Purpose:
In the present study novel compounds of 1,3,4-Thiadiazole derivatives were synthesized because they are constitutes an important class of compounds for new drug development these are synthesized by using optimised chemical reactions
Methods: All these newly synthesized compounds were screened for their in vitro antimicrobial activity by an agar plate diffusion method,antidiabetic activity by ex-vivo everted intestinal sac method.
Results:
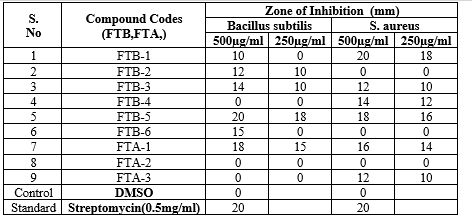
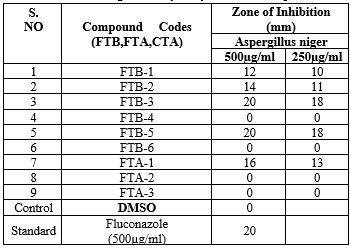
The structures of all the newly synthesized compounds were characterized by their IR, HNMR, mass spectral studies and elemental analysis. In Antibacterial activity FTB-5 and FTB-3 were effective against Bacillus subtilis and Streptococcus aureus. FTA-1 and FTB-6 is found have moderate activity against both Bacillus subtilis and Streptococcus aureus and FTB-1,FTB-2,FTB-4 and FTA-2 showed activity against Bacillus subtilis.In Antifungal activity FTB-1, FTB-5, FTA-1, FTB-3 were effective against Asregillus niger ,FTB-6, FTA-2 is found to have moderate activity.In Antidiabetic activity FTB-1 and FTA-1 were shown more significant activity when compared to standard of 1mg/ml conc.
conclusion:
The synthesized compounds FTB-3 having chlorine group in benzene ring and FTB-5 having acetoxy group in benzene ring exhibited good antimirobial activity,FTB-1 having both amine and chlorine groups in benzene ring and FTA-1having nitro group in benzene ring exhibited good antidiabetic activity .
There are number of five membered heterocyclic containing nitrogen and sulphur atom, have turned out to be a potential chemotherapeutic and pharmacotherapeutic agents but the interesting biological activities of a novel heterocyclic compound like thiadiazole has stimulated considerable research work. The biological profile of 1, 3, 4-Thiadiazole derivatives is very extensive. The broad and potent activities of thiadiazole categories such as antiinflammatory[7,8,14],anticonvulsant,[1,4,16]antihypertensive[5],antimicrobial[2,3,15,18],analgesic[17],antiepileptic[20],antiviral[10],antineoplastic[4,9]andcytotoxic[6,19],antitubercular[22,13], anti-depressant, anti-oxidant[11,12] properties, it also plays a prominent role in nature. For example, the thiazolium ring present in vitamin B1 serves as an electron sink and its coenzyme form is important for the decarboxylation of a-ketoacidosis. Furthermore, 1,3,4thiadiazoles exhibit broad spectrum of biological activities, possibly due to the presence of toxophoric N2C2S moiety. They find applications as antibacterials, antitumor agents, pesticides, herbicides, dyes, lubricants, and analytical reagents. Two schemes were used for synthesis of 1,3,4-thiadiazole derivatives in which the first step is same for two schemes and in first scheme use different benzoic acids in second step and in second scheme use different aldehydes in second step
MATERIALS AND METHODS
All chemicals and solvents were of commercial reagent grade and used as received from loba chemie and sigma aldrich Pvt.Ltd. Melting points were determined in open capillaries The purity of the compounds was checked by TLC using silica gel-G coated aluminum plates (Merck) and spots were visualized by exposing the dry plates to iodine vapours. The IR spectra were recorded onFT-IR-spercle Elmer Bruker spectrometer. The H NMR (DMSO-d6) spectra recorded on a Bruker NMR (400 MHz) and the chemical shifts were expressed in ppm (d scale) downfield from TMS. Mass spectral data were recorded on Agilent 1100 series LC-MSD .
Experimental
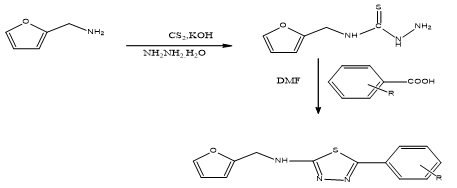
STEP-1: Synthesis of Furfuryl amino thiosemicarbazide:
Furfuryl amine (1) (0.1 mol 9.7 ml)was added to potassium hydroxide (30%,30 ml) then carbon disulfide (0.1 mol,) was added drop wise and stirred for 30 min. To this hydrazine hydrate (0.1 mol,) was added and the reaction mixture was refluxed on water bath .In between, the completion of reaction was checked by TLC. After completion of reaction, the reaction mixture was poured into the crushed ice and allow to stand for over night and then filtered the separated solid (2) , dried and recrystalised from methanol.
STEP-2: Synthesis of 1, 3, 4-Thiadiazole derivatives:
Furfuryl thiosemicarbazide (2) (0.01mol, 1.67g), various aromatic acids (0.01mol) in DMF (25ml) were taken and refluxed on water bath for 10-12 h. In between TLC was checked to the completion of reaction. After that the reaction mixture was slowly poured into crushed ice and kept overnight. The separated solid was filtered, washed with water, dried and purified by recrystallization from methanol.
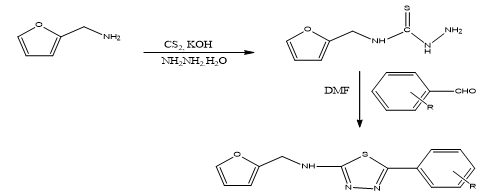
STEP-1: Synthesis of Furfuryl amino thiosemicarbazide:
Furfuryl amine (1) (0.1 mol 9.7 ml)was added to potassium hydroxide (30%,30 ml) then carbon disulfide (0.1 mol,) was added drop wise and stirred for 30 min. To this hydrazine hydrate (0.1 mol,) was added and the reaction mixture was refluxed on water bath .In between, the completion of reaction was checked by TLC. After completion of reaction, the reaction mixture was poured into the crushed ice and allow to stand for over night and then filtered the separated solid (2) ,dried and recrystalised from methanol.
STEP-2: Synthesis of 1, 3, 4-Thiadiazole derivatives:
Furfuryl thiosemicarbazide (2) (0.01mol, 1.67g), various aldehydes (0.01mol) in DMF (25ml) were taken and refluxed on water bath for 10-12 h. In between TLC was checked to the completion of reaction. After that the reaction mixture was slowly poured into crushed ice and kept overnight. The separated solid was filtered, washed with water, dried and purified by recrystallization from methanol.
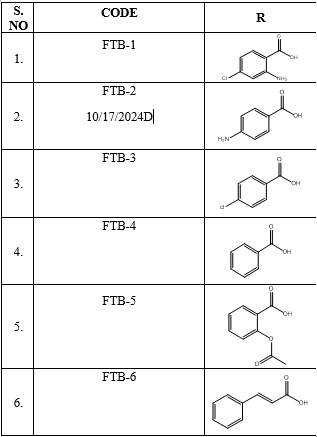
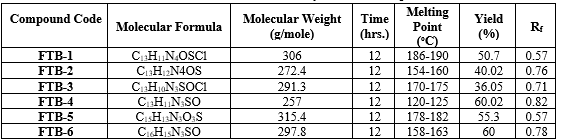
5-(2-amino-4-chlorophenyl)-N-((furan-2-yl)methyl)-1,3,4-thiadiazol-2-amine(FTB-1):
Yield 50.7% (methanol); M. P 186-190 °C; Rf = 0.57 (n-Hexane:Ethyl acetate 5:5v/v) IR(?incm1):C=C(1657.19Str),C=N(1581.60Str), C-N(1096.12Str),C-S(689.63Str), C-H(2830.82),N-H(3381.80Str),C-O(1242.47Str), C-Cl(785.04Str), N-H(3236.51Str)
1H NMR (DMSO-d6, ? in ppm): 4.649 ( 1H(NH)),7.595(2H,NH2),2.506-2.510 (2H,CH2) , 6.276-6.410 (6H,Ar-H) .
5-(4-aminophenyl)-N-((furan-2-yl)methyl)-1,3,4-thiadiazol-2-amine(FTB-2):
Yield 40.02% (methanol); M. P154-160 °C; Rf = 0.76 (n-Hexane:Ethyl acetate 5:5v/v)
IR ( ? in cm-1) :
C=C(1663.86Str),C=N(1599.90Str),C-N(1135.90Str),C-S(693.21Str),C-H(2923.21Str),N-H(3233.28Str),C-O(1282.93Str)
1H NMR (DMSO-d6, ? in ppm):
4.662 (1H,NH) , 7.591 (2H,NH2),3.701-3.842 (2H,CH2) , 6.262-6.568(7H,Ar-H).
5-(4-cholorophenyl)-N-((furan-2-yl)methyl)-1,3,4-thiadiazol-2-amine(FTB-3):
Yield 36.05% (methanol); M. P170-175 °C; Rf = 0.71 (n-Hexane:Ethyl acetate 5:5v/v) C=C(1673.26Str),C=N(1581.64Str),C-N(1096.12Str),C-S(676.84Str),C-H(2804.78Str),C-O(1274.68Str),C-Cl(752.82Str)N-H(3381.80Str)
1H NMR (DMSO-d6, ? in ppm):
3.839(1H,NH) ,3.163-3.696 (2H,CH2), 6.276-6.401(7H,Ar-H)
13CNMR(DMSOd6,ðinppm):
142.05(1C),109.41(2C),40.16(5C),148.32(6C),118.52(7C),128.32(8C),128.48(9C),128.66(10C),128.89(12C),129.21(13C),123.64(11C).
MS: m/z -271.2(M+)
N-((furan-2-yl)methyl)-5-phenyl-1,3,4-thiadiazol-2-amine(FTB-4):
Yield 60.02% (methanol); M. P120-125°C; Rf = 0.82 (n-Hexane:Ethyl acetate 5:5v/v)
IR (? in cm-1) :
C=C(1607.64Str),C=N(1529.30Str),C-N(1176.79Str),C-S(702.25Str),C-H(2928.84Str),N-H(3220.84Str),C-O(1322.00Str)
1H NMR (DMSO-d6, ? in ppm):
4.703(1H,NH), 2.510-3.841(2H,CH2) ,7.508-7.653 (8H,Ar-H)
2-(5-((furan-2-yl)methyl amino)-1,3,4-thiadiazol-2-yl)phenyl acetate(FTB-5):
Yield-50.3% (methanol); M. P178-182°C; Rf = 0.57(n-Hexane:Ethyl acetate 5:5v/v)
IR(? in cm-1):
C=C(1661.52Str),C=N(1607.03Str),C-N(1287.57Str),C-S(690.65Str),C-H(2941.37Str),N-H(3231.68Str),C-O(1329.43Str)
1HNMR(DMSOd6,?inppm):
5.229(1H,NH),2.5092.513,(H,CH2),6.275-6.432(7H,Ar-H). 13CNMR(DMSOd6,ðinppm):
142.10(1C),109.16(2C),40.17(5C),149.20(6C),117.01(7C),130.22(8C,9C,10C,12C,13C),119.05(11C), 171.86(12C). MS: m/z -316.9(M+)
5-cinnamyl-N-((furan-2-yl)methyl)-1,3,4-thiadiazol-2-amine(FTB-6):Yield-60% (methanol); M. P158-163 °C; Rf = 0.78(n-Hexane:Ethyl acetate 5:5v/v)
IR ( ? in cm-1) :
C=C(1676.25Str),C=N(1623.66Str),C-N(1181.73Str),CS(698.65Str),C-H(2923.42Str),N-H(3253.18Str),C-O(1310.60Str)
1H NMR (DMSO-d6, ? in ppm):
4.503(1H,NH ), 3.699 (2H,Ali-CH), 2.559-2.511(2H,CH2)6.290-6.65(8H,Ar-H)
N-((furan-2-yl)methyl)-5-(4-nitrophenyl)-1,3,4-thiadiazol-2-amine(FTA-1):
Yield 66% (methanol); M. P190-192°C; Rf = 0.65(n-Hexane:Ethyl acetate 5:5v/v)
IR ( ? in cm-1) :
C=N(1610.11Str),CN(1345.24Str),C-S(678.06Str),C-H(2993.53Str),N-H(3345.10Str),C-O(1273.03Str)NO2(1512.80Str)
1H NMR (DMSO-d6, ? in ppm):
3.339(1H,NH),4.8374.851(2H,CH2),6.303-6.422(7H,Ar-H) .
N-((furan-2-yl)methyl)-5-(3,4,5-trimethoxyphenyl)-1,3,4-thiadiazol-2-amine(FTA-2):
Yield 70% (methanol); M. P 225-227°C; Rf = 0.82 (n-Hexane:Ethyl acetate 5:5v/v)
IR (? in cm-1) :
C=N(1610.11Str),C-N(1345.24Str),C-S(678.06Str),C-H(2993.53Str),N-H(3345.10Str),C-O(1273.03Str)NO2(1512.80Str)
1H NMR (DMSO-d6, ? in ppm):
3.339(1H,NH),2.5062.510(2H,CH2),6.303-6.422(7H,Ar-H) .
4-(5-((furan-2-yl)methylamino)-1,3,4-thiadiazol-2-yl)phenol(FTA-3):
Yield-75.5% (methanol); M. P 194-197°C; Rf = 0.82 (n-Hexane:Ethyl acetate 5:5v/v)
IR ( ? in cm-1) :
C=N(1578.71Str),CN(1243.43Str),CS(641.16Str),CH(2990.48),NH(3190.80Str),CO(1323.68Str)O-H(3614.86Str)
1H NMR (DMSO-d6, ? in ppm):
4.808(1H,NH), 7.576 (2H,(OH), 3.367 2H,(CH2) , 6.289-6.795(7H,Ar-H
BIOLOGICAL EVALUATION
Antimicrobial activity:
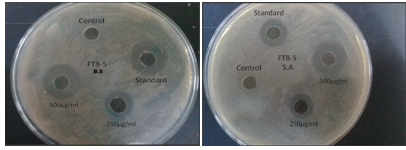
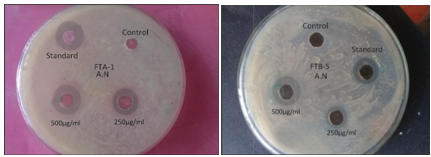
The newly synthesized compounds were screened against bacterial and fungicidal activities by an agar plate diffusion method and potato dextrose agar (PDA) diffusion method respectively . All the compounds were screened for their antibacterial activity against Bacillus subtilis, Staphylococcus aureus, as well as antifungal activity against Aspergillus niger , DMSO was used as a vehicle to get the desired concentration of compounds to test upon microbial strains. Streptomycin and fluconazole were used as standards for antibacterial and antifungal activities respectively. The experiment was done in triplicate, and average values were calculated. The results of antibacterial and antifungal are summarized in (table 5) and (table 4) respectively.
Antidiabetic activity:
Exvivo Rat Everted Sac Model:( Nithin Gupta et al.,2011)was used for antidiabetic activity Wister rats weighing 180-200g are anesthetized with petroleum ether. A midline incision is made & Small intestine from the ligament of treitz to the ileocaecal junction with a length of 6-7 cm is rapidly removed and everted with a glass rod. Sac is securely ligated at both ends and filled with Krebs-Henseleit bicarbonate buffer solution (ph7.4)containing 0.4% glucose and pregassed with 95% O2.Sac is incubated at 370C in a glass vessel containing the same buffer solution and gassed with O2.After 5 min ,the drug solution is added to the glass vessel and ,the preparation is further incubated.
Intestinal transport studies:
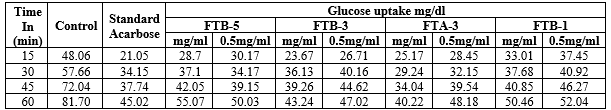
At the end of the incubation period (15,30,45,60 min) the sacs was removed from the organ bath ,blotted by a standardized procedure . The contents of the sac were drained through a small incision into a test tube . So as to empty the sac completely , gentle pressure was applied ;the glucose content was determined by using Gluco-kit and autoanalyser.Results were shown in table-6. I have obtain approval from the IAEC/ CPCSEA for this animal study.


 Darla Kristuraju*
Darla Kristuraju*
 Nandikatti Vijayalakshmi
Nandikatti Vijayalakshmi
 10.5281/zenodo.13944434
10.5281/zenodo.13944434