Abstract
The performance of an existing medicinal molecule in terms of patient compliance, safety, and efficacy can be greatly enhanced by evolving it from a traditional form to a unique delivery mechanism. An old medication molecule can be given new life as a Novel Drug Delivery System. The limitations of the conventional drug delivery methods are addressed by the innovative drug delivery system, which is a novel method of drug administration. A significant improvement in the ability to release a drug at a specified spot and rate is possible with a novel drug delivery system that is properly developed. Pharmaceutical companies are working to create novel drug delivery systems in order to give medications to patients effectively and with fewer side effects. The fundamentals of novel drug delivery systems, as well as their various varieties, are covered in this article. The scientific requirements to be incorporated in novel drug delivery systems, such as nanoparticles, microemulsions, matrix systems, solid dispersions, liposomes, solid lipid nanoparticles, and so on, can be met by modern phytopharmaceuticals research, though, by determining pharmacokinetics, mechanism of action, site of action, required precise dose, etc.
Keywords
Novel drug delivery system, Conventional drug delivery, Pharmaceutical companies, Pharmacokinetics.
Introduction
1.1 Overview of Drug Delivery System (DDS)
Drug delivery systems (DDS) are specialized technologies designed to transport therapeutic agents to targeted sites in the body with precision, efficiency, and controlled release. Over the past few decades, DDS have revolutionized the field of medicine by improving drug bioavailability, reducing side effects, and enhancing patient compliance.[1] Conventional drug delivery methods, such as oral tablets, injections, and topical applications, often suffer from limitations such as poor solubility, rapid clearance, and lack of specificity. As a result, advanced drug delivery systems have been developed to overcome these challenges and enhance therapeutic outcomes.
Modern DDS incorporate novel materials, nanotechnology, and bioengineering principles to ensure site-specific drug release, minimize systemic toxicity, and maintain therapeutic efficacy over prolonged periods.[2] These systems include liposomes, nanoparticles, polymeric micelles, and hydrogels, among others. The development of stimuli-responsive drug delivery systems has emerged as a promising approach to achieving controlled and targeted drug release based on specific internal or external triggers.
1.2 Need for stimuli-Response Drug Delivery
Stimuli-responsive drug delivery systems (SRDDS) offer an innovative approach to addressing the limitations of conventional DDS by responding to specific biological or external stimuli. These systems leverage environmental cues such as pH, temperature, enzymes, light, and magnetic fields to trigger the release of therapeutic agents in a controlled manner.[3]
The primary advantages of SRDDS include
Site-Specific Drug Release: By responding to localized stimuli, SRDDS minimize off-target effects and enhance drug concentration at the disease site.[4]
Reduced Side Effects: Controlled drug release reduces systemic toxicity, thereby improving patient safety.
Enhanced Drug Stability: Encapsulation of drugs within stimuli-responsive carriers protects them from premature degradation.
Therapeutic Outcomes: Timed and sustained drug release maintains optimal therapeutic levels, reducing the frequency of administration and improving patient adherence.[5]
1.3 Relevance to Personalized Medicine
Personalized medicine aims to tailor therapeutic interventions to individual patient characteristics, including genetic makeup, disease progression, and response to treatment. Stimuli-responsive DDS play a significant role in advancing personalized medicine by enabling:
Targeted Drug Delivery: Precision targeting based on disease biomarkers and physiological conditions ensures patient-specific treatment.[6]
Controlled Drug Release: Adaptive drug release mechanisms optimize therapeutic efficacy based on Individual needs.
Minimized Adverse Reactions: Personalized drug delivery minimizes side effects by delivering the right drug at the right dose to the right location.[7] By integrating SRDDS with diagnostic tools and patient-specific data, researchers and clinicians can develop customized treatment regimens that enhance therapeutic efficiency and improve patient outcomes.

Figure 1:- Steps on the road to personalised medicines
2. Types Of Stimuli-Responsive Drug Delivery
Stimuli-responsive DDS can be broadly classified into internal and external stimuli-responsive systems. Internal stimuli include pH, enzymes, and redox potential, while external stimuli involve external triggers such as light, heat, magnetic fields, and ultrasound. This section focuses on externally triggered stimuli-responsive drug delivery systems.[8]
2.1 External Stimuli
External stimuli-responsive DDS rely on externally applied triggers to initiate drug release.[9] These systems offer precise control over drug delivery and can be activated on demand. The primary external stimuli used in SRDDS include light, heat, magnetic fields, and ultrasound.
2.1.1 Light-responsive System
Light-responsive drug delivery systems utilize specific wavelengths of light (UV, visible, or near-infrared) to trigger drug release.[10] These systems incorporate photo-sensitive materials such as:
Photodegradable Polymers: Undergo light-induced cleavage to release drugs.
Photo-switchable Molecules: Change conformation in response to light, altering drug encapsulation and release.
Ophthalmic Drug Delivery: Light-triggered hydrogels release drugs for treating eye diseases.
Dermal Applications: Light-sensitive formulations provide controlled transdermal drug delivery.[11]
2.1.2 Heat-responsive System

Figure 2:- Schematic illustrating the multifactorial effects of temperature hypothermia from its stand alone cytotoxicity to inducing synergistic cytotoxic effects when combined with drug delivery system . Heat-sensitive drug delivery systems leverage temperature changes to trigger drug release.[12] These systems utilize materials such as:
Thermo-Responsive Polymers: Polymers like poly(N-isopropylacrylamide) (PNIPAM) undergo sol-gel transitions in response to temperature changes.
Lipid-Based Systems: Thermosensitive liposomes release drugs upon heating.
Gold and Carbon Nanomaterials: Convert external heat stimuli into localized temperature increases for drug activation.[13]
Applications:
Hyperthermia-Induced Drug Release: Enhances localized chemotherapy in tumors.
Localized Infection Treatment: Temperature-sensitive hydrogels deliver antibiotics to infected sites.
Transdermal Drug Delivery: Temperature-induced drug permeation enhances skin penetration.[14]
2.1.3 Magnetic-responsive System

Figure 3:- Magnetic particle options commonly used for magnetic soft composites
(a) magnetic flux density with respect tomagnetic field H curves of soft magnetic and hard magnetic particles
(b) actuation mechanism of soft magnetic composites
(c) actuation mechanism of hard magnetic composites
Magnetic-responsive DDS utilize external magnetic fields to control drug release. These systems are designed using:
Magnetic Nanoparticles (MNPs): Composed of iron oxide or cobalt, MNPs respond to external magnetic fields.
Magnetothermal Therapy: Magnetic fields generate heat to release drugs.[15]
Magnetically Guided Targeting: Magnetic fields steer drug carriers to specific locations.
Controlled Insulin Release: Magnetic fields regulate insulin release in diabetes reatment.
2.1.4 Ultrasound Responsive System

Figure 4:- Illustration of ultra- sound responsiveness of block copolymer nanoparticles (different colors represent different nanoparticles). The initially presented thermodynamic state of the nanoparticle dictates the ultrasound responsiveness. Regulating factors are the Ts and solvent. The ultrasound responsive rate is dictated by the Tu; a higher temperature leads to a faster response rate. Reproduced with permission from ultrasound-responsive drug delivery systems utilize acoustic waves
Gas-Encapsulated Microbubbles: Collapse under ultrasound to release drugs.[16]
Ultrasound-Responsive Liposomes: Release drugs upon ultrasound stimulation.
3. Design And Mechanism Of Action
3.1 Material Sciences: Polymers, Nanoparticles and Hydrogel
The development of drug delivery systems relies heavily on material science, particularly in designing polymers, nanoparticles, and hydrogels. Polymers, such as poly(lactic-co-glycolic acid) (PLGA), polyethylene glycol (PEG), and chitosan, are widely used for their biocompatibility, controlled degradation, and tunable release properties.[17]
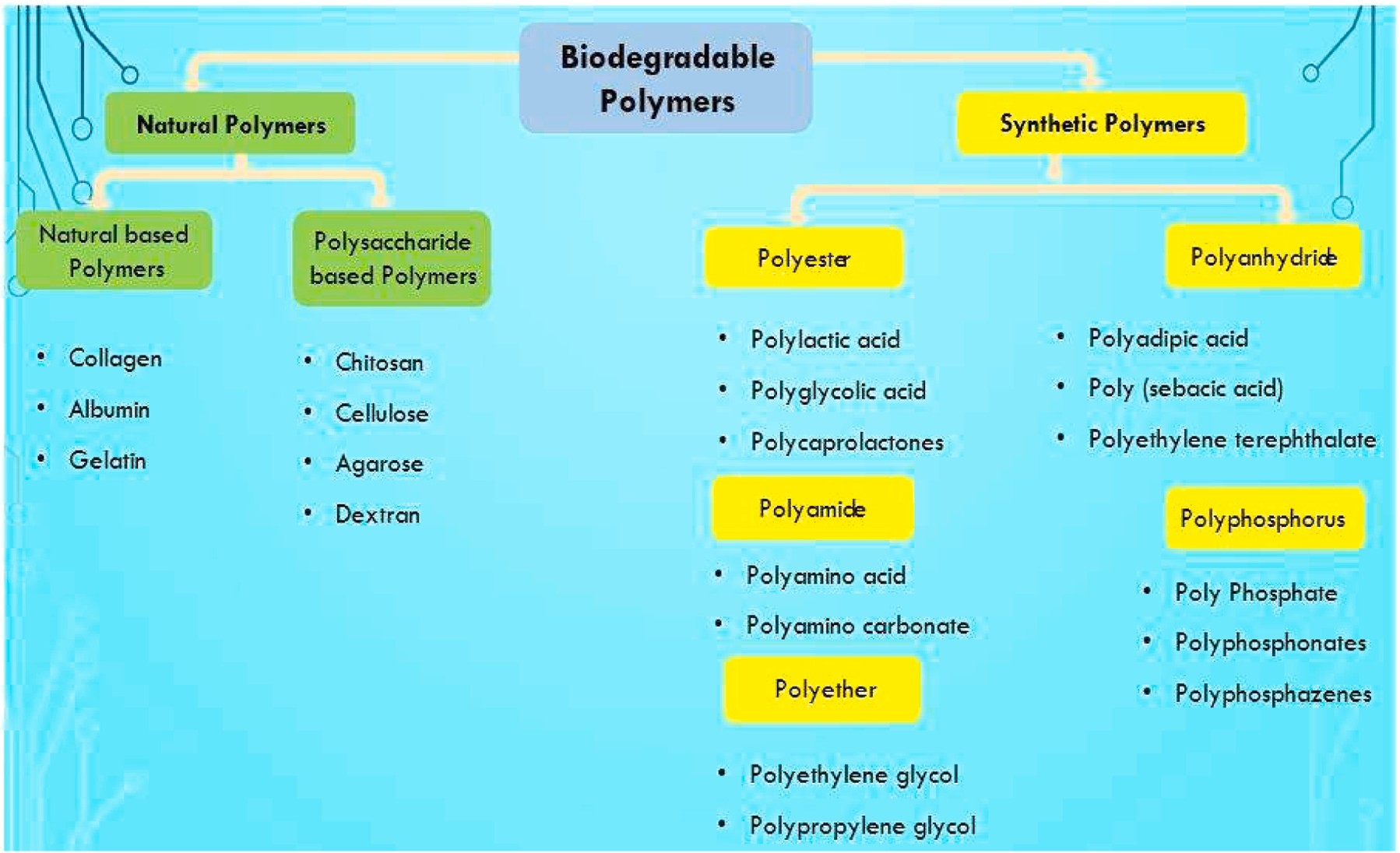
Figure 5:- Classification of polymers
Nanoparticles, including liposomes, dendrimers, and metal-based carriers, offer targeted delivery and improved drug solubility. Hydrogels, composed of crosslinked polymer networks, provide a hydrated environment for drugs and enable stimuli-responsive release.

Figure 6:- Multiscale properties of the hydrogel These materials collectively enhance the stability, bioavailability, and efficacy of therapeutic agents.
3.2 Surface Engineering for Drug Release Control
Surface engineering plays a critical role in controlling drug release kinetics, reducing immunogenicity, and improving targeting efficiency. Techniques such as PEGylation enhance circulation time by reducing opsonization and clearance. Functionalization with ligands (e.g., antibodies, peptides) allows for active targeting of specific cell receptors.[18] Layer-by-layer assembly and surface grafting enable precise modulation of drug diffusion and release profiles. Such surface modifications ensure that drugs reach their intended site of action with minimal off-target effects.[19]
3.3 Triggering Mechanisms: A Closer Look
Smart drug delivery systems incorporate triggering mechanisms to release drugs at the right time and location. pH-sensitive carriers exploit tumor or inflammatory site acidity to induce drug release. Temperature-sensitive systems respond to localized hyperthermia, often induced by external stimuli like infrared radiation.[20]
3.4 Targeting Mechanisms for Precision Medicine
Precision medicine aims to deliver therapeutics based on patient-specific factors, requiring advanced targeting mechanisms. Passive targeting leverages the enhanced permeability and retention (EPR) effect seen in tumors and inflamed tissues.[21] Active targeting uses molecular recognition by incorporating ligands that bind to disease-specific markers, such as folate receptors in cancer. [22]
4. Application In Personalized Medicine
4.1 Cancer Therapy
4.1.1 Tumor Microenvironment Targeting
The tumor microenvironment (TME) presents unique physiological features such as hypoxia, low pH, and high interstitial fluid pressure, which can be exploited for targeted drug delivery. Nanocarriers engineered with pH-sensitive coatings release drugs specifically in acidic tumor environments.[23]

Figure 7:- Depicts a full comprehension of cellular and non-cellular behavior and actions required for prevention and therapy of TME.
4.1.2 Overcoming Multidrug Resistance
Multidrug resistance (MDR) remains a major challenge in cancer therapy, often caused by efflux pumps such as P-glycoprotein (P-gp). Nanocarriers can bypass these mechanisms by facilitating intracellular drug release. Co-delivery systems incorporating chemotherapeutics with MDR inhibitors, such as siRNA targeting efflux proteins, enhance drug retention in cancer cells.[24]
4.2 Diabetes and Insulin Delivery
Nanotechnology-based insulin delivery systems aim to replace traditional injections with more patient-friendly options.

Figure 8:- Diabetes and insulin delivery
4.3 Cardiovascular Diseases

Table 1:- Example of clinical application of biomarkers and tests in cardiovascular disease
Targeted drug delivery in cardiovascular diseases (CVDs) focuses on reducing side effects and improving therapeutic outcomes.[25] Nanoparticles loaded with anti-inflammatory drugs selectively target atherosclerotic plaques, reducing inflammation without affecting systemic immunity.[23] Thrombolytic nanoparticles dissolve blood clots in a site-specific manner, minimizing the risk of hemorrhage. Gene therapy approaches using lipid nanoparticles deliver RNA-based treatments to correct genetic abnormalities associated with CVDs.[26]
4.4 Neurological Disorders
The blood-brain barrier (BBB) presents a significant challenge in treating neurological disorders. Nanocarriers engineered with BBB-penetrating ligands facilitate drug delivery to the brain. Liposomes, dendrimers, and polymeric nanoparticles can encapsulate neuroprotective agents, ensuring sustained release and prolonged therapeutic effects.[27]
4.5 Infectious Diseases
Advanced drug delivery systems play a crucial role in managing infectious diseases, particularly antibiotic-resistant infections.[28] Liposomal and polymeric nanoparticles enhance the bioavailability of antimicrobial agents, ensuring higher drug concentrations at the infection site. CRISPR-loaded nanoparticles provide a novel strategy for targeting bacterial genomes, eliminating resistant strains. Immunomodulatory nanoparticles enhance the host immune response, improving vaccine efficacy and infection control.[29]
5. Clinical Translation and Challenges
5.1 Safety and Biocompatibility
Ensuring the safety and biocompatibility of drug delivery systems (DDS) is a fundamental prerequisite for their clinical translation.[30] Nanoparticles and exosome-based DDS must be evaluated for cytotoxicity, immunogenicity, and potential long-term effects. Studies focus on understanding biodistribution, accumulation in non-target organs, and elimination pathways. Additionally, biocompatibility assessments consider inflammatory responses and potential genotoxic effects.[31]
Strategies for Enhancing Safety:
Surface modifications to improve biocompatibility Use of biodegradable materials and In-depth in vivo and in vitro toxicological studies [32].

Figure 9:- Discovery and preclinical phase of drugs
5.2 Regulatory Considerations
Regulatory approval for stimuli-responsive DDS presents significant challenges due to complex formulations and novel mechanisms of action. Agencies like the FDA and EMA require extensive preclinical and clinical evaluations to ensure efficacy and safety.[33]
5.3 Scale-up and Manufacturing Challenges

Figure 10:- Overview of the key milestones in the development of personalised medicine (PM) and also illustrates how the field has evolved overtime
The transition from laboratory-scale synthesis to large-scale production involves several challenges, including batch-to-batch consistency, reproducibility, and cost-effectiveness. Advanced manufacturing techniques such as microfluidics and high-throughput screening aid in overcoming these challenges.[34]
5.4 Patient-Specific Customization and Ethics
Personalized medicine requires DDS to be tailored to individual patient profiles, which raises ethical and logistical concerns. Challenges include ensuring equitable access, addressing privacy concerns in genetic profiling, and managing regulatory hurdles.[35]
Ethical Considerations:
Informed consent for personalized treatments , Addressing socioeconomic disparities in access and Developing frameworks for ethical AI applications in medic.[36]
6. Future Perspective
6.1 Emerging Technologies in Stimuli-Responsive DDS
New materials and mechanisms are being explored to enhance the precision and responsiveness of DDS. Examples include pH-responsive hydrogels, enzyme-triggered nanoparticles, and magnetically controlled systems.[37]
Innovations in the Field:
Smart polymers with tunable release properties , CRISPR-based gene delivery systemsand Hybrid nanocarriers combining multiple stimuli responses .[38]
6.2 Potential for AI and Machine Learning Integration

Figure 11:- Potential for AI and machine learning Integration
AI Applications in DDS:
Predictive modeling for drug formulation , machine learning-based screening of nanoparticle efficacy and AI-assisted diagnostics for personalized therapy selection .[39]
6.3 Nanotechnology in Personalized Medicine

Figure 12:- A schematic representation of nanotechnology used in personalised medicine
6.4 Future Research Directions and Innovations
Research efforts continue to focus on improving precision, reducing toxicity, and enhancing patient compliance in DDS applications.[40]
Future Innovations:
Development of biodegradable nanocarriers , Exploration of exosome-based DDS for gene therapy and Integration of multi-functional platforms for combination therapy.[41]
7. CONCLUSION
7.1 Summary of Current Innovations
The field of drug delivery systems (DDS) has witnessed remarkable advancements, particularly in the development of stimuli-responsive DDS, which hold the potential to revolutionize targeted therapy. These systems are designed to release drugs in response to specific physiological or external stimuli, such as pH, temperature, enzymes, or light, ensuring precise drug localization and minimizing systemic side effects.[42] One of the most promising applications of stimuli-responsive DDS is in oncology. Traditional cancer treatments, such as chemotherapy, often result in significant toxicity to healthy tissues.[43] However, advanced DDS can enhance the therapeutic index by selectively delivering chemotherapeutic agents to tumor sites based on unique tumor microenvironment triggers, such as acidic pH or hypoxia. This precision reduces adverse effects and improves patient compliance.[44] In neurology, DDS is making strides in overcoming the blood-brain barrier (BBB), a major challenge in treating neurodegenerative diseases and brain tumors.[45] Smart nanoparticles and liposomes capable of responding to endogenous triggers or external ultrasound stimulation have shown promise in enhancing drug penetration into the brain, thereby improving treatment efficacy for conditions like Alzheimer’s, Parkinson’s, and glioblastoma.[46]
Furthermore, infectious disease management has benefited from DDS innovations. Controlled-release antibiotic formulations can enhance drug bioavailability, reduce dosing frequency, and combat antibiotic resistance by maintaining optimal drug concentrations at the infection site.[47] Additionally, vaccine delivery via nanoparticles and hydrogels has improved immunogenicity and long-term protection against pathogens.[48] Despite these advances, several challenges remain. The safety profile of novel DDS, potential long-term toxicity, and immune system interactions need thorough investigation. Moreover, regulatory approval processes must evolve to accommodate the complexity of these systems. Scalable manufacturing techniques also require optimization to ensure cost-effectiveness and widespread accessibility.[49]
7.2 Future Impact on Personalized Healthcare

Figure 13:- Improvement of personalised medicines
As drug delivery technologies continue to evolve, their integration with artificial intelligence (AI), nanotechnology, and bioinformatics will drive the next frontier of personalized medicine. Personalized drug delivery focuses on tailoring treatment strategies to individual patient profiles based on genetic, epigenetic, and physiological data, significantly improving therapeutic efficacy and minimizing adverse reactions. The application of AI in DDS is transforming the way drugs are designed, formulated, and administered.[50] Machine learning algorithms can analyze vast datasets to predict patient responses, optimize drug dosages, and enhance DDS design for maximum efficiency. AI-driven systems can also facilitate real-time monitoring of treatment progress through wearable biosensors, allowing dynamic adjustments to drug release based on patient needs. Nanotechnology plays a pivotal role in advancing personalized healthcare. Engineered nanoparticles with tunable properties can be customized for targeted therapy, ensuring precise interaction with diseased tissues while sparing healthy cells. Functionalization with ligands, antibodies, or peptides allows highly specific targeting, making treatments more effective and reducing systemic toxicity. Furthermore, the convergence of biotechnology and data science will enable the development of smart DDS capable of responding to an individual’s real-time biological signals.[51] Implantable or injectable smart systems can adjust drug release rates based on continuous monitoring of biomarkers, creating a feedback loop that ensures optimal therapeutic outcomes. In the coming years, advances in 3D bioprinting and organ-on-a-chip technology will further refine personalized drug testing, reducing reliance on traditional trial-and-error approaches. These innovations will help predict individual drug responses more accurately, streamlining the drug development pipeline and expediting the delivery of effective treatments to patients.[52]
Overall, the integration of DDS with AI, nanotechnology, and personalized medicine will redefine modern healthcare, shifting from a one-size-fits-all approach to tailored therapeutic interventions. This transformation will not only enhance treatment outcomes but also improve patient quality of life and reduce healthcare costs. The future of drug delivery is poised to be dynamic, precise, and deeply personalized, ushering in a new era of medical innovation.[53]
REFERENCES
-
-
-
- Reddy PD, Swarnalatha D. Recent advances in novel drug delivery systems. Int J PharmTech Res. 2010;2(3):2025-7.
- Muller CC. Physicochemical characterization of colloidal drug delivery systems such as reverse micelles, vesicles, liquid crystals and nanoparticles for topical administration. Eur J Pharm Biopharm. 2004;58(2):343-56. doi:10.1016/j.ejpb.2004.03.028
- Sharma A, Sharma US. Liposomes in drug delivery: Progress and limitations. Int J Pharm. 1997;154(2):123-40. doi:10.1016/S0378-5173(97)00135-X
- Lau JR, Geho WB, Snedekar GH, inventors; SDG INC, an Ohio Corporation, assignee. Targeted liposomal drug delivery system. US patent 20100209492. 2010 Aug 19.
- Takagi A, Yamashita N, Sonobe T, inventors; Astellas Pharma INC, Tokyo, assignee. Intracellular drug delivery improving liposomes. US patent 20070286898. 2007 Dec 13.
- Lau JR, Geho WB, Snedekar GH, inventors. Targeted liposomal drug delivery system. US patent 20070104777. 2007 May 10.
- Zhang Y, Luo B, Iyer L, inventors. Liposomal delivery vehicle for hydrophobic drugs. US patent 20070014845. 2007 Jan 18.
- Yamauchi H, Morita H, Kikuchi H, inventors; Daiichi Pharmaceuticals Co. LTD, assignee. Liposomes and liposomal dispersion. US patent 20020182248. 2002 Dec 5.
- Verma RK, Garg S. Current status of drug delivery technologies and future directions. Pharm Tech On-Line. 2001.
- Torchilin VP. Structure and design of polymeric surfactant-based drug delivery systems. J Control Release. 2001.
- Turos E, Cormier R, Kyle DE, inventors; University of South Florida, FL, assignee. Polyacrylate nanoparticle drug delivery. US patent 20100278920. 2010 Nov 4.
- Sung H, Liang H, Tu H, inventors. Nanoparticle for protein drug delivery. US patent 20090155374. 2009 Jun 18.
- Jacobson GB, Zare RN, Markides KE, Shinde RR, inventors. Encapsulated nanoparticle for drug delivery. US patent 20080095856. 2008 Apr 24.
- Lobl TJ, Schloss JV, Nagy AI, Pananen JE, inventors; Neurosystec Corporation, Valencia, CA, assignee. Nanoparticle drug formulation. US patent 20080145439. 2008 Jun 19.
- Singh AN, Mahanti B, Bera K. Novel drug delivery system & its future: An overview. Int J Pharm Engin. 2021.
- Wu D, Chu CC, Carozza J, inventors. Injectable microspheres. US patent 20110151004. 2011 Jun 23.
- Sah HK, inventor; SK Chemicals Co. Ltd., KR, assignee. Method for producing microspheres loaded with drugs and microspheres loaded with drugs produced thereby. US patent 20090318569. 2009 Dec 24.
- Shah S. Novel drug delivery carrier: Resealed erythrocytes. Int J Pharm Biosci. 2011.
- Raut D, Sakhare R, Dadge K, Halle PD. Resealed erythrocytes drug delivery: A review. Int J Pharm Chem. 2013.
- Gupta A, Mishra AK, Bansal P, Kumar S, Gupta V, Singh R, Kalyan GS. Cell-based drug delivery system through resealed erythrocytes: A review. Int J Pharm Sci Drug Res. 2010.
- Magnani M, Rossi L, Biagiotti S, Bianchi M, inventors. Drug delivery system. US patent 20120141540. 2012 Jun 7.
- Grimald S, Lisi A, Cinti C, inventors; CNR Consiglio Nazionale Delle Ricerche, Roma, assignee. US patent 20110262415. 2011 Oct 27.
- Yang VC, Kwon YM, Chung HS, Yang AJ, inventors. Erythrocyte encapsulated L-asparaginase for enhanced acute lymphoblastic leukemia therapy. US patent 20100284982. 2010 Nov 11.
- Madhar NVS, Saini A. Niosomes: A novel drug delivery system. Int J Res Pharm Chem. 2011.
- Alcantor N, Williams EC, Toomey R, inventors; University of South Florida, FL, assignee. Niosome hydrogel drug delivery systems. US patent 20100068264. 2010 Mar 18.
- Arunachalam A, Karthikeyan M, Vinay Kumar D, et al. Transdermal drug delivery system: A review. Curr Pharm Res. 2010.
- Sharma B, Saroha K, Yadav B. Sonophoresis: An advanced tool in transdermal drug delivery system. Int J Curr Pharm Res. 2011.
- Easterbrook TJ, Gosden E, Meyer E, inventors. Transdermal drug delivery device. US patent 20110190716. 2011 Aug 4.
- Tang J, inventor. Stabilized transdermal drug delivery system. US patent 20110182949. 2011 Jul 28.
- Nisato G, Baret JC, inventors; Koninklijke Philips Electronics N.V., NC, assignee. Transdermal drug delivery patch. US patent 20100222751. 2010 Jun 10.
- Heiati H, Weimann L, inventors; Pharmapatch LLC, San Diego, CA, assignee. Multiple nozzle transdermal drug delivery system. US patent 20100143448. 2010 Sep 2.
- Vinod KR, Reddy R, Banji D, Reddy V, Sandhya S. Critical review on mucoadhesive drug delivery systems. Hygeia J Drugs Med. 2012.
- Yoon HJ, Jang WD. Polymeric supramolecular systems for drug delivery. J Mater Chem. 2010
- Zerbe HG, Paiement N, inventors. Oral mucoadhesive dosage form. US patent 20110028431. 2011 Feb 3.
- Sambasivam M, inventor; Convatec Technologies Inc., NV, assignee. Ostomy devices with mucoadhesives. US patent 2010010064. 2010 Apr 22.
- David AE, Zhang R, Park YJ, Yang AJM, Yang VC, inventors. Mucoadhesive vesicles for drug delivery. US patent 20090232899. 2009 Sep 17.
- Gupta S, Singh RP, Sharma R, Kalyanwat R, Lokwani P. Osmotic pumps: A review. Int J Comprehen Pharm. 2011.
- Patel H, Patel U, Kadikar H, Bhimani B, Daslaniya D, Patel G. A review on osmotic drug delivery system. Int Res J Pharm. 2012.
- Nghiem T, Jackson G. Multiparticulate osmotic delivery system. US patent 20090004281. 2009 Jan 1.
- Patel HB. Oral osmotic drug delivery system. US patent 20080248114. 2008 Oct 9.
- Kidane A, Bhatt PP. Osmotic drug delivery system. US patent 20070254032. 2007 Nov 1.
- Kumar A, Sharma P, Banik A. Microencapsulation as novel drug delivery system. Int Pharm Sci. 2011.
- Allen TM, Cullis PR. Liposomal drug delivery systems: From concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36-48. doi:10.1016/j.addr.2012.09.03
- Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov. 2014;13(11):813-27. doi:10.1038/nrd4333
- Rizzello L, Pompa PP. Nanosilver-based antibacterial drugs and devices: Mechanisms, methodological drawbacks, and guidelines. Chem Soc Rev. 2014;43(5):1501-18. doi:10.1039/C3CS60218D
- Huser V, Sincan M, Cimino JJ. Developing genomic knowledge bases and databases to support clinical management: Current perspectives. Pharmacogenomics Pers Med. 2014;7:275-83. doi:10.2147/PGPM.S49904
- Akhondzadeh S. Personalized medicine: A tailor-made medicine. Avicenna J Med Biotechnol. 2014;6(4):191. PMID:25414780
- Wilsdon T, Barron A, Edwards G, Lawlor R. Report of the benefits of personalised medicine to patients, society and health care system. European Biopharmaceutical Enterprises (EBE) and European Federation of Pharmaceutical Industries and Associations (EFPIA). 2018.
- Edward A, Ginsburg GS, Silver M. The Personalized Medicine Coalition: Goals and strategies. Am J Pharmacogenomics. 2005;5(6):345-55. doi:10.2165/00129785-200505060-00002. PMID:16336000
- Sanzo MD, Cipollini L, Borro M, Russa RL. Clinical application of personalized medicine: A new paradigm and challenge. Curr Pharm Biotechnol. 2017;18(3):194-203. doi:10.2174/1389201018666170224105600. PMID:28240172
- Juan MA, Rey CD, Bardey D, et al. Personalized medicine: Closing the gap between knowledge and clinical practice. Autoimmun Rev. 2016;15(8):833-42. doi:10.1016/j.autrev.2016.06.005. PMID:27302209
- Florence AT, Lee VHL. Personalized medicines: More tailored drugs, more tailored delivery. Int J Pharm. 2011;415(1-2):29-33. doi:10.1016/j.ijpharm.2011.04.047. PMID:21565262
- Chivda VP. Tailor-made medicine: A step towards future of diagnostic and therapeutic. PharmaTutor. 2015;3(11):25-28.


 Meet Agrawal*
Meet Agrawal*














 10.5281/zenodo.14835771
10.5281/zenodo.14835771