Abstract
The principal aim of this review is to systematically aggregate comprehensive data pertaining to herbal drugs employed in the therapeutic intervention of cancer, with a specific emphasis on the caspase-dependent apoptotic pathway. The primary focus encompasses the meticulous compilation of information encompassing various herbal drugs, elucidating details such as the specific botanical source, the targeted caspase mode of action, and the associated types of cancer. By synthesizing this information, the review aims to furnish an enhanced comprehension of the utility of herbal drugs in relation to conventional chemotherapeutic modalities, thereby contributing to a more nuanced understanding of their therapeutic potential in cancer treatment.
Keywords
Apoptosis, caspases, cancer therapy, herbal medicines, caspases inducer
Introduction
Global burden of cancer
Around the world, about 19.3 million people are diagnosed with cancer. The global incidence of cancer is expected to rise to 28.4 million by 2040, marking a 47% increase compared to 2020. The rise is expected to be more significant in countries that are transitioning (from 64% to 95%) compared to those that have already transitioned (from 32% to 56%). This increase is mainly due to changes in population, and it might be made worse by factors linked to globalization and economic growth. It's crucial to work on creating a reliable system to spread cancer prevention methods and provide cancer care in transitioning countries to effectively control cancer worldwide [1]
In 2022, it was estimated that around 14,61,427 people in India would be diagnosed with cancer, with a rate of 100.4 cases per 100,000 people. This means about one in nine individuals in India might get cancer during their life. The most common types of cancer in men and women were lung and breast cancers, respectively. For children aged 0-14 years, lymphoid leukemia was the most prevalent type. It's anticipated that the number of cancer cases will go up by 12.8% in 2025 compared to 2020[2]
On December 14th, IARC shared new information about the global cancer situation. The latest update, called Globocan 2020, shows that 2020, there were 19.3 million cancer cases and 10 million deaths attributed to cancer globally [3]
Limitations of conventional treatment
One big problem with usual treatments is that they aren't very specific. Take chemotherapy, for example. It goes after cells that divide quickly, but it doesn't distinguish between cancer cells and healthy ones. This lack of selectivity is why patients often suffer from severe side effects during these treatments [4]
Cancer cells can change and resist chemotherapy or radiation, making the treatment not work well, and the disease can come back. The reasons for this resistance are complicated and involve things like genetic changes, different pathways in the body, and special cancer cells called stem cells.[5] Regular treatments have improved, but they might not always completely get rid of cancer cells, especially when it spreads. Some cancer cells can stick around even after strong treatments, causing the cancer to come back and requiring more interventions [6]
Apoptosis and cancer
Apoptosis pathway
The nomenclature "apoptosis" is introduced to designate a previously underappreciated mechanism of regulated cellular deletion. This mechanism is observed to function as a complementary, albeit opposing, counterpart to mitosis in the orchestration of animal cell populations.[12] Apoptosis, a conserved process, entails distinct morphological changes like chromatin condensation and nuclear fragmentation [13]. Cellular rounding, reduced volume, and pseudopod retraction occur.[12] The plasma membrane remains intact initially, progressing to membrane blebbing and organelle changes. Phagocytosis often prevents apoptotic bodies. Failure leads to secondary necrosis, resembling necrosis. [14]
Apoptosis is a physiological phenomenon inherent in developmental processes and aging, serving as a homeostatic mechanism crucial for the regulation of cellular Populations within tissues. Moreover, apoptosis acts as a protective mechanism, especially during immune responses or when cellular damage occurs due to pathological conditions or harmful agents. [15]
The role of caspases in apoptosis
Apoptosis is a highly complex process involving an energy-dependent series of molecular events. [16,17].

Figure 1: Apoptotic Pathways
The initiation of apoptosis through extrinsic signaling pathways involves interactions mediated by transmembrane receptors. Specifically, death receptors, which belong to the tumor necrosis factor (TNF) receptor superfamily, play a crucial role in this process..[18] Positive stimuli like radiation, toxins, hypoxia, hyperthermia, viral infections, and free radicals cause changes in the inner mitochondrial membrane. This triggers the opening of the mitochondrial permeability transition (MPT) pore, leading to the loss of mitochondrial transmembrane potential. Consequently, pro-apoptotic proteins are released from the intermembrane space into the cytosol. [19]
T-cell mediated cytotoxicity, a form of type IV hypersensitivity, involves sensitized CD8+ T cells targeting and eliminating cells that present specific antigens. Cytotoxic T lymphocytes (CTLs) execute cell death primarily via the extrinsic pathway, with the interaction between Fas ligand (FasL) on the CTLs and Fas receptor (FasR) on the target cells being the main mechanism driving CTL-induced apoptosis. [20]

Figure 2: Different Natural Products used in Treatment of Cancer
These findings led to the identification of novel chemotypes exhibiting cytotoxic activities, such as taxanes and camptothecins. The development of these drugs took approximately three decades (1960-1990) before they were incorporated into clinical practice [11]
Implications for cancer therapy:
Upon apoptosis initiation, intracellular modifications ensue, marked by caspase activation. Caspases cleave vital cellular elements like cytoskeletal and nuclear proteins, disrupting normal cellular function. This enzymatic activity induces apoptotic cell contraction and triggers alterations in the plasma membrane, serving as signals for macrophage response [21]
Apoptosis is executed by caspases, a class of cysteine proteases specifically targeting and cleaving proteins.[22] Caspase protease activity is essential for effective apoptosis, as these enzymes cleave hundreds of different proteins [23][22]
![Caspases and their Role [22].png](https://www.ijpsjournal.com/uploads/createUrl/createUrl-20240624215451-3.png)
Table 1: Caspases and their Role [22]
The universal characteristics of cancer, found in all cancer cells irrespective of their origin or type, encompass unbridled proliferation, angiogenesis, and resistance to apoptosis [24,25]
Treating cancer involves controlling cell growth, often by utilizing the cell's programmed death mechanism, apoptosis. Targeting apoptosis is a successful non-surgical approach effective across various cancer types. Many anticancer drugs focus on different stages in intrinsic and extrinsic apoptotic pathways [26,27,28]
Rationale for focusing on herbal drugs:
Herbal drugs, made from plants or combinations of plants, have been in use for thousands of years, predating modern pharmaceuticals. Today, these natural remedies remain relevant and are still employed as alternative or complementary treatments.[7] With the onset of the industrial revolution and the introduction of contemporary pharmaceuticals, the utilization of herbal plants underwent a temporary cessation [7] However, impediments to the investigation of natural compounds have recently diminished predominantly through the application of modern techniques.[8] This has led to more interest in using natural substances in making medicines [9] Out of 121 drugs prescribed for cancer treatment, 90 originate from herbal medicine. According to a study, among the 65 newly registered drugs for cancer treatment between 1981 and 2002, 48 were derived from natural products [10]
Natural and herbal drugs overview:
Importance of natural and herbal drugs.
Natural compounds derived from plants, microorganisms, and marine life exhibit strong anticancer properties by triggering cell death through diverse pathways such as apoptosis, autophagy, necroptosis, paraptosis, parthanatos, or mitotic catastrophe. These effects entail complex molecular mechanisms, including epigenetic regulation, protein kinase cascades, and nuclear/mitochondrial processes, as extensively discussed in the literature. [29,30-35,36-43]
Historical context of herbal medicine in cancer treatment:
Traditional Chinese Medicine (TCM), practiced for centuries, is widely acknowledged as an alternative cancer treatment. It outlines TCM's molecular mechanisms, emphasizing phytochemicals (curcumin, resveratrol, berberine), the role of oncogenes and tumor suppressor genes, epigenetic influences (DNA methylation, histone modification, noncoding RNAs), and TCM's impact on the tumor microenvironment and cancer stem cells. The comprehensive information provided serves as a foundational resource for future research in TCM-based cancer therapy [87]

Table 2: In-depth Discussion of Specific Herbal Compounds known for Inducing Caspases
Mechanism of action:
Medications used in cancer treatment often trigger signaling pathways that lead to the activation of caspases, a group of cysteine proteases pivotal in numerous cell death processes [88]
- Exploration of the mechanisms through which natural compounds induce caspases:
Caspases can be activated through two main mechanisms. In the induced proximity model, initiator caspases like caspase-8 or caspase-9 are activated within distinct complexes, such as caspase-8 within the death-inducing signaling complex (DISC) and caspase-9 within the apoptosome [89-93]
In an alternative pathway, caspases can be activated by undergoing enzymatic processing of their inactive forms at specific cleavage sites. [90] Activation of caspases can originate from various sources, such as signaling at the cellular membrane through death receptors (receptor pathway) or from within the mitochondria (mitochondrial pathway) [89,90]
Activation of death receptors from the tumor necrosis factor (TNF) receptor superfamily, such as CD95 (APO-1/Fas) or TRAIL receptors, leads to receptor clustering and the recruitment of the adaptor protein Fas-associated death domain (FADD) along with caspase-8 [91-93]
- Regulation of caspase activation:
Caspases play a crucial role in drug-induced apoptosis, influencing sensitivity or resistance to cancer treatments. Surprisingly, tumors show low caspase mutations, but rather, epigenetic downregulation, emphasizing the potential of restoring functional caspase systems to overcome resistance [94-96]
- Caspase expression:

Figure 3: Caspase Expression
synergistic effects or interactions with conventional cancer treatments
Recent advancements in combined chemotherapy, involving multiple drugs targeting diverse biochemical and molecular pathways, enhance efficacy while minimizing adverse effects [97,98]
Experimental findings provide evidence for the synergistic impact of natural compounds in conjunction with chemotherapy, demonstrating the potential of combining antitumoral agents with various natural compounds to augment efficacy in cancer therapy.
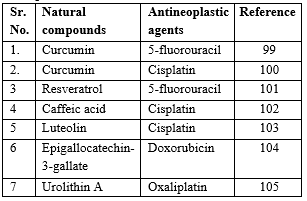
Table 3: Synergistic Effects of Herbal Compounds with Conventional Cancer Treatments
Safety and Side Effects:
Herbal medicines, while potentially causing fewer side effects compared to conventional drugs, can still lead to complications. Negative interactions with chemotherapy drugs or impaired blood clotting post-surgery are possible issues associated with certain herbs [106]
Although commonly viewed as safe because of their natural origins, herbal medicines can produce adverse effects, varying from mild to severe, including allergic reactions, asthma, headaches, nausea, vomiting, and diarrhea. It's important to recognize that, similar to conventional prescription drugs, herbal medicines should be administered by qualified and licensed practitioners. Seeking guidance from professional organizations affiliated with the specific therapy is recommended to access a roster of certified practitioners in your locality [107]
Top of Form
Conclusion and Future Directions:
Given that nearly 50% of existing pharmaceuticals have plant origins, exploring natural sources, particularly plants, for effective cancer treatment is evident. Historically, deriving Medications derived from natural sources, such as botanicals, was a time-consuming process. However, modern techniques have significantly expedited the discovery of active plant compounds, leading to a resurgence in the use of herbal plants. The revitalization of medications sourced from herbs, particularly in the treatment of cancer, immunological, and central nervous system (CNS) disorders, holds great importance. Presently, more than 60 herbal complexes are under investigation for their potential as anti-cancer agents. Despite this progress, the efficacy and safety of herbal products remain insufficiently understood. Neglecting the common usage of such products underscores the need for further research to enhance the appropriate utilization of plant-derived products. This is particularly crucial in addressing the gaps in knowledge regarding the effectiveness and safety of herbal interventions.
REFERENCE
- Sung, Hyuna, Jacques Ferlay, Rebecca L. Siegel, Mathieu Laversanne, Isabelle Soerjomataram, Ahmedin Jemal, and Freddie Bray. "Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries." CA: A Cancer Journal for Clinicians 71, no. 3 (May 2021): 209-249. doi:10.3322/caac.21660
- Sathishkumar, Krishnan, Meesha Chaturvedi, Priyanka Das, S. Stephen, and Prashant Mathur. "Cancer Incidence Estimates for 2022 & Projection for 2025: Results from National Cancer Registry Programme, India." Indian Journal of Medical Research 156, no. 4&5 (October-November 2022): 598-607. doi: 10.4103/ijmr.ijmr_1821_22.
- "17 December 2020. 'GLOBOCAN 2020: New Global Cancer Data.' News."
- Makrilia, N., Kollias, A., Manolopoulos, L., & Syrigos, K. (2009). Cell adhesion molecules: Role and clinical significance in cancer. Cancer Investigation, 27(10), 1023–1037
- Holohan, C., Van Schaeybroeck, S., Longley, D. B., & Johnston, P. G. (2013). Cancer drug resistance: an evolving paradigm. Nature Reviews Cancer, 13(10), 714–726.
- Gerlinger, M., Rowan, A. J., Horswell, S., Math, M., Larkin, J., Endesfelder, D., ... & Swanton, C. (2012). Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. New England Journal of Medicine, 366(10), 883–892.
- Pal SK, Shukla Y. Herbal medicine: current status and the future. Asian Pac J Cancer Prev. 2003;4(4):281–8.
- Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat Rev Drug Discov . 2005;4(3):206–20
- Saklani A, Kutty SK. Plant-derived compounds in clinical trials. Drug Discov Today
- Wang Z, Wang N, Chen J, Shen J. Emerging glycolysis targeting and drug discovery from chinese medicine in cancer therapy. Evid Based Complement Alternat Med. 2012; 2012:873175
- Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacol . 2005;100(1):72–9.
- Kerr, J. F. R., Wyllie, A. H., & Currie, A. R. "Apoptosis: A Basic Biological Phenomenon with Wideranging Implications in Tissue Kinetics." British Journal of Cancer 26 (1972): 239–257. Published 01 August 1972
- Kroemer G, El-Deiry WS, Golstein P, Peter ME, Vaux D, Vandenabeele P, Zhivotovsky B, Blagosklonny MV, Malorni W, Knight RA, Piacentini M, Nagata S, Melino G: Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005, 12: 1463-1467
- Norbury CJ, Hickson ID. Cellular responses to DNA damage. Annu Rev Pharmacol Toxicol. 2001; 41:367–401.
- Danial NN, Korsmeyer SJ (2004). Cell death: critical control points. Cell, 116: 205-219
- Kurokawa M, Kornbluth S (2009). Caspases and Kinases in a Death Grip. Cell, 138: 838-854.
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001; 104:487–501
- Saelens X, Festjens N, Vande Walle L, van Gurp M, van Loo G, Vandenabeele P. Toxic proteins released from mitochondria in cell death. Oncogene. 2004; 23:2861–74.
- Brunner T, Wasem C, Torgler R, Cima I, Jakob S, Corazza N. Fas (CD95/Apo-1) ligand regulation in T cell homeostasis, cell-mediated cytotoxicity and immune pathology. Semin Immunol. 2003; 15:167–76
- Hassan M., Watari H., AbuAlmaaty A., Ohba Y., Sakuragi N. Apoptosis and molecular targeting therapy in cancer. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/150845.
- Zaman S., Wang R., Gandhi V. Targeting the apoptosis pathway in hematologic malignancies. Leuk. Lymphoma. 2014; 55:1980–1992. doi: 10.3109/10428194.2013.855307.
- Lopez J., Tait S.W.G. Mitochondrial apoptosis: Killing cancer using the enemy within. Br. J. Cancer. 2015; 112:957–962. doi: 10.1038/bjc.2015.85.
- Arbiser J.L., Bonner M.Y., Gilbert L.C. Targeting the duality of cancer. NPJ Precis. Oncol. 2017;1 doi: 10.1038/s41698-017-0026-x.
- Xu W., Jing L., Wang Q., Lin C.-C., Chen X., Diao J., Liu Y., Sun X. Bas-PGAM5L-Drp1 complex is required for intrinsic apoptosis execution. Oncotarget. 2015; 6:30017–30034. doi: 10.18632/oncotarget.5013.
- Liu Y., Zhu X. Endoplasmic reticulum-mitochondria tethering in neurodegenerative dieseaes. Transl. Neurodegener. 2017; 6:21. doi: 10.1186/s40035-017-0092-6.
- Villa-Pulgarín J.A., Gajate C., Botet J., Jimenez A., Justies N., Varela-M R.E., Cuesta-Marbán A., Müller I., Modolell M., Revuelta J.L., et al. Mitochondria and lipid raft-located FoF1-ATP synthase as major therapeutic targets in the antileishmanial and anticancer activities of ether lipid edelfosine. PLoS Negl. Trop. Dis. 2017;11: e0005805. doi: 10.1371/journal.pntd.0005805.
- Bao H., Zhang Q., Zhu Z., Xu H., Ding F., Wang M., Du S., Du Y., Yan Z. BHX, a novel pyrazoline derivative, inhibits breast cancer cell invasion by reversing the epithelial-mesenchymal transition and down-regulating Wnt/?-catenin signaling. Sci. Rep. 2017; 7:9153. doi: 10.1038/s41598-017-09655-7.
- Kravchenko J., Corsini E., Williams M.A., Decker W., Manjili M.H., Otsuki T., Singh N., Al-Mulla F., Al-Temaimi R., Amedei A., Colacci A.M., Vaccari M., Mondello C., Scovassi A.I., Raju J., Hamid R.A., Memeo L., Forte S., Roy R., Woodrick J., Salem H.K., Ryan E.P., Brown D.G., Bisson W.H., Lowe L., Lyerly H.K. Chemical compounds from anthropogenic environment and immune evasion mechanisms: potential interactions. Carcinogenesis. 2015;36(Suppl. 1): S111–S127. doi: 10.1093/carcin/bgv033.
- Zhang S.F., Wang X.L., Yang X.Q., Chen N. Autophagy-associated targeting pathways of natural products during cancer treatment. Asian Pac. J. Cancer Prev. 2014;15(24):10557–10563. doi: 10.7314/APJCP.2014.15.24.10557.
- Kim J., Park E.J. Cytotoxic anticancer candidates from natural resources. Curr. Med. Chem. Anticancer Agents. 2002;2(4):485–537. doi: 10.2174/1568011023353949.
- Kinghorn A.D., Chin Y.W., Swanson S.M. Discovery of natural product anticancer agents from biodiverse organisms. Curr. Opin. Drug Discov. Devel. 2009;12(2):189–196.
- Meiyanto E., Hermawan A. Anindyajati. Natural compounds for cancer-targeted therapy: citrus flavonoids as potent chemopreventive agents. Asian Pac. J. Cancer Prev. 2012; 13:427–436. doi: 10.7314/APJCP.2012.13.2.427
- Wang N., Feng Y. Elaborating the role of natural compounds-induced autophagy in cancer treatment: achievements and artifacts in the state of the art. 2015.
- Gali-Muhtasib H., Hmadi R., Kareh M., Tohme R., Darwiche N. Cell death mechanisms of plant-derived anticancer drugs: beyond apoptosis. Apoptosis. 2015;20(12):1531–1562. doi: 10.1007/s10495-015-1169-2
- Luo X., Yu X., Liu S., Deng Q., Liu X., Peng S., Li H., Liu J., Cao Y. The role of targeting kinase activity by natural products in cancer chemoprevention and chemotherapy (Review). Oncol. Rep. 2015;34(2):547–554.
- Barrera L.N., Cassidy A., Johnson I.T., Bao Y., Belshaw N.J. Epigenetic and antioxidant effects of dietary isothiocyanates and selenium: potential implications for cancer chemoprevention. Proc. Nutr. Soc. 2012;71(2):237–245. doi: 10.1017/S002966511200016X.
- Shen M., Chan T.H., Dou Q.P. Targeting tumor ubiquitin-proteasome pathway with polyphenols for chemosensitization. Anticancer. Agents Med. Chem. 2012;12(8):891–901. doi: 10.2174/187152012802649978.]
- Cerella C., Dicato M., Jacob C., Diederich M. Chemical properties and mechanisms determining the anti-cancer action of garlic-derived organic sulfur compounds. Anticancer. Agents Med. Chem. 2011;11(3):267–271. doi: 10.2174/187152011795347522.
- Huang J., Plass C., Gerhauser C. Cancer chemoprevention by targeting the epigenome. Curr. Drug Targets. 2011;12(13):1925–1956. doi: 10.2174/138945011798184155.
- Fulda S. Modulation of apoptosis by natural products for cancer therapy. Planta Med. 2010;76(11):1075–1079. doi: 10.1055/s-0030-1249961.
- Wang S., Penchala S., Prabhu S., Wang J., Huang Y. Molecular basis of traditional Chinese medicine in cancer chemoprevention. Curr. Drug Discov. Technol. 2010;7(1):67–75. doi: 10.2174/157016310791162794.
- Sarkar F.H., Li Y. Harnessing the fruits of nature for the development of multi-targeted cancer therapeutics. Cancer Treat. Rev. 2009;35(7):597–607. doi: 10.1016/j.ctrv.2009.07.001.
- Gull, Sheereen, Kokab Farooq, Asima Tayyeb, Muhammad Imran Arshad, and Naveed Shahzad. "Ethanolic Extracts of Pakistani Euphorbiaceous Plants Induce Apoptosis in Breast Cancer Cells through Induction of DNA Damage and Caspase-Dependent Pathway." Gene 824 (25 May 2022): 146401 Volume 824, 25 May 2022,
- Nath, Rumki, Saswati Roy, Biplab De, and M. Dutta Choudhury. "Anticancer and Antioxidant Activity of Croton: A Review.”, International Journal of Pharmacy and Pharmaceutical Sciences, volume 5, 2013
- Alshuail, Nora, Zeyad Alehaideb, Sahar Alghamdi, Rasha Suliman, Hamad Al-Eidi, Rizwan Ali, Tlili Barhoumi, Mansour Almutairi, Mona Alwhibi, Bandar Alghanem, Abir Alamro, Amani Alghamdi, and Sabine Matou-Nasri. "Achillea fragrantissima (Forssk.) Sch.Bip Flower Dichloromethane Extract Exerts Anti-Proliferative and Pro-Apoptotic Properties in Human Triple-Negative Breast Cancer (MDA-MB-231) Cells: In Vitro and In Silico Studies." Pharmaceuticals 15, no. 9 (2022): 1060. doi: 10.3390/ph15091060.
- Bin Break, Mohammed Khaled, Kareem Mahmoud Younes, Salem Elkahoui, Rahamat Unissa, Suliman Ayad Alfahidat, Khalid Salem Alshawi, and Amr S. Abouzied. "Achillea fragrantissima (Forssk.) Sch.Bip. methanolic extract exerts potent antimicrobial activity and causes cancer cell death via induction of caspase-dependent apoptosis and S-phase arrest." Natural Product Research 36, no. 18 (2022): 4639-4644.
- Piryaei, Mohammad, Bahareh Mehrparvar, Ali Mohammadian, Fatemeh Shahriari, and Mohammad Amin Javidi. "Anti-cancer impact of Hypericin in B-CPAP cells: Extrinsic caspase dependent apoptosis induction and metastasis obstruction." European Journal of Pharmacology 910 (November 5, 2021): 174454.
- Yazdani, Mohammad, Alireza Hallaj, Farzaneh Salek, and Javad Baharara. "Potential of the combination of Artemisia absinthium extract and cisplatin in inducing apoptosis cascades through the expression of p53, BAX, caspase 3 ratio, and caspase 9 in lung cancer cells (Calu-6)." European Journal of Integrative Medicine 56 (December 2022): 102193.
- Sultan, Muhammad H., Zuwaiel Alanazi A., Moni, Sivakumar S., Alshahrani, Saeed, Alqahtani, Saad S., Madkhali, Osama, & Elmobark, Mohamed E. "Bioactive Principles and Potentiality of Hot Methanolic Extract of the Leaves from Artemisia absinthium L in vitro Cytotoxicity Against Human MCF-7 Breast Cancer Cells, Antibacterial Study and Wound Healing Activity." Current Pharmaceutical Biotechnology 21, no. 15 (2020): 1711-1721. doi: 10.2174/1389201021666200928150519.
- Klaophimai, Sirinthip, Phisit Pouyfung, Ariyaphong Wongnoppavich, and Kongthawat Chairatvit. "Induction of S arrest and apoptosis in human oral cancer cells by Rhinacanthin-C extracted from Rhinacanthus nasutus via modulating Akt and p38 signaling pathways." Journal of Ethnopharmacology 317 (December 5, 2023): 116813.
- Vichitsakul, Kanokkorn, Khanittha Laowichuwakonnukul, Boonchoy Soontornworajit, Natwadee Poomipark, Arunporn Itharat, and Pichayanoot Rotkrua. "Anti-proliferation and induction of mitochondria-mediated apoptosis by Garcinia hanburyi resin in colorectal cancer cells." Heliyon 9, no. 6 (June 2023): e16411.
- Guefack, Michel-Gael F., Francois Damen, Armelle T. Mbaveng, Simplice Beaudelaire Tankeo, Gabin T. M. Bitchagno, ?lhami Çelik, James D. Simo Mpetga, and Victor Kuete. "Cytotoxic Constituents of the Bark of Hypericum roeperianum towards Multidrug-Resistant Cancer Cells." Evidence-Based Complementary and Alternative Medicine 2020 (2020): 4314807. doi: 10.1155/2020/4314807
- Guefack, Michel-Gael F., Debojit Talukdar, Rimi Mukherjee, Subhabrata Guha, Debarpan Mitra, Depanwita Saha, Gaurav Das, François Damen, Victor Kuete, and Nabendu Murmu. "Hypericum roeperianum bark extract suppresses breast cancer proliferation via induction of apoptosis, down319, Part 1 (January 30, 2024): 117093.
- Kumar, Sunil, Rashmi Malhotra, and Dinesh Kumar. "Euphorbia hirta: Its chemistry, traditional and medicinal uses, and pharmacological activities." Pharmacognosy Reviews 4, no. 7 (January-June 2010): 58–61. doi:10.4103/0973-7847.65327.
- Mahdavi, Behnam, Habibeh Zare, Maryam Qorbani, Hadi Atabati, Mohammad Reza Vaezi Kakhki, Amir Raoofi, and Vahid Ebrahimi. "Euphorbia granulata Forssk: Evaluation of antioxidant activity, cytotoxicity, and apoptosis induction in breast cancer cells." South African Journal of Botany 150 (November 2022): 576-582.
- [57] Seo, Hee Won, Huiwon No, Hye Jin Cheon, and Jin-Kyung Kim. "Sappanchalcone, a flavonoid isolated from Caesalpinia sappan L., induces caspase-dependent and AIF-dependent apoptosis in human colon cancer cells." Chemico-Biological Interactions 327 (August 25, 2020): 109185.
- Park, Cheol, Min-Ho Han, Shin-Hyung Park, Su-Hyun Hong, Gi-Young Kim, Sung-Kwon Moon, Wun-Jae Kim, and Yung Hyun Choi. "Induction of apoptosis by Moutan Cortex Radicis in human gastric cancer cells through the activation of caspases and the AMPK signaling pathway." Revista Brasileira de Farmacognosia 27, no. 3 (May-June 2017): 315-323.
- Marzhoseyni, Zeynab, Mohammad Shayestehpour, Morteza Salimian, Davoud Esmaeili, Mahmood Saffari, and Hadis Fathizadeh. "Designing a novel fusion protein from Streptococcus agalactiae with apoptosis induction effects on cervical cancer cells." Microbial Pathogenesis 169 (August 2022): 105670.
- Ekiz, Elif, Emel Oz, A. M. Abd El-Aty, Charalampos Proestos, Charles Brennan, Maomao Zeng, Igor Tomasevic, Tahra Elobeid, Kenan Çad?rc?, Muharrem Bayrak, and Fatih Oz. "Exploring the Potential Medicinal Benefits of Ganoderma lucidum: From Metabolic Disorders to Coronavirus Infections." Foods 12, no. 7 (April 2023): 1512. doi:10.3390/foods12071512.
- Jiao, Chunwei, Wang Chen, Xupeng Tan, Huijia Liang, Jieyi Li, Hao Yun, Chunyan He, Jiaming Chen, Xiaowei Ma, Yizhen Xie, and Burton B. Yang. "Ganoderma lucidum spore oil induces apoptosis of breast cancer cells in vitro and in vivo by activating caspase-3 and caspase-9." Journal of Ethnopharmacology 247 (January 30, 2020): 112256.
- Qian, Yanfang, Chenying Shi, Chen Cheng, Dengwei Liao, Junping Liu, and Gui-tang Chen. "Ginger polysaccharide UGP1 suppressed human colon cancer growth via p53, Bax/Bcl-2, caspase-3 pathways and immunomodulation." Food Science and Human Wellness 12, no. 2 (March 2023): 467-476.
- Alshabi, Ali Mohamed, Saad Ahmed Alkahtani, Ibrahim Ahmed Shaikh, Mohamed A.A. Orabi, Basel A. Abdel-Wahab, Ismail A. Walbi, Mohammed S. Habeeb, Masood Medleri Khateeb, Joy H. Hoskeri, Arun K. Shettar, and Syed Mohammed Basheeruddin Asdaq. "Phytochemicals from Corchorus olitorius methanolic extract induce apoptotic cell death via activation of caspase-3, anti-Bcl-2 activity, and DNA degradation in breast and lung cancer cell lines." Journal of King Saud University - Science 34, no. 7 (October 2022): 102238.
- Lee, Se-Eun, Zhanna Okhlopkova, Chiyeon Lim, and Suin Cho. "Dracocephalum palmatum Stephan extract induces apoptosis in human prostate cancer cells via the caspase-8-mediated extrinsic pathway." Chinese Journal of Natural Medicines 18, no. 10 (October 2020): 793-800.
- Rawat, Sandeep, Arun K. Jugran, Indra D. Bhatt, and Ranbeer S. Rawal. "Hedychium spicatum: A Systematic Review on Traditional Uses, Phytochemistry, Pharmacology and Future Prospectus." Journal of Pharmacy and Pharmacology 70, no. 6 (2018): 687-712. doi:10.1111/jphp.12890.
- Ray, Asit, Ayushman Gadnayak, Sudipta Jena, Ambika Sahoo, Jeetendranath Patnaik, Pratap Chandra Panda, and Sanghamitra Nayaka. "Hedychium spicatum Rhizome Essential Oil Induces Apoptosis in Human Prostate Adenocarcinoma PC-3 Cells via Mitochondrial Stress and Caspase Activation." Heliyon 9, no. 3 (2023): e13807. Published online February 17, 2023. doi: 10.1016/j.heliyon. 2023.e13807. PMCID: PMC9981923. PMID: 36873474.
- de Medeiros, Daniel S. S., Tiago B. Rego, Ana P. de A. dos Santos, Adriana S. Pontes, Leandro S. Moreira-Dill, Najla B. Matos, Juliana P. Zuliani, Rodrigo G. Stábeli, Carolina B. G. Teles, Andreimar M. Soares, Angelo R. de M. Sperotto, Dinara J. Moura, Jenifer Saffi, Cleópatra Alves da Silva Caldeira, Daniel Carvalho Pimenta, and Leonardo A. Calderon. "Biochemical and Biological Profile of Parotoid Secretion of the Amazonian Rhinella marina (Anura: Bufonidae)." BioMed Research International 2019 (2019): 2492315. Published online February 21, 2019. doi:10.1155/2019/2492315. PMCID: PMC6535847. PMID: 31214612.
- Garcia, Israel José Pereira, Gisele Capanema de Oliveira, Jéssica Martins de Moura Valadares, Felipe Finger Banfi, Silmara Nunes Andrade, Túlio Resende Freitas, Evaldo dos Santos Monção Filho, Hérica de Lima Santos, Gerardo Magela Vieira Júnior, Mariana Helena Chaves, Domingos de Jesus Rodrigues, Bruno Antonio Marinho Sanchez, Fernando P. Varotti, and Leandro Augusto Barbosa. "New Bufadienolides Extracted from Rhinella marina Inhibit Na,K-ATPase and Induce Apoptosis by Activating Caspases 3 and 9 in Human Breast and Ovarian Cancer Cells." Steroids 152 (December 2019): 108490. Published by Elsevier.
- Mohammadi, Ali, Behzad Mansoori, Elham Safarzadeh, Sahar Gholizadeh, and Behzad Baradaran. "Anacyclus pyrethrum Extract Significantly Destroyed Lung Cancer Cell Line (A549) by Inducing Apoptosis." Journal of Herbal Medicine 39 (June 2023): 100649. Published by Elsevier.
- Elazzouzi, Hanane, Kamal Fadili, Ali Cherrat, Smail Amalich, Nadia Zekri, Hannou Zerkani, Imane Tagnaout, Christophe Hano, Jose M. Lorenzo, and Touria Zair. "Phytochemistry, Biological and Pharmacological Activities of the Anacyclus pyrethrum (L.) Lag: A Systematic Review." Plants 11, no. 19 (2022): 2578.
- Hongwei, Wang, Hailong Li, Fengqin Chen, Jun Luo, Jing Gu, Huping Wang, Hongyan Wu, and Yan Xu. "Baicalin Extracted from Huang Qin (Radix Scutellariae Baicalensis) Induces Apoptosis in Gastric Cancer Cells by Regulating B Cell Lymphoma (Bcl-2)/Bcl-2-Associated X Protein and Activating Caspase-3 and Caspase-9." Journal of Traditional Chinese Medicine 37, no. 2 (April 2017): 229-235.
- Wilson, Rachel B., Jason J. Lee, J. Geoffrey Pickering, and Nica M. Borradaile. "Natural Products in Regeneration." In Regenerative Nephrology (Second Edition), , 419-437. 2022.
- Isaq, Mona, Yarappa Lakshmikanth Ramachandra, Padmalatha S. Rai, Ashajyothi Chavan, Rajkumar Sekar, Meng-Jen Lee, and Prathap Somu. "Biogenic Synthesized Silver Nanoparticles Using Fungal Endophyte Cladosporium oxysporum of Vateria indica Induce Apoptosis in Human Colon Cancer Cell Line via Elevated Intracellular ROS Generation and Cell Cycle Arrest." Journal of Molecular Liquids 386 (September 15, 2023): 122601. Published by Elsevier.
- Zhou, Wei-Jie, Sheng Wang, Zhuang Hu, Zhen-Yu Zhou, and Cai-Juan Song. "Angelica sinensis Polysaccharides Promotes Apoptosis in Human Breast Cancer Cells via CREB-Regulated Caspase-3 Activation." Biochemical and Biophysical Research Communications 467, no. 3 (November 20, 2015): 562-569. Published by Elsevier.
- Tan, Bee Ling, and Norhaizan Mohd Esa. "Manilkara zapota (L.) P. Royen Leaf Water Extract Triggered Apoptosis and Activated Caspase-Dependent Pathway in HT-29 Human Colorectal Cancer Cell Line." Biomedicine & Pharmacotherapy 110 (February 2019): 748-757. Published by Elsevier.
- Agrawal, Shikha, Deepika Bablani Popli, Keya Sircar, and Aman Chowdhry. "A review of the anticancer activity of Azadirachta indica (Neem) in oral cancer." Journal of Oral Biology and Craniofacial Research [italicized] 10, no. 2 (2020): 206–209. doi: 10.1016/j.jobcr.2020.04.007.
- Zhang, Manchao, Hongpeng Liu, Zhenkun Tian, Brian N. Griffith, Min Ji, and Q. Quentin Li. "Gossypol Induces Apoptosis in Human PC-3 Prostate Cancer Cells by Modulating Caspase-Dependent and Caspase-Independent Cell Death Pathways." Life Sciences 80, no. 8 (January 30, 2007): 767-774. Published by Elsevier.
- Dey, Aditi, Subhankar Manna, Sourav Chattopadhyay, Dipankar Mondal, Dipankar Chattopadhyay, Anupam Raj, Subhajit Das, Braja Gopal Bag, and Somenath Roy. "Azadirachta indica Leaves Mediated Green Synthesized Copper Oxide Nanoparticles Induce Apoptosis Through Activation of TNF-? and Caspases Signaling Pathway Against Cancer Cells." Journal of Saudi Chemical Society 23, no. 2 (February 2019): 222-238.
- Moga, Marius Alexandru, Andreea B?lan, Costin Vlad Anastasiu, Oana Gabriela Dimienescu, Carmen Daniela Neculoiu, and Claudia Gavri?. "An Overview on the Anticancer Activity of Azadirachta indica (Neem) in Gynecological Cancers." International Journal of Molecular Sciences 19, no. 12 (December 2018): 3898. https://doi.org/10.3390/ijms19123898. Published online December 5, 2018. PMCID: PMC6321405. PMID: 30563141.
- Alzohairy, Mohammad A. "Therapeutic Role of Azadirachta indica (Neem) and Their Active Constituents in Diseases Prevention and Treatment." Evidence-Based Complementary and Alternative Medicine 2016 (2016): 7382506. https://doi.org/10.1155/2016/7382506. Published online March 1, 2016. PMCID: PMC4791507. PMID: 27034694.
- Hajiaghaalipour, Fatemeh, M.S. Kanthimathi, Junedah Sanusi, and Jayakumar Rajarajeswaran. "White Tea (Camellia sinensis) Inhibits Proliferation of the Colon Cancer Cell Line, HT-29, Activates Caspases and Protects DNA of Normal Cells Against Oxidative Damage." Food Chemistry 169 (February 15, 2015): 401-410. Published by Elsevier.
- Esghaei, Maryam, Hadi Ghaffari, Bahman Rahimi Esboei, Zienab Ebrahimi Tapeh, Farah Bokharaei Salim, and Manijeh Motevalian. "Evaluation of Anticancer Activity of Camellia Sinensis in the Caco-2 Colorectal Cancer Cell Line." Asian Pacific Journal of Cancer Prevention [italicized] 19, no. 6 (2018): 1697–1701. doi: 10.22034/APJCP.2018.19.6.1697.
- Limam, Inès, Mohamed Abdelkarim, Rym Essid, Ahlem Chahbi, Mayssa Fathallah, Salem Elkahoui, and Fatma Ben Aissa-Fennira. "Olea europaea L. cv. Chetoui Leaf and Stem Hydromethanolic Extracts Suppress Proliferation and Promote Apoptosis via Caspase Signaling on Human Multiple Myeloma Cells." European Journal of Integrative Medicine 37 (August 2020): 101145. Published by Elsevier.
- Hashmi, Muhammad Ali, Afsar Khan, Muhammad Hanif, Umar Farooq, and Shagufta Perveen. "Traditional Uses, Phytochemistry, and Pharmacology of Olea europaea (Olive)." Volume 2015, Article ID 541591.
- Rajagopal, Anitha, and Subashini Rajakannu. "Cassia auriculata Linn. Extracts Induce Apoptosis and Cell Cycle Arrest of A549 Lung Cancer Cell Lines: An In Vitro Approach." South African Journal of Botany 147 (July 2022): 275-285. Published by Elsevier.
- Xia, Wen, Er-sheng Gong, Yanyun Lin, Bisheng Zheng, Wenhan Yang, Tong Li, Sheng Zhang, Peng Li, and Rui-hai Liu. "Wild Pink Bayberry Free Phenolic Extract Induces Mitochondria-Dependent Apoptosis and G0/G1 Cell Cycle Arrest Through p38/MAPK and PI3K/Akt Pathway in MDA-MB-231 Cancer Cells." Food Science and Human Wellness 12, no. 5 (September 2023): 1510-1518. Published by Elsevier.
- Xiang, Yuening, Zimu Guo, Pengfei Zhu, Jia Chen, and Yongye Huang. "Traditional Chinese Medicine as a Cancer Treatment: Modern Perspectives of Ancient but Advanced Science." Cancer Med 8, no. 5 (2019): 1958-1975. doi:10.1002/cam4.2108.
- Fulda, Simone, and Klaus-Michael Debatin. "Caspase Activation in Cancer Therapy." In Madame Curie Bioscience Database, National Center for Biotechnology Information.
- Hengartner M O. The biochemistry of apoptosis. Nature. 2000; 407:770–777.
- Thornberry N, Lazebnik Y. Caspases: Enemies within. Science. 1998; 281:1312–1316.
- Schulze-Osthoff K, Ferrari D, Los M. et al. Apoptosis signaling by death receptors. Eur J Biochem. 1998; 254:439–459
- Krammer P H. CD95's deadly mission in the immune system. Nature. 2000; 407:789–795.
- Walczak H, Krammer P H. The CD95 (APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp Cell Res. 2000; 256:58–66.
- Mandruzzato S, Brasseur F, Andry G. et al. A CASP-8 mutation recognized by cytolytic T lymphocytes on a human head and neck carcinoma. J Exp Med. 1997;186:785–793
- Teitz T, Wei T, Valentine M B. et al. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat Med. 2000;6:529–535.
- Fulda S, Kufer M U, Meyer E. et al. Sensitization for death receptor- or drug-induced apoptosis by re-expression of caspase-8 through demethylation or gene transfer. Oncogene. 2001;20:5865–5877
- Vieira e Silva, Fábio França, María Elena Padín-Iruegas, Vito Carlo Alberto Caponio, Alejandro I. Lorenzo-Pouso, Paula Saavedra-Nieves, Cintia Micaela Chamorro-Petronacci, José Suaréz-Peñaranda, and Mario Pérez-Sayáns. "Caspase 3 and Cleaved Caspase 3 Expression in Tumorogenesis and Its Correlations with Prognosis in Head and Neck Cancer: A Systematic Review and Meta-Analysis." International Journal of Molecular Sciences."
- M.T. Seymour, T.S. Maughan, J.A. Ledermann, C. Topham, R. James, S.J. Gwyther, D.B. Smith, S. Shepherd, A. Maraveyas, D.R. Ferry, A.M. Meade, L. Thompson, G.O. Griffiths, M.K. Parmar, R.J. Stephens,” Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial. Lancet, 370 (2007), pp. 143-152
- M. Koopman, N.F. Antonini, J. Douma, J. Wals, A.H. Honkoop, F.L. Erdkamp, R.S. de Jong, C.J. Rodenburg, G. Vreugdenhil, O.J. Loosveld, A. van Bochove, H.A. Sinnige, G.J.M. Creemers, M.E. Tesselaar, P.H.T.J. Slee, M.J. Werter, L. Mol, O. Dalesio, C.J. Punt “Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial Lancet, 370 (2007), pp. 135-142
- M. Shakibaei, P. Kraehe, B. Popper, P. Shayan, A. Goel, C. Buhrmann. “Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer” BMC Cancer, 15 (2015)
- B.H. Park, J.E. Lim, H.G. Jeon, S. Il Seo, H.M. Lee, H.Y. Choi, S.S. Jeon, B.C. Jeong. “Curcumin potentiates antitumor activity of cisplatin in bladder cancer cell lines via ROS-mediated activation of ERK1/2” Oncotarget, 7 (2016), pp. 63870-63886
- C. Buhrmann, M. Yazdi, B. Popper, P. Shayan, A. Goel, B.B. Aggarwal, M. Shakibaei. “Resveratrol chemosensitizes TNF-?-induced survival of 5-FU-treated colorectal cancer cells Nutrients”, 10 (2018)
- R. Sirota, D. Gibson, R. Kohen. “The timing of caffeic acid treatment with cisplatin determines sensitization or resistance of ovarian carcinoma cell lines” Redox Biol., 11 (2017), p. 170
- H. Wang, Y. Luo, T. Qiao, Z. Wu, Z. Huang. “Luteolin sensitizes the antitumor effect of cisplatin in drug-resistant ovarian cancer via induction of apoptosis and inhibition of cell migration and invasion” J. Ovarian Res., 11 (2018)
- W. Wang, D. Chen, K. Zhu. “SOX2OT variant 7 contributes to the synergistic interaction between EGCG and Doxorubicin to kill osteosarcoma via autophagy and stemness inhibition” J. Exp. Clin. Cancer Res., 37 (2018)
- E. Norden, E.H. Heiss, Urolithin. “A gains in antiproliferative capacity by reducing the glycolytic potential via the p53/TIGAR axis in colon cancer cells” Carcinogenesis, 40 (2019), pp. 93-101
- Whitmer, Michelle. "Herbal Medicine and Cancer." Edited by Walter Pacheco. Medically Reviewed by Tejal Parekh. The Mesothelioma Center, Accessed January 16, 2024.
- "Herbal Medicine." Better Health Channel. Accessed January 16, 2024.


 Chetana Shewale*
Chetana Shewale*
 Prithviraj Deoda
Prithviraj Deoda


![Caspases and their Role [22].png](https://www.ijpsjournal.com/uploads/createUrl/createUrl-20240624215451-3.png)



 10.5281/zenodo.12519010
10.5281/zenodo.12519010