Abstract
Breast cancer is the most common cause of cancer in women and the second most common cause of cancer death in women in the U.S. Breast cancer refers to cancers originating from breast tissue, most commonly from the inner lining of milk ducts or the lobules that supply the ducts with milk. Cancer develops if the immune system is not working properly and / or the amount of cells produced is too great for the immune system to eliminate. The rate of DNA and RNA mutations can be too high under some conditions such as; unhealthy environment (due to radiation, chemicals, etc.), poor diet (unhealthy cell environment) people with genetic predispositions to mutations and people of advanced age (above 80). There are different kinds of breast cancer which are Non-Invasive Breast Cancer, Invasive Breast Cancer, Lobular carcinoma in situ, Ductal carcinoma in situ, Infiltrating ductal carcinoma. Targeted drug therapy uses medicines that are directed at (target) proteins on breast cancer cells that help them grow, spread, and live longer. Targeted drugs work to destroy cancer cells or slow their growth. They have side effects different from chemotherapy. Some targeted therapy drugs, for example, monoclonal antibodies, work in more than one way to control cancer cells and may also be considered immunotherapy because they boost the immune system. Like chemotherapy, these drugs enter the bloodstream and reach almost all areas of the body, which makes them useful against cancers that have spread to distant parts of the body.
Keywords
Breast cancer, Targeted therapy, HER2, Antibody-drug conjugate, Triple negative breast cancer, BRCA 1/2
Introduction
In December 2020, the World Health Organization announced that breast cancer has become the most commonly diagnosed cancer globally, surpassing lung cancer [1]. Treatment for breast cancer includes various modalities such as surgery, radiation therapy, chemotherapy, endocrine therapy, and notably, targeted therapy. The selection of these therapies is influenced by the subtype of breast cancer and the stage at diagnosis [2]. The National Cancer Institute’s SEER summary identifies four primary types of female breast cancer:
1. Hormone receptor (HR) positive / Human Epidermal Growth Factor Receptor 2 (HER2) negative
2. HR negative/HER 2 negative
3. HRR positive / HER2 positive
4. HR negative / HER2 positive [3].
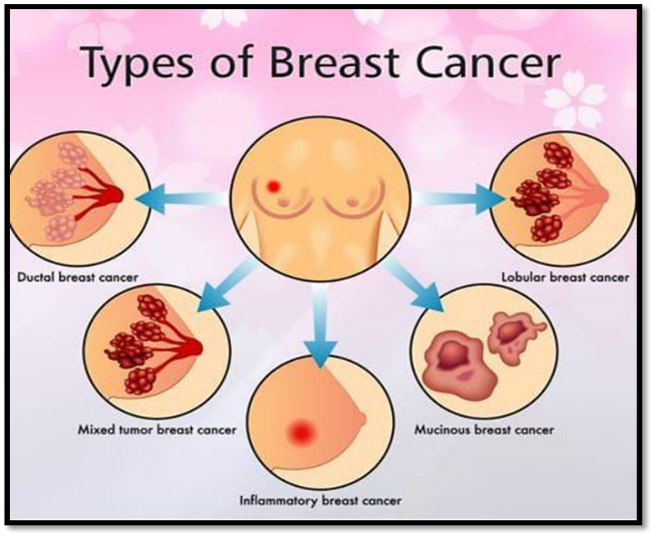
Figure No 1:- Types of breast cancer
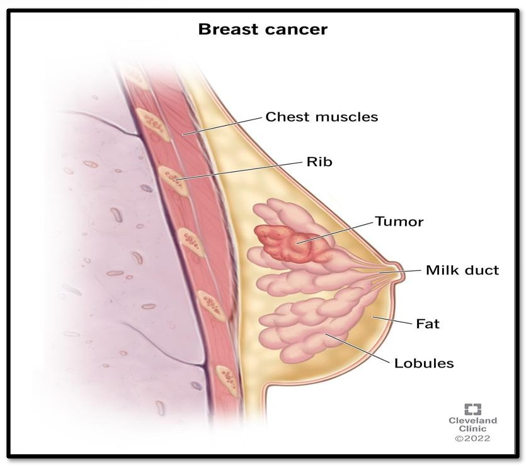
Figure No 2:- Breast cancer
Advances in Targeted Therapies
Over the past 25 years, significant progress has been made in the development of targeted therapies for breast cancer, driven by an enhanced understanding of the biological mechanisms underlying tumor growth. Initiatives like The Cancer Genome Atlas (NCI/NIH) have contributed to the molecular drivers of breast cancer. This review aims to outline the evolution of targeted therapies, organized by breast cancer subtype, and discuss the development of various drug classes alongside their impact on patient outcomes.
Key Developments in Targeted Therapies :-
? HER 2 positive breast cancer Following the approval of trastuzumab in 1998 subsequent advancements have produced a variety of therapies aimed at HER2-positive breast cancer [4]. This has led to improved outcomes for patients with this subtype.
? HR Positive / HER2 Negative Breast Cancer Targeted therapies for HR-positive breast cancer have evolved to include agents that modulate hormone receptor activity and block growth factor signaling pathways, enhancing treatment effectiveness.
? Triple Negative Breast Cancer (TNBC) and BRCA Mutations The development of targeted therapies for triple-negative breast cancer, characterized by the absence of HR and HER2 expression, has gained momentum. Drugs targeting the BRCA1/2 mutations have emerged, providing new therapeutic options for this challenging subtype
Clinical Indications and Future Directions:
A summary of targeted therapy drug classes, organized by FDA approval year, is provided in. This timeline illustrates the progressive development of therapies, highlighting key advancements in targeted approaches for specific breast cancer subtypes. Ongoing research aims to uncover novel targets and enhance existing therapies, paving the way for improved patient outcomes.

Figure No3: Targeted therapy drug classes/drugs for breast cancer.
1. Targeted therapies for HER2+ breast cancer-
1.1 Anti-HER2 antibodies
A notable success for targeted therapies in breast cancer is the development of drugs against HER2, also known as ERBB2. A diagram summarizing the classes of drugs targeting HER2 is provided in Fig. 3
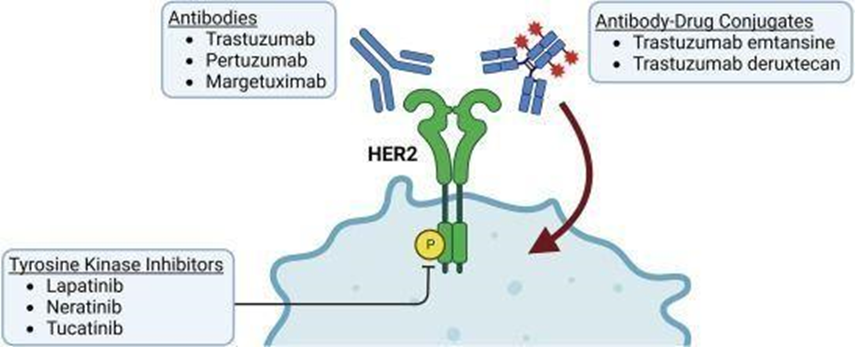
Figure No4: drug classes and drugs that target HER2
HER2 is a transmembrane tyrosine kinase receptor that mediates cell proliferation, survival, differentiation, angiogenesis, invasion and metastasis [4]. HER2 heterodimerization with Epidermal Growth Factor Receptor (EGFR/HER1) results in tyrosine phosphorylation that stimulates a mitogenic response [5]. Salmon, et al. were the first to relate amplification of the HER2 gene with disease outcomes in breast cancer. They reported HER2 amplification in 53/189 (28%) primary human breast tumors analyzed and correlated this with a higher risk of relapse and death. These findings provided a clear rationale to develop agents against HER2. The first such drug that was developed and approved for clinical use was trastuzumab, a humanized monoclonal antibody directed against an extracellular epitope on HER2 [4]. Binding of trastuzumab to HER2 induces receptor downregulation, suppressing proliferation and survival in HER2-overexpressing cancer cells, H0648g was a pivotal trial to evaluate trastuzumab plus chemotherapy in the first-line treatment of metastatic HER2+ breast cancer. Patients were randomized into four treatment arms. Based on existing standard of care, patients who had prior anthracycline exposure were given paclitaxel alone or in combination with trastuzumab. Patients who had not received a prior anthracycline were given an anthracycline plus cyclophosphamide alone or in combination with trastuzumab. In the intention-to-treat population, adding trastuzumab increased the time to disease progression from 4.5 to 6.9 months. The benefit was most pronounced for the paclitaxel- trastuzumab combination. In this treatment group, the median time to progression increased from 3.0 with paclitaxel alone to 6.9 months when trastuzumab was added. Overall survival in the intention-to-treat population was also prolonged by the addition of trastuzumab, but survival outcomes in the individual subgroups did not reach significance, perhaps owing to their small sample size. Based on results of the H0648g, trastuzumab was granted FDA approval for use in combination with paclitaxel as a first-line therapy for HER2+ metastatic breast cancer. A subsequent Phase II trial, M77001 tested an alternative combination of trastuzumab plus docetaxel. One rationale for using docetaxel instead of paclitaxel was based on results from a previous Phase III trial (TAX-311) that showed better outcomes for docetaxel in the treatment of advanced breast cancer . Like the H0648g trial, results from M77001 also favored the use of a trastuzumab-taxane combination over a taxane alone. But unlike H0648g, M77001 showed a significant overall survival benefit for the trastuzumab-docetaxel combination. In light of these and other clinical trials, the choice of paclitaxel vs. docetaxel remains a subject of debate, although both drugs are widely considered as valid clinical options.
1.2 Tyrosine kinase inhibitors
The significance of HER2 as an oncogenic driver of breast cancer prompted the development of orally-bioavailable, small molecules that inhibit HER2 catalytic activity. The first tyrosine kinase inhibitor approved for breast cancer, lapatinib, competitively inhibits EGFR and HER2 with similar affinity, and was shown in preclinical development to cause selective growth arrest and apoptosis in HER2-overexpressing breast cancer cell lines [6].
EGF100151 was a phase III clinical trial of lapatinib in combination with the chemotherapeutic capecitabine for the second line therapy of advanced HER2+ breast cancer. Patients enrolled in the trial had HER2+ clinical stage IIIb-IV breast cancer that had progressed following taxanes, anthracyclines, or trastuzumab. Patients were treated with capecitabine alone or in combination with lapatinib. Median time to progression at the time of the interim analysis was 4.4 months on capecitabine alone, and 8.4 months for the lapatinib-capecitabine combination. The study had originally planned to enroll 528 patients. Enrollment was halted when lapatinib showed a larger-than-expected benefit for time to progression at the interim analysis. In the final analysis, adding lapatinib extended the median time to progression by 1.9 months. The benefit was smaller than predicted by the interim analysis, but was still statistically significant. Overall survival was also extended by 2.8 months. Significance for overall survival was not reached, possibly on account of a smaller-than-planned enrollment size, along with several control patients having crossed over to the lapatinib arm. Based on these data, the FDA approved lapatinib plus capecitabine as a second-line therapy for advanced breast cancer in 2007 [7]. According to the SEER database, about 4 in 5 breast cancers are HR+ and express the estrogen and/or progesterone receptors . Endocrine therapy is the cornerstone of practice for HR+ breast cancer, and has a proven role in the neoadjuvant, adjuvant, and metastatic settings. One common challenge in treating HR+ breast cancers is resistance to endocrine therapy. Around 15–20% of HR+ tumors have intrinsic resistance to endocrine therapy, while another 30–40?velop resistance over time. Several mechanisms contribute to this process, including the overexpression of HER2, which drives resistance to endocrine therapy through signaling crosstalk with the estrogen receptor (ER) . Therefore, a combination of endocrine and HER2- targeted therapy was proposed to overcome drug resistance and improve disease outcomes. The effectiveness of this approach in the first-line treatment for locally-advanced and metastatic breast cancer was tested in a phase III clinical trial. Patients included postmenopausal women with HR+ breast cancer, clinical stages IIIb-IV, who received the nonsteroidal aromatase inhibitor (NSAI), letrozole in combination with lapatinib or placebo. Effects were modest for the intention-to-treat population, which included both HER2+ and HER2- patients. Compared with letrozole alone, the combination of letrozole and lapatinib increased progression-free survival by just over 1 month, and there was no difference in overall survival [8]. However, patients in the HER2+ subgroup had a significant benefit from lapatinib, which more than doubled median progression-free survival. Surprisingly, lapatinib also had a large benefit for HER2- patients who had experienced relapse within 6-months of discontinuing tamoxifen. These patients were defined in the trial as being hormone-resistant, and the benefit of lapatinib in this subgroup was similar in size to HER2+ patients, increasing progression-free survival from 3.1 to 8.3 months. The clinical benefit of lapatinib for tamoxifen-resistant HER2- patients, although not statistically significant, is consistent with the upregulation of HER2 expression by hormone therapy and supports the use for lapatinib in this population. Results from this trial led to accelerated approval by the FDA for lapatinib plus letrozole for HR+/HER2+ metastatic breast cancer.
1.3 HER2 antibody-drug conjugates
The introduction of trastuzumab in the late 1990?s significantly enhanced progression and survival outcomes for patients with HER2+ breast cancer, and helped to preserve their quality of life. Dual HER2 blockade with trastuzumab-pertuzumab further improved disease outcomes in the neoadjuvant, adjuvant, and metastatic settings. The next major advance in HER2-directed therapy was the introduction of trastuzumab emtansine, an antibody-drug conjugate (ADC) between trastuzumab and the antimitotic maytansinoid derivative, DM1. Prior studies demonstrated in vitro synergy for the combination of trastuzumab with antimetabolites, such as docetaxel or vinorelbine. Maytansinoid derivatives are semi-synthetic antimetabolites that bind to ?-tubulin and inhibit tubulin assembly in a manner similar to vinca alkaloids, but with significantly higher potency . The use of DM1 as a conventional therapeutic is not practical due to its high toxicity. Therefore, trastuzumab emtansine was designed to enhance the tumor specificity of DM1 for HER2+ cancer cells, lowering the risk of systemic adverse effects. Trastuzumab emtansine has the added benefit of eliciting an antitumor immune response, which theoretically contributes to therapeutic outcomes. The anti-HER2 antibody in trastuzumab emtansine is attached to the DM1 payload with a nonreducible thioester linker. The attachment of DM1 does not alter the binding affinity of trastuzumab to HER2. Once bound, the antibody- receptor complex is internalized by endocytosis, and an active DM1 metabolite is released by proteolytic digestion of the antibody. Moreover, efficacy for trastuzumab emtansine was demonstrated in both trastuzumab-sensitive and trastuzumab-resistant preclinical models, expanding its potential use to patients with relapsed/refractory disease following the use of trastuzumab [9]. The EMILIA trial evaluated trastuzumab emtansine for advanced HER2+ breast cancer. Eligible patients had advanced disease that progressed during or after treatment with trastuzumab and a taxane, and patients who had relapsed within 6 months of treatment for early-stage disease. Patients received either trastuzumab emtansine or a combination of lapatinib and capecitabine.
Results from EMILIA favored the use of trastuzumab emtansine. Median progression-free survival was significantly longer with trastuzumab emtansine compared with lapatinib plus capecitabine, at 9.6 and 6.4 months, respectively. In the final analysis of EMILIA, median overall survival was also significant with trastuzumab emtansine having a 4-month survival benefit over lapatinib plus capecitabine. Based on results from EMILIA, trastuzumab emtansine (ado-trastuzumab emtansine) received FDA approval in 2013 for metastatic, HER2+ breast cancer. Several therapeutic options had become available for relapsed or refractory HER2+ breast cancer. TH3RESA was a phase III trial designed to compare the efficacy of trastuzumab emtansine to the treatment of physician’s choice (TPC) in the third-line treatment of advanced disease. Participants in the study had experienced progression following two or more regimens of HER2-directed therapy that must have included trastuzumab and lapatinib. The difference in median progression-free survival was significant at 6.2 months with trastuzumab emtansine, and 3.3 months with TPC. A significant survival benefit was also observed, with trastuzumab emtansine prolonging overall survival by approximately 7 months over TPC.
In summary, TH3RESA confirmed the utility of trastuzumab emtansine in the management of treatment- resistant disease. trastuzumab emtansine compared with lapatinib plus capecitabine, at 9.6 and 6.4 months, respectively. In the final analysis of EMILIA, median overall survival was also significant with trastuzumab emtansine having a 4-month survival benefit over lapatinib plus capecitabine. Based on results from EMILIA, trastuzumab emtansine (ado-trastuzumab emtansine) received FDA approval in 2013 for metastatic, HER2+ breast cancer. Several therapeutic options had become available for relapsed or refractory HER2+ breast cancer. TH3RESA was a phase III trial designed to compare the efficacy of trastuzumab emtansine to the treatment of physician’s choice (TPC) in the third-line treatment of advanced disease. Participants in the study had experienced progression following two or more regimens of HER2-directed therapy that must have included trastuzumab and lapatinib. The difference in median progression-free survival was significant at 6.2 months with trastuzumab emtansine, and3.3 months with TPC. A significant survival benefit was also observed, with trastuzumab emtansine prolonging overall survival by approximately 7 months over TPC. In summary, TH3RESA confirmed the utility of trastuzumab emtansine in the management of treatment- resistant disease.
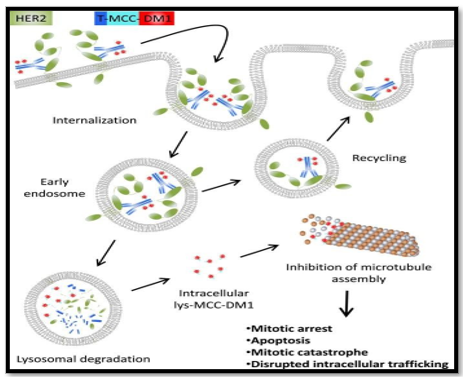
Figure No 5: Mechanism of action of T-DM1
2 Targeted therapies for HR+/HER2- breast cancer
2.1 CDK4/6 inhibitors
The CCND1 gene is amplified in approximately 15% of breast cancer cells, and cyclin D1 is overexpressed in up to 67% . Overexpression of cyclin D1 enhances cyclin-dependent kinases 4 and 6 (CDK4/6) and stimulates cell division, making this pathway an attractive target in cancer therapy. Abemaciclib (LY2835219) is a small molecule inhibitor of cyclin dependent kinases CDK4 (IC50 = 2 nM), CDK6 (IC50 = 10 nM), and CDK9 (IC50 = 4 nM) . Amplification of CCND1 and overexpression of cyclin D1 protein is commonly observed in ER+ breast cancers, implying greater sensitivity to CDK4/6 inhibition. Indeed, in a panel of 44 breast cancer cell lines, those that were ER+ exhibited the highest sensitivity to growth inhibition by abemaciclib.
MONARCH 2 evaluated abemaciclib for HR+/HER2- advanced breast cancer that progressed during or after endocrine therapy. Patients received abemaciclib or placebo in addition to the ER antagonist, fulvestrant. Abemaciclib significantly lowered the risk of disease progression or death. Median progression-free survival was 16.4 months in the abemaciclib arm and 9.3 months with placebo. A subsequent follow-up of MONARCH 2 reported that abemaciclib significantly increased median overall survival by 9.4 months (25%) compared with placebo. Based on the positive results of MONARCH 2, the FDA approved abemaciclib in combination with fulvestrant as a second-line therapy for HR+/HER2- disease. Abemaciclib was also approved as a second-line, single-agent therapeutic based on results from MONARCH 1, a separate phase II study [10]. MONARCH 3 tested the efficacy of abemaciclib in the first-line treatment of HR+/HER2- advanced breast cancer in postmenopausal women. Patients were given abemaciclib or placebo plus a non-steroidal aromatase inhibitor. Adding abemaciclib to endocrine therapy increased median progression-free survival by more than 1-year, from 14.8 months in the placebo arm to 28.2 months with abemaciclib. . Based on the findings of MONARCH 3, abemaciclib was approved by the FDA as a first-line agent for HR+/HER2- metastatic breast cancer in combination with an aromatase inhibitor. Additional studies have continued to demonstrate efficacy for abemaciclib in combination with other endocrine agents, including tamoxifen [11]
2.2 I3K inhibitors
Approximately 40% of HR+/ HER2- breast cancers possess activating mutations in the PIK3CA gene, causing hyperactivation of the p110? catalytic subunit of phosphatidylinositol 3- kinase (PI3K) . PI3K p110? mutations promote tumor-like behavior in breast epithelial cells and mediate resistance to endocrine and conventional chemotherapy, correlating with poor clinical outcomes. Alpelisib is a PI3K inhibitor with activity in cell culture and tumor xenograft models. The presence of PIK3CA mutations correlates positively with sensitivity to this drug class The SOLAR-1 trial enrolled postmenopausal patients with HR+/HER2- advanced breast cancer who experienced disease progression during or after therapy with an aromatase inhibitor. Participants were given alpelisib or placebo in combination with the ER antagonist, fulvestrant. Results were analyzed according to PIK3CA status. A modest clinical benefit was observed in the PIK3CA non-mutated subgroup. Alpelisib increasing progression-free survival by 1.8 months, but the effect was not statistically significant. However, for patients with PIK3CA mutations, median progression-free survival was nearly doubled, from 5.7 months with placebo to 11.0 months with alpelisib [215]. The efficacy of alpelisib in patients with known PIK3CA mutations underscores the significance of PI3K in mediating disease progression, and the value of personalized medicine in achieving favorable treatment outcomes. Based on the positive outcomes of SOLAR-1, the FDA approved alpelisib in combination with fulvestrant for HR+/HER2- and PI3KCA-mutated advanced breast cancer in postmenopausal women and men [12].
3. Targeted therapies for triple negative breast cancer
According to data from 2015 to 2019 in the NCI SEER database, approximately 13% of breast cancer cases are HR-/HER2-, also referred to as triple negative breast cancer or TNBC. These patients are not candidates for treatment with endocrine therapy or HER2-directed agents. Until recently, drug treatment for TNBC has consisted of platinum-based chemotherapy. Among breast cancer subtypes, patients with TNBC have the lowest 5-year survival rates (77.1% vs 90.6% overall for all breast cancer subtypes according to SEER). A long-term goal of targeted therapy development has been the introduction of agents that would treat this patient population. Progress in this area has recently occurred with approval of an antibody-drug conjugate and the immune checkpoint inhibitors.
3.1 TROP2 Antibody-Drug conjugates
Sacituzumab govitecan is an antibody-drug conjugate where an antibody is used to deliver a cytotoxic chemotherapy. The antibody is directed against the Human trophoblast cell-surface antigen (Trop-2), a glycoprotein that is highly expressed in carcinomas and is linked to proliferation and invasion . The payload of sacituzumab govitecan, SN-38, is the same active metabolite generated by the prodrug, irinotecan (CPT-11) . Conjugation of SN-38 to the anti- Trop-2 antibody delivers the payload to tumor cells, where it is internalized and the hydrolysable linker is cleaved, releasing the active drug. SN-38 is also released at the cell surface within the tumor microenvironment. Studies have shown comparable efficacy between sacituzumab govitecan and SN-38 in apoptosis induction and inhibition of tumor growth . Also, SN-38 in sacituzumab govitecan is protected from glucuronidation, which helps to increase drug exposure and reduce its toxicity [13].
IMMU-132–01 was a phase I/II basket trial that evaluated sacituzumab govitecan in patients with epithelial cancers experiencing disease progression after prior chemotherapy. For patients in the trial with HR+/HER2- metastatic breast cancer, median progression-free and overall survival were both favorable . Following the positive outcomes of IMMU-132–01, TROPiCS- 02 is Phase III trial to investigate sacituzumab govitecan for HR+/HER2- metastatic breast cancer . IMMU-132–01 reported positive outcomes for patients with TNBC, as progression-free and overall survival were 5.5 and 13.0 months, respectively . Based on these outcomes, sacituzumab govitecan was granted accelerated approval by the FDA as a third-line treatment for metastatic TNBC .
3.2 Immune checkpoint inhibitors
Immune checkpoints are critical for maintaining self-tolerance and regulating the magnitude and duration of immune responses. Some cancers can co-opt these processes to their own advantage, thereby gaining immune resistance. Many immune checkpoints are regulated by ligand-receptor interactions, one example being the association of programmed death-ligand 1 (PD-L1) with programmed cell death protein 1 (PD1). The binding of PD-L1 on antigen-presenting cells with PD1 expressed on T-cells has a suppressive effect on immune activity and promotes T-cell exhaustion . PD-L1 is frequently upregulated in cancer, where the degree of PD-L1 expression on tumor cells correlates with a poor prognosis. A growing number of antibody-based therapeutics are now available that disrupt immune checkpoints and stimulate anti-tumor immune responses [14]. Pembrolizumab is a humanized monoclonal antibody against PD1. The KEYNOTE-355 trial tested the efficacy of pembrolizumab as a first-line agent against TNBC. Patients with advanced disease were administered pembrolizumab or placebo in addition to taxane or anthracycline- based chemotherapy. Median progression-free survival in the intention-to-treat population was 5.6 months in the placebo arm, and 7.5 months with pembrolizumab . Overall survival did not achieve statistical significance in the ITT population. However, in a subgroup of patients with high PD-L1 expression, median overall survival was significant at 16.1 months with placebo and 23.0 months with pembrolizumab. The enhanced efficacy of pembrolizumab with PD-L1 enrichment is consistent with clinical outcomes against other cancer types, such as melanoma, lung, renal, and biliary tract cancers .
The KEYNOTE-522 trial evaluated the efficacy of pembrolizumab in the (neo)adjuvant setting for non-metastatic TNBC, in clinical stages II-III. Patients were administered pembrolizumab or placebo in combination with neoadjuvant chemotherapy. Pembrolizumab or placebo were continued as a single-agents therapy after definitive surgery. At 3-years after randomization, event-free survival was 85% in the pembrolizumab group and 77% with placebo, showing a clear benefit for pembrolizumab in this setting. Curiously, using pembrolizumab in (neo)adjuvant care increased the rate of pathological complete responses irrespective of the PD- L1 expression level. The percentage of patients with a pathological complete response was 14.2% for PD-L1 positive patients; and 18.3% for PD-L1 negative patients. This is in contrast to the KEYNOTE-355 trial, where better outcomes were observed in patients with high PD-L1 expression. The reason for the benefit to PD-L1 negative patients with non-metastatic breast cancer is unclear. The authors speculate that PD-L1 expression may have distinct roles in immune checkpoint inhibition early, compared with advanced TNBC. Of potential relevance, tumor cells can induce the expression of PD-L1 on natural killer (NK) cells. In this context, the addition of anti-PD-L1 antibodies enhances the anti-tumor actions of PD-L1-expressing NK cells, representing a potential mechanism by which PD-L1 antibodies are effective against PD- L1 negative tumors [15]. In 2021, the FDA granted approval for pembrolizumab in the (neo)adjuvant therapy of non-metastatic and TNBC with high PD-L1 expression.
4. Targeted therapies for BRCA1/2-mutated breast cancer
Mutations in genes for BRCA 1 and 2 predispose patients to the development of breast cancer. Cumulative risk of breast cancer development by the age of 80 years old is 72% for BRCA1 mutation carriers and 69% for BRCA2 carriers.
4.1 PARP inhibitors
Poly (ADP-ribose) polymerase (PARP) 1 and 2 participate in DNA repair through the base excision repair pathway, transferring ADP-ribosyl groups onto itself and other proteins and promoting their recruitment to sites of DNA damage. BRCA 1 and 2 proteins are involved in double-strand break repair through homologous recombination. Two studies performed in 2005 demonstrated that tumors with BRCA 1 and 2 mutations are especially sensitive to PARP inhibition, which results in chromosomal instability and apoptosis . This synthetic lethality is mainly attributed to a deficiency in homologous recombination in BRCA1/2-mutated cancers, which prevents the repair of PARP inhibitor-induced damage at replication forks . PARP inhibitors also sensitize BRCA1/2-mutated cells to chemotherapeutics that damage DNA. The PARP inhibitors olaparib and talazoparib were both shown to inhibit BRCA1/2-deficient tumors synergistically with cisplatin or carboplatin in murine tumor models. Olaparib (AZD2281) is an orally-active, competitive inhibitor of NAD+ at the catalytic site of PARP1 and PARP2 . The OlympiAD trial compared the efficacy of olaparib to the physician’s choice of single-agent chemotherapy (TPC; capecitabine, eribulin, or vinorelbine) for metastatic HER2- breast cancer with germline BRCA1 or BRCA2 mutations (gBRCAm). Participants may have received up to two prior chemotherapy regimens for metastatic breast cancer, although approximately 20% were chemotherapy-naïve. In the intention-to-treat population, olaparib significantly extended progression-free survival to a median of 7.0 months, compared to 4.8 months with TPC, reducing the risk of disease progression of death by 42% . The relative benefit of olaparib on progression-free survival was similar between chemotherapy-naïve patients, and those who received prior chemotherapy. Although olaparib improved progression- free survival in the intention-to-treat population, it did not significantly improve overall survival relative to TPC. However, subgroup analysis showed that olaparib had a clinically-meaningful survival benefit that was selective in the treatment-naïve population. When used as a first-line drug for metastatic disease, overall survival with olaparib was 7.9 months longer than TPC, conferring a 49% reduction in the risk of death. But when used as a second- or third-line agent, olaparib prolonged median survival by a more moderate 1.6 months over TPC. Based on results with the primary trial outcome of progression-free survival, in 2018 the FDA approved olaparib for patients with gBRCAm HER2- metastatic breast cancer who had been treated with chemotherapy in the neoadjuvant, adjuvant, or metastatic settings.
OlympiA is an ongoing clinical trial to evaluate olaparib in the adjuvant care of gBRCAm, HER2- high-risk early breast cancer. Following surgery plus neoadjuvant or adjuvant chemotherapy, patients were given olaparib or placebo for 1-year. At 3-years after randomization olaparib significantly reduced the risk of invasive disease or death [16]. Moreover, efficacy was demonstrated in patients with TNBC, where 3-year invasive disease- free survival increased from 77% with placebo, to 86% with olaparib. Based on outcomes of the OlympiA trial, the FDA approved olaparib for the adjuvant care of patients with gBRCAm and HER2- high-risk early breast cancer following neoadjuvant or adjuvant chemotherapy.
CONCLUSION: -
Treatment options for breast cancer are evolving towards nontoxic, targeted therapies that can be tailored to each patient's tumor. Targeted therapies are now available for nearly all patients. resistance to these therapies is a continuous challenge and opportunity for learning. Biomarkers of response or prediction can be used in conjunction with robust clinical trials. Recent advances in targeted therapy have provided a more selective and effective treatment option for breast cancer patients. The primary goal of targeted therapy is to inhibit specific chemicals that promote tumor growth and survival. Some targeted medications, alone or in combination with other drugs, have already received FDA approval for the treatment of certain breast cancer subtypes, and many of them are currently in clinical trials.
REFERENCES
- W.H. Organization, 2020.https://www.who.int/news/item/03-02-2021-breast-cancer- now-most-common-form-of-cancer-who -taking -action
- N.M. Grogan Fleege, E.F. Cobain, Breast cancer management in 2021: A primer for the obstetrics and gynecology, Best Practice & Research Clinical Obstetrics and Gynecology in press (2022).
- E. Surveillance, and End Results (SEER), NCI, Cancer Stat Facts: Female Breast Cancer Subtypes, 2022.
- C. Gutierrez, R. Schiff HER2: biology, detection, and clinical implications
- H.A. Burris 3rd Dual kinase inhibition in the treatment of breast cancer: initial experience with the EGFR/ErbB-2 inhibitor lapatinib 6
- Rusnak, K. Lackey, K. Affleck, E.R. Wood, K.J. Alligood, N. Rhodes, B.R. Keith, D M. Murray, W.B. Knight, R.J. Mullin, T.M. Gilmer The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo Mol. Cancer Ther., 1 (2) (2001), pp. 85-94
- Q. Ryan, A. Ibrahim, M.H. Cohen, J. Johnson, C.W. Ko, R. Sridhara, R. Justice, R. Pazd urFDA drug approval summary: lapatinib in combination with capecitabine for previously treated metastatic breast cancer that overexpression HER-2
- s. Johnston, J. Pippen Jr., X. Pivot, M. Litchinitser, S. Sadeghi, V. Dieras, H.L. Gomez, G. Romieu, A. Manikhas, M.J. Kennedy, M.F. Press, J. Maltzman, A. Florance, L. O'Ro urke, C. Oliva, S. Stein, M. Pegram Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer
- M. Barok, H. Joensuu, J. Isola Trastuzumab emtansine: mechanisms of action and drug resistance Breast Cancer Res., 16 (2) (2014), p. 209
- E.A. Musgrove, C.E. Caldon, J. Barraclough, A. Stone, R.L. Sutherland Cyclin D as a therapeutic target in cancer
- E.P. Hamilton, J. Cortes, O. Ozyilkan, S. Chen, K. Petrakova, A. Manikhas, G. Jerusalem, R. Hegg, J. Huober, S.C. Chapman, E.L. Johnston, M. MartinnextMONARCH: Final overall survival analysis of abemaciclib monotherapy or in combination with tamoxifen in patients with HR+, HER2- metastatic breast cancer
- FDA, Drug Safety Announcement. FDA warns about rare but severe lung inflammation with Ibrance, Kisqali, and Verzenio for breast cancer., 2019. https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-rare- severe-lung-inflammation-ibrance-kisqali-and-verzenio-breast-cancer.
- D.M. Goldenberg, T.M. Cardillo, S.V. Govindan, E.A. Rossi, R.M. Sharkey Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC) Oncotarget, 6 (26) (2015), pp. 22496-2251
- R.K. Vaddepally, P. Kharel, R. Pandey, R. Garje, A.B. Chandra Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence Cancers (Basel), 12 (3) (2020)
- W. Dong, X. Wu, S. Ma, Y. Wang, A.P. Nalin, Z. Zhu, J. Zhang, D.M. Benson, K. He, M.A. Caligiuri, J. Yu The Mechanism of Anti-PD-L1 Antibody Efficacy against PD-L1-Negative Tumors Identifies NK Cells Expressing PD-L1 as a Cytolytic Effector
- A.N.J. Tutt, J.E. Garber, B. Kaufman, G. Viale, D. Fumagalli, P. Rastogi, R.D. Gelber, E. de Azambuja, A. Fielding, J. Balmana, S.M. Domchek, K.A. Gelmon, S.J. Hollingsworth, L.A. Korde, B. Linderholm, H. Bandos, E. Senkus, J.M. Suga, Z. Shao,A.W. Pippas, Z. Nowecki, T. Huzarski, P.A. Ganz, P.C. Lucas, N. Baker, S. Loibl, R. McConnell, M. Piccart, R. Schmutzler, G.G. Steger, J.P. Costantino, A. Arahmani, N. Wolmark, E. McFadden, V. Karantza, S.R. Lakhani, G. Yothers, C. Campbell, C.E. Geyer, Jr., A.C.T.S.C. Olympi, Investigators, Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer, N Engl J Med 384(25) (2021)


 Mukta Raut*
Mukta Raut*
 Shivprasad Deokar
Shivprasad Deokar
 Dr. Kawade Rajendra
Dr. Kawade Rajendra





 10.5281/zenodo.14249523
10.5281/zenodo.14249523