Abstract
Because liposomes are highly biocompatible, biodegradable, and immunogenicity-free, they are now thought to be the most widely employed nanocarriers for a variety of hydrophobic and hydrophilic compounds that have the potential to be active. Moreover, liposomes have demonstrated improved drug solubility, regulated dispersion, and surface modification ability for focused, extended, and sustained release. Liposomes can be thought of as having developed from traditional, long-circulating, immune-system-targeting liposomes to actively targeted liposomes that respond to stimuli based on their composition. Numerous liposomal-based drug delivery methods are presently clinically licensed to treat a variety of illnesses, including viral, fungus, and cancer infections; more liposomes have advanced to the stage of clinical trials. The structure, composition, preparation techniques, and clinical uses of liposomes are covered in this article.
Keywords
Drug delivery systems, phospholipids, liposomes, parts of a liposome.
Introduction
Liposomes are vesicular structures that are colloidal in nature, consisting of one or more lipid bilayers encircling an equivalent number of aqueous compartments1. The liquid interior of the sphere-shaped shell was filled with compounds including hormones, enzymes, peptides, proteins, antibiotics, antifungals, and anticancer agents. A free medicine injected into the bloodstream usually only temporarily reaches the therapeutic level because of elimination and metabolism. Long-term therapeutic levels of drugs encapsulated in liposomes are achieved because the medication must be released from the liposome prior to metabolism and excretion. These are tiny, synthetic, spherical vesicles that can be made from natural, non-toxic phospholipids and cholesterol. In addition to their biocompatibility, liposomes’ size and hydrophobic and hydrophilic characteristics make them attractive drug delivery vehicles. Liposome characteristics vary greatly depending on the lipid content and surfaceCost, dimensions, and preparation technique. Moreover, the charge and rigidity or fluidity of the bilayer are decided by the selection of bilayer components. For example, natural sources of unsaturated phosphatidylcholine (soy or egg) produce far more permeable and less stable bilayers than saturated phospholipids with lengthy acyl chains. (Dpalmitoyl-phosphatidyl choline, for instance) combine to form a stiff, rather impermeable bilayer structure2. Phospholipids have been shown to spontaneously form closed structures when hydrated in aqueous solutions. Depending on the type of medication, these vesicles with one or more phospholipid bilayer membranes can carry either lipid or aqueous medications. Lipids are amphipathic in aqueous conditions, meaning they are both hydrophobic and hydrophilic.Thermodynamic phase parameters and self-assembling features affect the hydro-phobic sections’ entropically focused confiscation into spherical bilayers. Lamellae are the names for those layers. Liposomes are typically spherical vesicles with particle sizes ranging from few micrometres to 30 nm. The polar head groups of these entities are orientated in the direction of the interior and exterior aqueous phases, and they are surrounded by one or more lipid bilayers around aqueous units. On the other hand, polar lipids can self-assemble into a variety of colloidal particles instead of being restricted to traditional bilayer structures, which depend on temperature, molecule shape, and preparation and environmental factors. A liposome is a spherical vesicle made of lipid bilayer that is artificially manufactured. The liposome can be administered using as a vehiclea lipid bilayer that is artificially manufactured. The liposome can be administered using as avehicleadministered using as a vehiclea lipid bilayer that is artificially manufactured. The liposome can be administeredusing as a vehicle vehicleadministered using as a vehiclea lipid bilayer that is artificially manufactured. The liposome can be administered using as a vehicle using as a vehicle
What is Liposome :
A liposome is a spherical vesicle with a structure akin to cell membranes, consisting of one or more phospholipid bilayers.
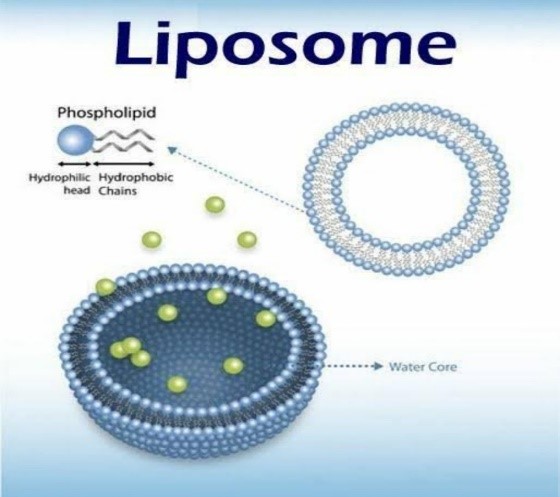
Due to their capacity to encapsulate hydrophilic or lipophilic medications, liposomes have developed into effective drug delivery vehicles.Liposomes are categorised into four groups based on the size and quantity of bilayers in their structures: multilamellar vesicles (MLV), multivesicular vesicles (MVV), large unilamellar vesicles (LUV), and small unilamellar vesicles (SUV). In a multilamellar structure, liposomes resemble onions, but in a unilamellar structure, they feature a monophospholipid bilayer. When several unilamellar vesicles are formed within bigger liposomes, MVV forms a multilamellar configuration with concentric phospholipid spheres [37]. For hydrophilic substances exclusively, liposome encapsulation effectiveness falls with the number of bilayers and rises with liposome size [38]. The size of the vesicles is a key element that regulates the circulation half-life of liposomes. The quantity of the medicine enclosed is influenced by the size and quantity of bilayers. Whenever liposomes are used in
History of Liposome:
Alec Douglas Bangham, a British hematologist, initially characterized liposomes in 1961 at the Babraham Institute in Cambridge. His discoveries were published in 1964. When Bangham and R. W. Horne added negative stain to dry phospholipids to test the institute’s new electron microscope, they made the finding.
Structure of Liposome :
Phospholipid
• Naturally occurring phospholipids used in liposome:
oPhosphatidylethanolamine
o Phosphatidylcholine
o Phsphatidylserine
•. Synthetic phospholipids used in the liposomes are:
o Dioleoyl phosphatidylcholine
o Disteroyl phosphatidylcholine
o Dioleoyl phosphatidylethanolamine
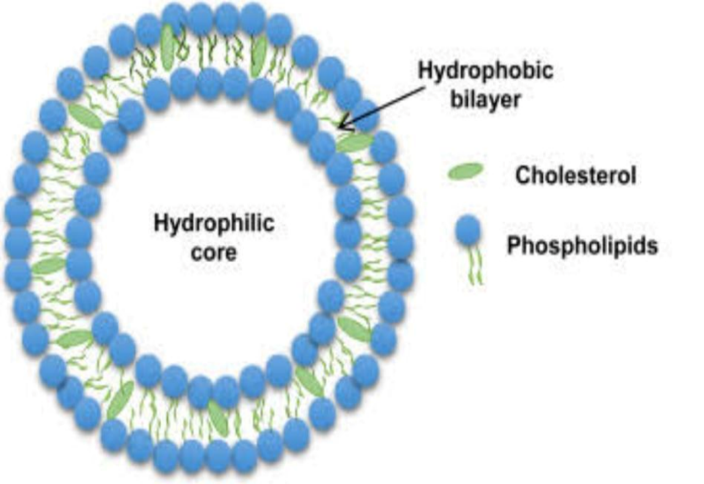
Cholesterol:
In phospholipid membranes, cholesterol can be present in very high concentrations, up to 1:1 or 2:1 molar ratios between cholesterol and phospatidylcholine. Because it is an amphipathic molecule, cholesterol inserts into the membrane with its hydroxyl group facing the aqueous floor and its aliphatic chain parallel to the acyl chains in the middle of the bilayers. It also increases the distance between choline head organizations and eliminates the regular hydrogen bonding and electrostatic interactions .
Advantage:
• Suitable for delivery of hydrophobic, hydrophilic and amphipatic drugs and agents
•The increase in number of layers (e.g., kinetic constraints) may be beneficial to prevents or delays the release of active molecules.
•Used as carriers for controlled and sustained drug delivery
•Improved pharmacokinetic effects (reduced elimination, increased circulation life time).
•site avoidance effect.
Disadvantages:
•Possibillity of phospholipid oxidation and hydrolysis-like reaction.
•Reduced bioavailability compared to nanoliposomes.
•Higher physical instability during storage.
•Drug leakage
•Some times phospholipids undergoes hydrolysis and oxidation reactions
•High cost of manufacture.
•Rapid clearance from circulation due to non specific uptake by the cells of RES.
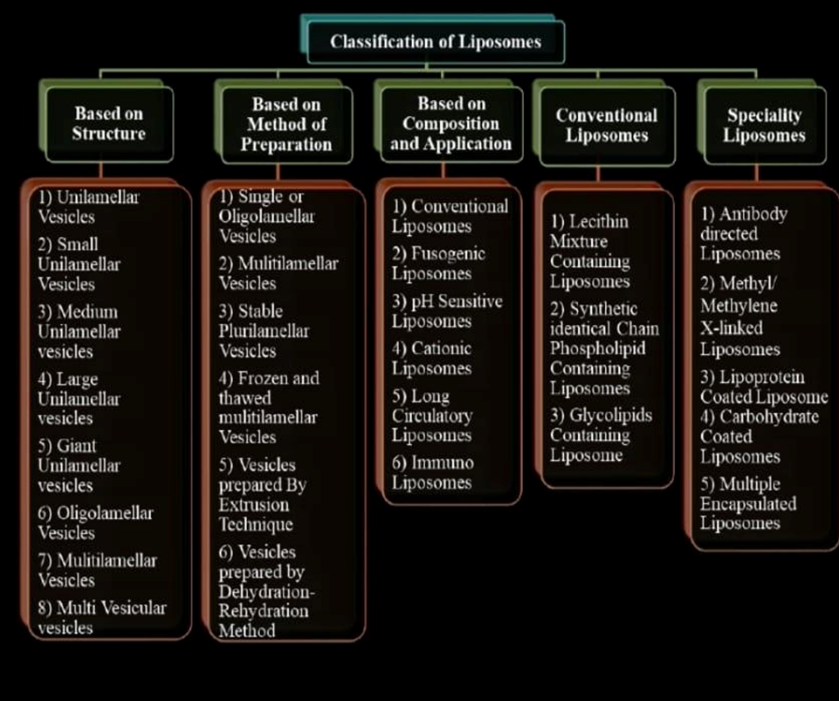
Types of Liposome:
Liposome are classified based on
Based on Structural Parameters=
Unilamellar Vesicles
• Small unilamellar vesicles (SUV)
• Medium unilamellar vesicles (MUV)
• Large unilamellar vesicles (LUV)
Oligolamellar Vesicles (OLV)
These are made up of 2-10 bilayers of lipids surrounding a large
Internal volume.
Multilamellar vesicles (MLV)
MLV made up of several bilayers. And the preparation method
Differs from other vesicle preparation methods. They are arranged
In a manner like an onion in which concentric spherical bilayers of
LUV/MLV enclosing a large number of SUV etc.
Based on Method of Preparation:
1.Reverse phase evaporation method (REV): single or oligolamellar Vesicles.
2.MLV-REV: multilamellar vesicles made by a reverse-phase Evaporation method.
3.SPLV: stable multilamellar vesicles
4.FATMLV: frozen and thawed MLV.
5.VET: vesicles prepared by the extrusion method.

< Mechanical>
• Sonication:
The most popular technique for creating small unilamellar vesicles is sonication. When LMV suspensions are disrupted with sonic energy (sonication), tiny Unicelamellar vesicles (SUV) with dimensions ranging from 15 to 50 nm are usually produced. For the manufacture of sonicated particles, bath and probe tip sonicators are the most often used equipment. Even though they are less common, cup-horn sonicators have successfully created SUVs. Two methods of sonication exist:
• Probe Sonication:
A sonicator tip is submerged in the liposome solution while using the probe sonication method, which is often utilised for tiny volumes. To prevent the high energy given by the tip from causing a local warming and deterioration of the lipidic solution, the bath vessel is submerged in a water/ice bath.This leads to the coupling of energy at the tip.Consequently, the vessel needs to be submerged in an ice or water bath due to the surrounding heat. It is possible to de-esterify over 5% of the lipids with one hour of result Sonication. Furthermore, using the probe sonicator
• Bath Sonication:
The liposome dispersion is placed into a sterile vessel (equipped with a temperature-control system) or under an inert atmosphere in the bath sonication method, which is typically employed for large volumes. The liposome dispersion solution is used in the bath sonicator, where it is placed in a cylinder and heated to the desired temperature of the lipid dispersion. The material that has been sonicated can be put in a covered, sterile vessel.
Classification of Liposome:
Mechanisms of actions:
The way that liposomes work Up until now, you have been familiar with the many processes involved in liposome synthesis, loading, and surface modification. Li et al., 2005; Gilbert and Knight, 1996; Koff and Fidler, 1985; Pollock et al., 2008; Mahmoud et al., 2021; G.F. Smaisim et al., 2022; Kianfar, 2023; Kianfar and Sayadi, 2022; Fattah et al., 2023) discuss the liposome transport process as well as the drug’s release mechanism. The intended material (such as medication) is encapsulated in liposomes, which are then administered to tissues or organs. As per the treatment strategy (Logue et al., 2010; Straubinger and Papahadjopoulos, 1983; Mannino and Gould-Fogerite, 1988; Nicolau et al., 1987; Bokov et al., 2021; Chupradit et al.,
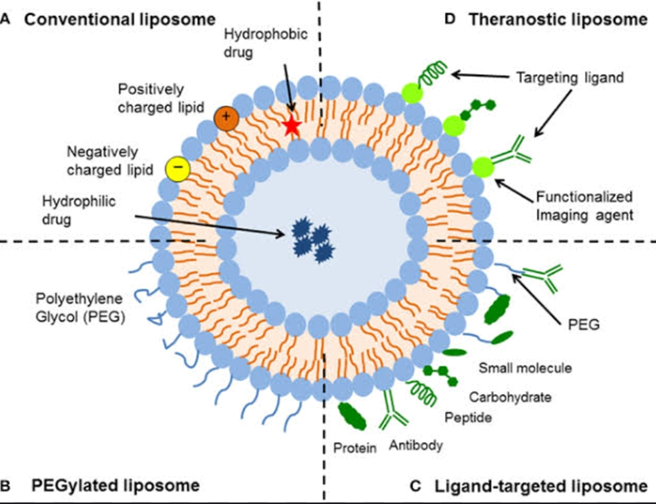
Application of Liposome:
Liposomes are used for drug delivery due to their unique properties. A liposome encapsulates a region on aqueous solution inside a hydrophobic membrane; dissolved hydrophilic solutes cannot readily pass through the lipids. Hydrophobic chemicals can be dissolved into the membrane, and in this way liposome can carry both hydrophobic molecules and hydrophilic molecules. There are three types of liposomes- MLV (multilamillar vesicles) SUV (Small Unilamellar Vesicles) and LUV (Large Unilamellar Vesicles) used to deliver different types of drugs. Liposomes are used as models for artificial cells. Liposomes can also be designed to deliver drugs in other ways.
CONCLUSION:
In summary In less than 30 years, liposomal drug delivery has been successfully developed thanks to the collaborative efforts of colloid science, physics, chemistry, biology, pharmacology, and medicine. The strong theoretical and experimental foundation that has been established promises future advancements and products1, 88, 98, 99, and 100. Which of the aforementioned applications and conjectures turns out to be successful will only be determined with time. Still, given the items that are already on the market, we may state that liposomes Liposomes have proven to be an effective medication delivery method for treating a variety of illnesses, including pain management and cancer. The formulation of the water-insoluble, poorly bioavailable, and extremely toxic medication was improved in terms of pharmacokinetics and pharmacodynamics by the biocompatible, biodegradable, and low immunogenicity liposomes. To get over their early drawbacks, liposomes underwent multiple evolutions in terms of their components and production method. Currently, a number of liposomal formulations are approved for use in treating different disorders and over five hundred liposomal formulations are undergoing various stages of clinical development. However, the chemical and physical stability of liposomes presents significant hurdles. Consequently, the development of liposomes with high stability is crucial since it greatly influences their therapeutic applicability. Consequently, in computational research and silico simulation
REFERENCES
- Mishra H, Teotia D, Kumar K, Chauhan V. A thorough analysis of liposomes, a cutting-edge medicine delivery technology. 2018; 8(6):400–404; Journal of Drug Delivery and Therapeutics. The doi: 10.22270/jddt.v8i6.2071 is available.
- Papahadjopoulos D., Lasic D. Liposomes revisited. 1995; Science 267(5202). 10.1126/science.7871422 is the URL to be used. Khar RK, Vyas SP.
- Novel Carrier Systems for Targeted and Controlled Drug Delivery, 2006:421-427. Liposomal Drug Delivery Systems: An Updated Review, Samad A, Sultana Y, Aqil M. Drug Delivery Today. 2007; 4:297–305. The doi: 10.2174/156720107782151269 Riaz M.
- Preparation techniques for liposomes. Journal of Pharmaceutical Sciences in Pakistan, 1996, pp. 65-77.
- Gregoriadis G, Florence AT. Drug Delivery Using Liposomes. Drugs. 1993; 15–28. 10.2165/00003495-199345010-00003 is the URL to be used. Kriss JP, Aragon S, Rooke JD, and Dunnick JK. Modification of Mammalian Cells by Artificial Lipid Vesicles Interaction. (1976) Cancer Research 36: 2385–2389.
- Gregoriadis G. Liposome Technology, Volumes I, II, and III, CRC Press, Boca Raton, 1984. 15. Gregoriadis G, 2nd Ed., Liposome Technology. Volumes I, II, and III, CRC Press, Boca Raton, 1992. Maierhofer, G. (1985, June). Mayhew E., Nikolopoulos G.T., King J.J., and Silviano A.A. (1985).
- Am. Clinical Products. 33. 17. Pharmaceutical Manufacture.2: 18. 18. Browning, I., Payne, N.I., and Hynes, C.A. (1986). Science J. Pharmacol. 75: 330. 19. Spargo, B.J., Crow, L.M., and Crow, J.H. (1987). United States. Proc. Natl. Acad. Sci. 84: 1537. 20.
- “Current Status of Drug Delivery Technologies and Future Directions,” by Rajan K. Verma and Sanjay Garg 25 (2), 1–14 in Pharmaceutical Technology On-Line (2001). 21. Barani, H. & Montazer, M. “A Review on Applications of Liposomes in Textile Processing. The Liposome Research Journal


 Utkarsha Gadekar *
Utkarsha Gadekar *
 Ulka Mote
Ulka Mote





 10.5281/zenodo.14211476
10.5281/zenodo.14211476