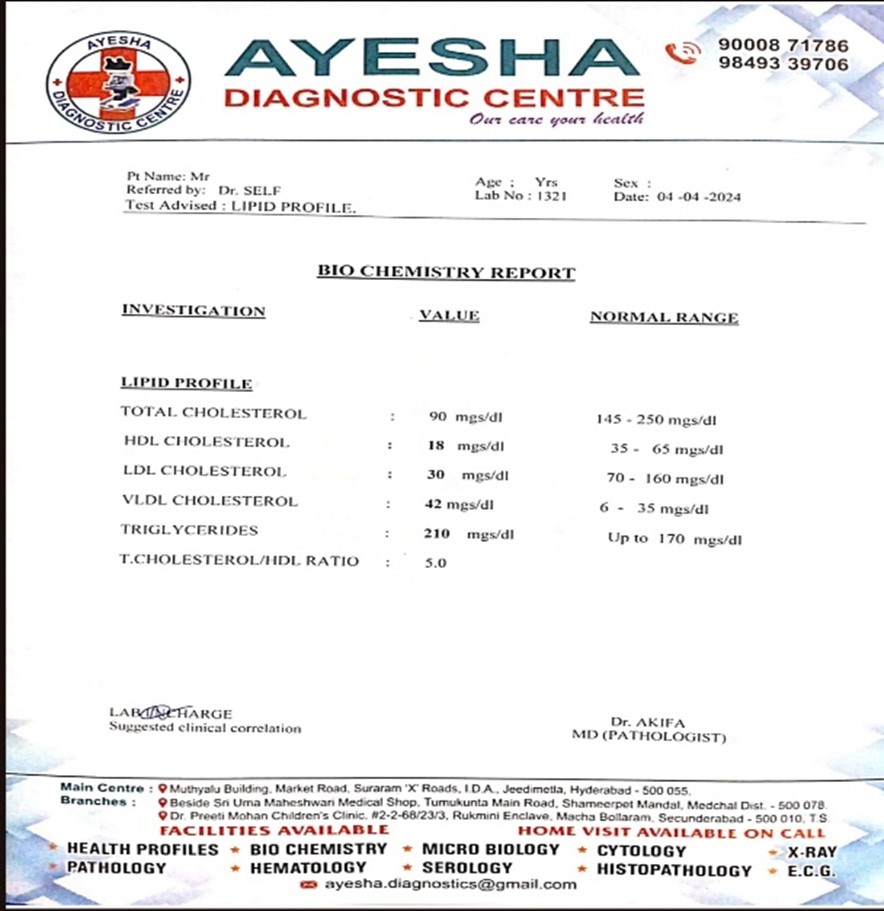
After seven days of drug administration the blood sample was collected through the tail vein technique, under the light concentration of chloroform anesthesia. Upto 1ml blood were collected from the rats and stored in heparin tubes. Then samples are sent to near by Ayesha diagnostic center.
The lab reports are given below ( lipid profile )
PRELIMINARY PHYTOCHEMICAL SCREENING :
Phytochemical test were done to identify the presence of bioactive chemical constituents such as terpenoids, tannins, flavonoids, phenols, saponins, alkaloids, glucosides and carbohydrates by the following procedure.
Test for Terpenoids :
- 2ml chloroform is added to 5ml plant extract which was evaporated in a water bath. After that, 3ml conc.H2SO4 is added while it is boiled in a water bath. And the solution turned to gray colored solution, that indicates the presence of Terpenoids.
Test for Tannins :
- Braymer’s test :
1ml filtrate is dissolved in 3ml distilled water and 3 drops 10?rric chloride solution is added in the solution. Then the solution turns into blue green color, it indicate the positive response for test of Tannins.
Test for Glucosides :
- 10% NaOH Test :
1ml dil. H2SO4 is added to 0.2ml extract and the solution is boiled for 15 min, and allowed to cool. It is neutralized with 10% NaOH and 0.2 ml fehling’s solution A and B. A brick red precipitation is formed which indicates the presence of glucosides.
Test for Phenols :
- Lead acetate test :
The leaf extract is dissolved in 5ml distilled water after which 3ml of 10% lead acetate solution is added to the solution. Due to formation of white precipitate the presence of phenolic compounds are identified.
Test for Carbohydrates :
- Molisch test :
In 2ml filtrate, 2 drops of alcoholic alpha naphthol and 1ml conc. H2SO4. The violet ring formed at the separation of the liquid, and indicate the presence of carbohydrates.
Test for Alkaloids :
- Hangers test :
Add a few ml filtrate with 1-2ml Hangers reagents. The positive results gives a creamy white precipitate.
- Dragendroff’s test :
Few ml of filtrate is added with 1-2ml. dragendroffs reagents. Presence of alkaloids indicated by a reddish – brown precipitate.
Test for Saponins :
a. Fronth test :
0.5gm leaf extract is added in 2ml tap water and shaken vigorously. A persistent foam is observed for 10 minutes in presence of Saponins.
Test for Flavonoids :
- Alkaline reagent test :
2-3 drops of H2SO4 were added to 2ml of extract. Initially, deep yellow color appeared then it gradually became colorless by adding of few drops of dil. HCL, it indicate that flavonoids were present.
RESULTS :
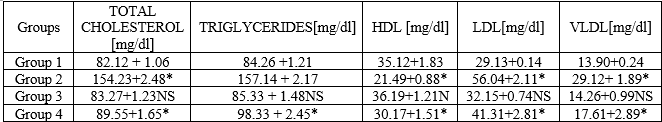
The present study establishes the hypolipidemic activity of ethanolic leaf extraction of Piper Betel in wistar rats. The results of different hypolipidemic parameters are shown in table. Atarvostatin treated animals significantly decreased the hypolipidemic activity. Ethanolic extract of piper betel can treated animals significantly decreased hypolipidemic condition.
Table 1. Effect of treatment of piper betel extract on cholesterol levels in high fat diet induced wistar rats.
DISCUSSION :
The objective of the present study is to evaluate hypolipidemic action of ethanolic extract of piper betel leaves. And high fat diet induced hyperlipidemia in female wistar rats. Development of atherosclerosis disease is complicated process involving accumulation of lipid containing particles in the walls of coronary arteries and other major arteries within the body. A high fat diet increased cholesterol levels to people which leads to obesity. High cholesterol diet increased serum levels and LDL-C significantly a rise deposition of cholesterol in arteries and aorta and it is a direct risk factor for coronary heart diseases. Piper Betel a well known traditional medicinal plant it has been used to treat diabetics and related hyperlipidemic condition.
CONCLUSION:
In conclusion from the above results, it can be suggested that the ethanolic extract of piper betel is an effective hypolipidemic activity, which supports the claim that the plant can be used to treat Hypolipidemic condition. The present study also provides basis for traditional use of Piper Betel in treatment of diabetes insipidus.
REFERENCES
- Kearney PM, whelton M. Reynolds K. Munter P, whelton PK. He J. Global burden of hypertension analysis of worldwide data. Lancet 2005, 365: 217-223.
- Howard-Alpe GM, Sear JM, Forx P. Methods of detecting atherosclerosis in non-cardiac surgical patients, the role of biochemical markers. Br J Anaesthesia 2006; 97:758-769.
- Sattivel A, Rao H, Balajiaraghavendran. Anti peroxidative and anti hyperlipidemic nature of ulva lactuca crude polysaccharide on D-galactose amine induced hepatitis in rats. Food chem toxicol 2000; 46: 3262-3267.
- Durrington P. Dyslipidemia. Lancet 2003; 362: 717-731.
- Speight TM. Avery’s drug treatment principles and practice of clinical pharmacology and therapeutics. Australia : Maclennan and petty; 1987.
- WHO. Resolution- promotion and development of training and research in traditional medicine, Geneva: WHO; 1977, P.49-49.
- WHO. Geneva legal status of traditional medicine and complementary/Alternative medicine: A worldwide Revicu, Geneva: world health organization; 2001. P. 129-143.
- Harbone JB. Phytochemical methods. A guide to modern techniques of plant analysis. 1st Edn., London: chapman and hall: 1973.
- Solowuta A. medicinal plant and traditional medicine in Africa. 2nd Edn. Ibadan: Spectrum books; 1996, u. 112.
- Tyler VE. Phytomedicines: back to the future. J Nat Prod 1999; 62: 1589-1592.
- Kumari K, Augusti KT. Lipid lowering effect of S-methyl cysteine sulfonide from allium crpe lints in high cholesterol diet fed rats. J ethnopharmacol 2007, 109: 367-371.
- Son 15. Kim JH,sohn HY, Son KIH, kim JS, kwon CS. Antioxidative and hypolipidemic effect of dingenin, a steroidal saponin of yam ( Dioscorea spp.), on high-cholesterol led rata. Binnes biotechnol biochem 2007; 71: 3063-3071.
- Anonymous. The wealth of india raw materials, Pb-Re, vol VIJ. New delhi national institute of science communication and plomatis remures ( NISCAIR), 2005, P. 84-04
- Ramji N, lyer R, chandrawkaran S. pediatric from piper betel in the prevention of the halitosis. JE 2002:16 461-466.
- Chenalbury D, kale RK. Antioxidant and non to preparation of piper betel leaf extract: In vitro and in vivo studies. Plytather res 2002; 16; 461-466.
- Tattarharya s, mola s, gamre s, kamat jp, Bandyopadhyay k Chattopadhyay s. inhibitory property of piper betel extract sp photo sensitization induced damages to lipids and proteins. Food chem 2007: 100: 1474- 1480.
- Bhattacharya S, subramania M, Bauri A. kamat JP. Bandyopadhyay SK, Chattopadhyay S. Radio protecting property of the ethanolic extract of piper betel leaf. JRadiat res 2005, 46 165-171.
- Srinivasan k, patole ps, kanl CL, Ramarao P. Reversal of glucose intolarence by pioglitazone in high fat diet fed cats. Meshadi find exp clinical pharmacology 2004; 26:327-333
- Reed MJ. Meszaros k. Entes L J, ClaypooL MD,Pinkett JG. Gadbois TM, et al. A newrat model of type 2 diabetes the ft-fed, streptozotocin-treated tat. Metabolism 2000, 49-1390-1394.
- Parekh AC, jung DM. Cholesterol determination with ferric acetate – uranum acetate and sulphuric acid, ferrous sulphate reagents. Anal chem 1970: 42: 1423-1427.
- Rice LB. determination of triglycerides ( enzymatic method) chem 1970, 31(5): 746-750.
- 1.yos TJ. Lipependein glycosides and its metabolic complication of diabetes 1992, 41(2): 67-73.
- Abbey M and Nestel PJ (1994). Plasma cholesteryl ester transfer protein activity is increased when trans- elaidic acid is substituted for cis-oleic acid in the diet. Atherosclerosis. 106: 99-107.. Ahmed RS, seth V and Banerjee RD (2000) influence of dietary ginger ( Zingiber officinale) on the antioxidant defense.
- In rat comparison with ascorbic acid. Indian journal of experimental biology 38:604-606.
- Akhani SP, Vishwakarma SL. And goal RK (2004). Anti -diabetic activity of zingiber officinale in streptozotocin-induced type 1 diabetic. Journal of pharmacy and pharmacology 56: 101-105.
- A and willett WC (1997). Health effects of trans fatty acids. The American journal of clinical nutrition 66: 1006-1010.
- Chang NW and huang PC (1998). Effects of the ratio of polyunsaturated and mono saturated fatty acid to saturated fatty acid on rat plasma and liver lipid concentration. Lipid 33: 481-483 colandre ME,Diez RS and CA (2003). Metabolic effects of trans fatty acids on an experimental dietary model.
- British journal of nutrition 89: 631-639. Dwivedi s (2004) atherosclerosis revisited. Indian journalof cardiology 7:6-12
- Estruch R, martinez MA, corella D, salas -salvado j ruiz- Gutierrez V. covas MI, fio M,10. Gomez- Gracia E lopez- sabater MC, vinyoles E, aros F, conde M, Lahoz C, lapetra j, saez G, Ros E (2006). Effects of a mediterranean -style.
- Diet on cardiovascular risk factors: A randomized trial. Annals of internal medicine 145:1-11.. Fuhrman B, roseblate M, hayek T, coleman R and aviram M (2000), ginger extract consumption reduces plasma cholesterol, inhibits LDL oxidation and attenuates development of atherosclerosis in atherosclerotic, apolipoprotein E – deficient in mice.
- The journal of nutrition 130: 1124-1131. Gridley MF (1960), mamual of histologic and special straining technique. MacGraw- hill book company USA. Pp. 28-29.
- Heeba GH, Abd- Elghany MI(2010). Effect of combined administration of ginger (zingiber officinale) and atorvastatin on the liver of rats. Phytomedicine 17: 1076-1081.
- Ibrahim A, natrajan s, Ghafoorunisaa R (2005). Dietary trans-fatty acids altera dipocyte plasma membrane fatty acids 17 composition and insulin sensitive rats. Metabolism 54: 240-246.
- Indian journal of experimental biology 47: 748-753. Katan Mb, mesink RP, zockPL. (1995). Trans fatty acids and their effect on lipoproteins in humans.
- Annual report of nutrition 15:473-493.
- Gujaral S, Bhumra H, Swaroop M (1978). Effect of ginger oleo resin on serum and hepatic cholesterol levels in cholesterol -fed rats. The journal of nutrition 17: 183-187.15
- Serum paraoxonase activity in healthy men and women. Metabolism 51: 1534-1537.
- Food chemistry 102: 764-770. Tanabe M. chen YD, saito K, kano Y (1993), cholesterol biosynthesis inhibitory component from zingiber officinale.
- Chemical and pharmaceutical bulletin 41: 710-713. Zaman OA, banoo H, Chowdhury s, Chowdhury ASR, khaleque A (1983). Effect of garlic oil on serum cholesterol and blood.


 P. VIJAY KUMAR *
P. VIJAY KUMAR *
 G. GOPALA KRISHNA
G. GOPALA KRISHNA
 D. VINUTHNA
D. VINUTHNA













 10.5281/zenodo.13337279
10.5281/zenodo.13337279