Abstract
Pharmacovigilance defined by the world health association as" the wisdom and conditioning relating to the discovery, assessment, understanding, assessment and forestallment of adverse goods or any other medicine related problems". It plays a crucial part in icing that cases admit safe medicines. Our knowledge of a medicine’s adverse responses can be increased by colorful means, including robotic reporting, ferocious monitoring and database studies. New processes both at a nonsupervisory and scientific position are being developed with the end of strengthening pharmacovigilance.
Keywords
Pharmacovigilance, Adverse drug Reaction monitoring.
Introduction
According to WORLD HEALTH Association Pharmacovigilance is defined as the wisdom and exertion relating to the discovery, assessment, understanding and forestallment of adverse effect or any other medicine related problem, particularly long term and short term adverse effect of drug( ).
Pharmacovigilance is an important and integral part of clinical exploration (2). Pharmacovigilance greatly concentrated on adverse dug response (ADRs) Adverse medicine response is defined as any response to a medicine which is dangerous and unintended including lack of efficacy used for the prophylaxis, analysis or remedy of illness or for revision of physiological function (3). The under- reporting of adverse medicine responses (ADRs) is the major reversal worldwide which may be attributed to the lack of time and report forms. It has been known that the world health association (WHO) has initiated the program of reporting all adverse responses held by the medicines (4). PV possesses colorful places similar as identification, quantification, and attestation of medicine- related problems which are responsible for medicine- related injuries (5,6). farther, public PV Programs have been introduced which occupies a high part in adding the public mindfulness about medicine safety(7,8). Pharmacovigilance supports safe and applicable use of medicine by a) promoting the discovery of preliminarily unknown ADRs and commerce and increases in frequence of known ADRs, b) identify threat factors for the development of ADRs, c) estimating quantitative aspects of benefits threat analysis and propagating information to ameliorate medicine prescribing and regulation ( 9). Pharmacovigilance is not new to India and has infact been going on from 1998 (10). Pharmacovigilance (PV) with its ultimate thing of minimising pitfalls and maximasing the benefits of medicinal products serves as an important public health tool. The World Health Organization( WHO) defines PV as “ the wisdom and conditioning relating to the discovery, assessment, understanding and for estallment of adverse goods or any other medicine related problem, previous to blessing by nonsupervisory authorities, medicine products are needed to suffer expansive testing and rigorous evaluation during clinical trials, to establish their safety and efficacy, The explanation for post-marketing PV is grounded on the need to alleviate the limitations of pre- marketing/ enrollment clinical trials including small population sizes, a short length of time and the rejection of special population groups( e.g. pregnant women and children). thus, unanticipated or severe adverse medicine responses( ADRs) are frequently not linked before nonsupervisory blessing performing in increased morbidity, mortality and fiscal loss( 11).
History
clearly, let’s cave into further detail on the crucial points in the history of pharmacovigilance
- Thalidomide
Tragedy (1950s- 1960s) Thalidomide, firstly specified as a opiate and antiemetic, caused severe birth blights in thousands of babies. This catastrophe underlined the necessity for systemic monitoring of medicine safety. The fate led to increased mindfulness of the implicit detriment medicines could cause, especially during gestation.
- conformation of WHO Program( 1968) In response to the thalidomide incident, WHO established the transnational medicine Monitoring Program in 1968. This program laid the foundation for a global network of pharmacovigilance centers, fostering collaboration in collecting and assaying data on adverse medicine responses ADRs).
- FDA and AERS( 1970s) The FDA initiated the Adverse Event reporting system( AERS) in 1970s. AERS come a vital tool for collecting, managing and assaying data on adverse events associated with medicines, enabling the FDA to cover and regulate medicine safety in united countries.
- ICH Guidelines (1990s)
The transnational conference on adjustment ( ICH) played a pivotal part in homogenizing pharmacovigilance practices encyclopedically. ICH guidelines, similar as E2B, handed harmonized frame. for the collection and exchange of safety data, fostering transnational cooperation among nonsupervisory authorities.
- EU Pharmacovigilance system( 2005)
The European Union introduced a comprehensive pharmacovigilance system, strengthening the monitoring and supervision of medicinal products.
The European medicinal agency( EMA) played acentral part in coordinating safety assessments and threat operation strategies.
- Periodic safety update reports( PSURs) PSURs come a standard demand for marketing authorization holders. These reports involve the regular submission of safety data to nonsupervisory authorities, icing nonstop evaluation of a medicines safety profile throughout its lifecycle.
- Digital Era and Signal Detection( 21st century) Technological advancement in the 21st century eased the integration of big data and digital platforms in pharmacovigilance. Automated signal discovery system, exercising algorithms and data mining ways, enhanced the effectiveness of relating implicit safety enterprises from large datasets.
- Global collaboration (present) ultramodern pharmacovigilance emphasizes global collaboration enterprise like WHO global individual case safety Reports (ICSRs) platform grease regularize reporting and information exchange across countries. This collaborative approach involves regulatory agencies, pharmaceutical companies, healthcare professionals, and patients in monitoring and ensuring the safety of medicinal products. (12)
Importance of PV: It's the wisdom which deals with the complex process of the understanding and explaining the nature of ADR passed in a case taking either oral or parenteral or intravenous (I.V) medicines for an disease. The medicines being retailed worldwide passed a whole array of tests and also passed clinical trials in creatures and mortal subjects to assess the safety of the medicine for a particular complaint and to know the exact side good s associated with it. Still there's a major part of it goes undetected and some of the ADR are detected in post marketing surveillance. It's estimated that there's significant quantum of ADRs which decreases the quality of life, increase hospitalization stay and increases the mortality. A corner study by Lazarou in 1998 described, ADRs to be the fourth to sixth leading cause of death in the US and ADRs are estimated to beget 3- 7 of all sanitarium admissions (13).
Methods used in PV:
Many researchers developed different methods of causality assessment of ADRs by utilizing different criteria like chronological relationship between the administration of the drug and the occurrence of the ADR, screening for non-drug related causes, confirmation of the reaction by in vivo or in vitro tests, and antecedent information on homogeneous events attributed to the suspect drug or to its therapeutic class, etc., to define ADRs in different categories [14]. Currently, there is no universally accepted method for assessing causality of ADRs [15]. Currently, there are many algorithmic methods of causality assessment but no single algorithm is accepted as the gold standard because of the shortcomings and discordances that subsist between them [16].
We would explicate them in short as listed below.
Dangaumou’s French method [17]
This rule of thumb has been used by the French government agency since 1977. The way of doing thing separates an intrinsic imputability (possible case between abused substance and dispassionate event) from an extrinsic imputability (bibliographical data) by the agency of seven criteria (three connected and four semiological) in two different tables. The criteria are (i) drug challenge, (ii) dechallenge, and (iii) rechallenge by the overall score of four possible categories. The semiological criteria are (i) semiology (clinical signs) using per se (suggestive or other), (ii) favoring component, (iii) arbitrary non-drug related (none or possible), and (iv) laboratory tests show with three possible outcomes (positive, negative or no test for the event-drug pair). Scores are grouped as possible and dubious.
Kramer et al. method [18]
This method applies when the offending drug is administered and a single adverse drug event has taken place. Each adverse event is assessed independently and assessment is prepared. One of the advantages of this algorithm is its transparency. However, certain levels of experience, expertise, and time are required to use this method effectively.
Naranjo et al. method (Naranjo scale) [19]
It is utilized to verify causality in a variety of clinical situations utilizing the categories and definitions of definite, probable, possible, and doubtful. It consists of ten questions which are answered as yes, no and unknown. The event is assigned to a probability category predicated on the total score after totaling. A total score of ?9 is definite, probable is 5-8, possible is 1-4 and doubtful ?0. This scale is more powerful when the adverse event is associated with only one drug, but when multiple drugs are involved or there is any interactions between drugs, this scale fails to identify the offending agent.
Balanced assessment method [20] This method evaluates a case report on various visual analog scale (VAS) models that each criterion is fulfilled individually. It has an added advantage that it considers an alternative causative factor as a possibility and not just as a separate factor. Each case is assessed independently by different assessors and the evaluation depends on the assessor’s skills knowledge.
Ciba-Geigy method [21]
Expert consensus meetings have resulted in Ciba-Geigy method. Experts used their clinical judgment to assess adverse drug events and assign causality on a VAS. This method uses a checklist which is composed of 23 questions, which is split into three sections: (i) History of present adverse reaction, (ii) patient’s past adverse-reaction history, and (iii) monitoring-physician’s experience. This updated method was found to have a high degree of agreement (62%) when compared with evaluator’s assessments.
Loupi et al. method [22]
This method developed to assess the teratogenic potential of drug. The first sections of the algorithm sanction for the drug to be omitted if not implicated in the inception of the abnormality. The second section weighs the bibliographical data. The three questions consider alternative etiological candidates other than the drug; chronology of the suspect drug and other bibliographical data, to arrive at a conclusion on causality.
Roussel Uclaf causality assessment method [23]
This method is used in disease states such as liver and dermatological problems. A retrospect assessment of the reproducibility of this method among four experts had showed a 37-99% agreement rate.
Australian method [24]
Australian method involves the evidence which helps in to draw the conclusion, such as timing, and laboratory information from case reports presented and the antecedent cognizance on the suspect drug profile is deliberately omitted in the assessment.
Adverse Drug Reaction
At a normal cure occasionally the given specifics may harm the cases which are called Adverse medicine responses( ADR). Adverse medicine response is different from side effect. The evaluation of ADRs is most critical in the field of pharmacovigilance. Concerning retailed remedies, a suitable description of an adverse medicine response is as follows.
- Unlisted/Unexpected Adverse drug Reaction
An adverse response is the nature or harshness of medicine which isnt dependable with the proper product data available at the time of clinical trials. Company is demanded help during investigators folder for an unapproved medicine. detail summary of medicine data distance for an sanctioned product. ( 25)
2.Listed/ Anticipated Adverse medicine response
The information about ADR like nature or inflexibility and particularity of medicines is formerly recorded (26)
Adverse Medicine Responses Reporting
When the adverse response to medicines is potentially serious or clinically important, all health care workers including doctors , druggists, nurses and other health experts are requested to clarify it. It's necessary to report an adverse medicine response to pharmacovigilance.
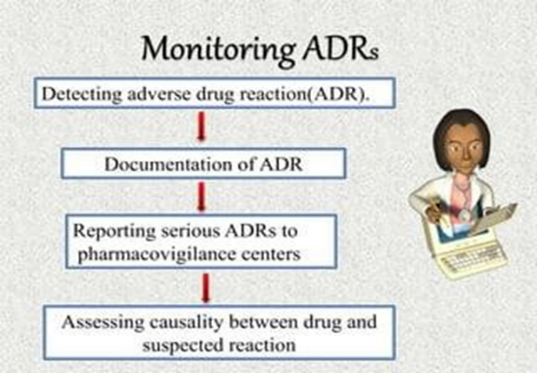
Fig No.2 (Monitoring of ADRs)
Spontaneous Reporting System:
- Regionalization
- Repossession of further data
- Access to all important pre and post marketing information.
- Detailed drug utilization data.
- Standardized Evaluation of causality and significance.
- Encouragement
Documentation of ADRs:
The pharmacovigilance class conveyed worldwide motivate that all suspected medicine affiliated adverse events should be outlined. It takes interests on reports of the following.
(A) Every adverse effect suspected or passed by new medicines and medicines of current issue( B) Attestation of colorful medicines that beget ADRs, which include death, life- hanging conditions disability ,hospitalization and natural abnormalities . The significant adverse response of any medicine should be notified within seven days. The other data related toad verse events should be informed within eight days.( Bates et al. 1995 Classen et al 1997). The ADR form can be collected through any pharmacovigilance center. After reviewing the form, the center forwards it to the indigenous center and after that, it's propelled to the zonal center (Goldman 1998 Palaian et al. 2006 Ravi Shankar et al. 2010). The details are also statistically audited and encouraged to WHO- Uppsala Monitoring commission (UMC). ( 27)
Procedure For Reporting ADRS:
It is the first duty of any pharmacovigilance center to report all suspected adverse events of the drug if found. Information regarding ADRs that should be reported and tabulated. Elements in ADR reporting Necessary information Others What should be reported Adverse reaction of drugs Medication over dose, ph. defect Who can report Doctors, Pharmacists, Nurses All government and private hospital staff When it can be reported Any adverse reaction if noticed -- How to report Through completely filled yellow form -- Where it can be reported Complete filled ADR form should be submitted to PV pI. -- Monitoring of ADRs ADR monitoring is the practice of continuously monitoring the undesirable effects caused using any drug. Pharmacovigilance plays an imperative impersonation in monitoring ADRs. It's essential for pharmaceutical controllers to screen their pharmaceutical products in the request and record if any suspected adverse responses are linked. ADRs can do by use of colorful pharmaceutical products, herbal medicines, cosmetics, medical bias, natural etc. Introducing this monitoring procedure intends at warranting that cases to admit safe and salutary medicinal products. {Karch and Lasagna 1997}. still, it may affect in noxious and serious goods of remedial products, If any of the adverse events arent stated. Therefore duly conducting ADR monitoring programs will help to reduce the dangerous goods of remedial products.
Benefits For Adr Monitoring:
An ADR monitoring and reporting program can furnish following benefits,
- It caters information about quality and safety of pharmaceutical products.
- It initiates threat- operation plans.
- It prevents the predictable adverse goods and helps in measuring ADR adherence.
- It instructs health care platoon i e .cases, druggists and nurses about adverse medicine goods and creates mindfulness regarding ADRs.
The main ideal of ADR monitoring is to expose the quality and frequency of ADRs and to identify the threat factors that can beget the adverse responses.( 28)
Serious Adverse Event
A serious adverse event( SAE) in mortal medicine trials are defined as any untoward medical circumstance
that's caused at any cure
- Results in death
- Is life hanging
- Bear in- case hospitalization
- extension of being hospitalization
- Causes natural anomaly/ birth disfigurement( 29).
Investigators in human clinical trial are obliged to report these events in clinical study reports.
Research suggests that these events are frequently deficiently reported in intimately available reports( 30).
Pharmacoviligance In India:
Fig no.3 (Pharmacovigilance in India)
India has further than half a million good qualified and 15,000 hospitals having a bed strength of. It's the fourth largest patron of medicinal. in the world. It's arising as an important trial mecca in the world. numerous new medicines are introduced in our country. thus, there's a need for a vibrant pharmacovigilance system in the country to cover the population from the implicit detriment that may be caused by some of these new medicines. easily apprehensive of the enormity of task the Central medicines Standard Control Organization( CDSCO) has initiated a well structured and largely participative National pharmacovigilance program. It's largely grounded on the recommendations the WHO document named" safety monitoring of medicinal products Guidelines for setting up and running a pharmacovigilance center”.( 31)
Future Aspects of Pharmacoviligance in India
Fig no. 4(future aspect pharmacovigilance in India)
The following proposals must be followed
With further and further clinical trials and other clinical exploration conditioning being conducted in India ,there an immense need to understand the significance of pharmacovigilance and how it impacts the life cycle of product. Given this situation, the DCGI should act snappily to ameliorate pharmacovigilance so as to integrate good pharmacovigilance practice in to the processes and procedures to insure nonsupervisory compliance and enhance clinical trial safety and post marketing surveillance.
A duly working pharmacovigilance system is essential if drugs are to be used safely. It'll profit all parties including health care professionals, nonsupervisory authorities, pharmaceutical companies and the consumers. It helps pharmaceutical companies to cover their drugs for threat and to concoct and apply effective threat operation
plans to save their medicines in delicate circumstances. The following proffers must be followed
- structure and maintaining a robust pharmacovigilance system.
- Making pharmacovigilance reporting obligatory and introducing pharmacovigilance
examinations.
- High- position conversations with colorful stake holders
•Strengthen the DCGI office with trained scientific and medical assessors for
pharmacovigilance
•Creating a single country specific adverse event reporting form to be used by all
•Creating a clinical trial and post marketing data base for SAEs SUSARs and ADRs for signal
discovery and
access to all applicable data from colorful stake holders
•List all new medicines suggestions by maintaining a standard data base for every medicinal
company
•Education and training of medical scholars, druggists and nursers in area of pharmacovigilance
•uniting with Pharmacovigilance associations in enhancing medicine safety with advancements in information technology there has been the emergence of new openings for public and transnational.
•erecting a network of pharmacovigilance and pharmacoepediologists in India. (32).
Aims Of Pharmacovigilance
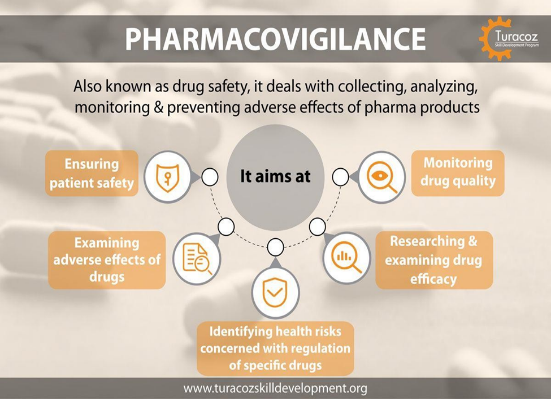
Fig no.5(The aims of Pharmacovigilance)
The aims of pharmacovigilance are ;
The identification and quantification of preliminarilyun-recognized adverse medicine response (ADR).
- The identification of sub-groups of cases at particular threat of ADRs (the threat relating to cure, age, genderand underpinning complaint).
- The continued monitoring of a safety of a product, throughout the duration of it use, to insure that its pitfalls and benefits remains respectable. This includes safety monitoring following significant recently approved suggestions.
- The relative adverse medicine response profile of products within the same remedial class.
- The discovery of unhappy tradition administration.
- The farther explication of a product pharmacological/ toxicological parcels and the medium by which it
produces adverse medicine responses.
- The discovery of significant medicine- medicine relations between new products and co-therapy with agents formerly established on the request, which may only be detected during wide use.( 33)
Importance Of Pharmacovigilance
Significance Of Pharmacovigilance
When a pharmaceutical medicine is introduced in the request there are still a lot of effects that are unknown about the safety of the new medicine.
These drugs are used by colorful cases for different conditions who might be using several other medicines and must be following different traditions and diets which may negatively affect the impact of drug in them. Also the same drug might differ in the manner of their product and constituents. also adverse medicine responses might also do when medicines are taken along with traditional and herbal drugs which should be covered through pharmacovigilance. In some cases, adverse medicine responses of certain drug might do only in one country or region.
To help all overdue physical, internal and fiscal suffering of cases, pharmacovigilance proves to be an important monitoring system for the safety of drugs in a country with the support of croakers , druggists, nurse and other health professionals of the country.
The significance of pharmacovigilance is as follows.
- Safety monitoring of medicinal products.
- Clinical trials.
- Pharmacoepidemiological studies.
- Case reports.
- Developing case series.
- Analysis of case series.
- Use of data mining to identify product- event combination.
- robotic reporting.( 34)
Steps In Pharmacovigilance Programme

Fig no.6(steps involve in pharmacovigilance programme)
1.Chancing the threat of a medicine
2.Clinical trials
3.Pharmacoepidemiological study
4.Case report
5.Developing case series
6.Analysis of case series
7.Use of data mining to identify product- event combination
8.robotic reporting.
Conditioning In Pharmacovigilance Operations
•Case Registry
•Triage
•Registry
•Registration
•Processing
•Data Entering
•Coding
•Labelling Medical Review
•Serious Case
•Medical Review
•Non-Serious Listing Review
•Aggregate Report Review
•Aggregate Reports
•Analysis And Creation of IND/ NDA Reports
-
- Analysis And Creation of Padre Reports
- Analysis And Creation of Psur & Bridge Reports
- Parteners In Pharmacovigilance
A complex and vital relationship exists between wide rangeo mates in the practice of medicine safety monitoring. Sustained collaboration and commitment are vital if unborn challenges in pharmacovigilance areto be met in order to develop and flourish.
-
- Government
- Assiduity
- Sanitarium and academia (35)
- Bane information centers
- Health professionals
- Cases
- Consumers
- Media
- WHO (36,37)
Pharmacist's Role in Pharmacovigilance:

Fig no7. (Pharmacist role in pharmacovigilance)
druggists enthrall a central and necessary part in the realm of pharmacovigilance, laboriously contributing to the identification ,assessment, and mitigation of adverse medicine responses( ADRs). Their unique position at the frontline of patient care equips them with the moxie to fete and manage drug- related pitfalls. druggists play a critical part in comforting cases about implicit side goods, icing proper specifics age, and conducting drug reviews to identify any signs of ADRs. Beyond patient relations, druggists are integral in the reporting and attestation of ADRs, acting as watchful doorkeepers who contribute precious data to pharmacovigilance databases.
Their part extends to promoting a culture of safety within healthcare settings, emphasizing the significance of accurate medicine allocating, covering medicine relations, and laboriously engaging in nonstop education. As the healthcare geography evolves, druggists are decreasingly using digital tools and electronic health records to enhance their pharmacovigilance benefactions, eventually fostering a safer and further informed approach to drug operation.( 38,39)
CONCLUSION
Pharmacovigilance is the only way to insure the safety of the medicine throughout the life cycle. It's veritably important pivotal as the clinical trials have limitation to descry the rare and veritably rare ADRs.
The knowledge and information available regarding safety of any medicine is veritably important to take applicable decision by medicine controllers to safe guard public health. Health care professionals are the main journalists of the ADRs. still there are high probabilities of under- reporting reported encyclopedically. It's the major challenge of moment. In malignancy of those limitations, robotic reporting system remains as amost extensively habituated system to report ADRs and is suitable to induce signal of rare and veritably rare types of ADRs. However, we can make our world safer than what's moment, If all the health care professionals take ADR reporting as an ethical obligation and a major responsibility. Every reporting by health care professionals is important, indeed though focus on the serious unlabelled types of ADRs is more important. There are significant goods on the pharmacovigilance to make it more functional after theconception has surfaced and day by day we're getting near to the fortune. It's our responsibility to insure well performing of pharmacovigilance system. ADR reporting should be taken as a veritably important duty not as an redundant clinical burden by health care professionals to insure the safer medicines use throughout the world.
REFERENCES
- World Health Organization The importance of pharmacovigilance- safety monitoring of medicinal products .WORLD HEALTH ORGANIZATION, Geneva,2002.
- WHO. Pharmacovigilance: Ensuring the Safe Use of Medicines. Geneva: WHO; 2004.
- Shuka SS,Gidwani bina,pandey R.Rao SP,Singh V and Vyas Amber,’’Importance of pharmacovigilance in Indian pharmaceutical industry’’ Asian Journal of Research In pharmaceutical science,2,2012,04-08
- WHO. Policy Perspectives on Medicines. Geneva: WHO; 2004.
- Skalli S, Soulaymani Bencheikh R. Safety monitoring of herb-drug interactions: A component of pharmacovigilance. Drug Saf 2012;35(10):785-91.
- Arnott J, Hesselgreaves H, Nunn AJ, Peak M, Pirmohamed M, Smyth RL, et al. What can we learn from parents about enhancing participation in pharmacovigilance? Br J Clin Pharmacol 2013;75(4):1109-17.
- Gerritsen R, Faddegon H, Dijkers F, van Grootheest K, van Puijenbroek E. Effectiveness of pharmacovigilance training of general practitioners: A retrospective cohort study in the Netherlands comparing two methods. Drug Saf 2011;34(9):755-62.
- Kshirsagar N, Ferner R, Figueroa BA, Ghalib H, Lazdin J. Pharmacovigilance methods in public health programmes: The example of miltefosine and visceral leishmaniasis. Trans R Soc Trop Med Hyg 2011;105(2):61-7. 8
- WORLD HEALTH ORGANIZATION. Safety monitoring of medicinal products; guidelines for setting up and running a pharmacovigilance center, WORLD HEALTH ORGANIZATION ;2OOO Google scholer
- Pipasha,B. Arun .K.B ‘ Setting standards for proactive pharmacovigilance in India ; The way foreword, Indian J pharmacol , 2007;39[3];124-128.
- Garashi HY, Steinke DT, Schafheutle EI. A Systematic Review of Pharmacovigilance Systems in Developing Countries Using the WHO Pharmacovigilance Indicators. Ther. Innov. Regul. Sci. 2022;56:717-743.
- Dr R. history And Development of pharmacovigilance. and others,editor;. p. 1–10
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA 1998;279(15):1200-5.
- Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: Application to drug-induced liver injuries. J Clin Epidemiol 1993;46(11):1323-30.
- Agbabiaka TB, Savovic J, Ernst E. Methods for causality assessment of adverse drug reactions: A systematic review. Drug Saf 2008;31(1):21-37.
- Macedon AF, Marques FB, Ribeiro CF, Texeira F. Causality assessment of adverse drug reactions: Comparison of the results obtained from published decisional algorithms and from the evaluations of an expert panel. Pharmacoepidemiol Drug Saf 2005;14(12):885-90.
- Dangoumau J, Evreux JC, Jouglard J. Mehtod for determination of undesirable effects of drugs. Therapie 1978;33(3):373-81. 1
- Kramer MS, Leventhal JM, Hutchinson TA, Feinstein AR. An algorithm for the operational assessment of adverse drug reactions. I. Background, description, and instructions for use. JAMA 1979;242(7):623-32
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30(2):239-45.
- Logier G, Vincens M, Castot A. Imputability in drug monitoring. Principles of the balanced drug reaction assessment method and principal errors to avoid. Therapie 1983;38(3):303-18.
- Venulet J, Ciucci A, Berneker GC. Standardized assessment of drug-adverse reaction associations –
- Rationale and experience. Int J Clin Pharmacol Ther Toxicol 1980;18(9):381-8.
- Loupi E, Ponchon AC, Ventre JJ, Evreux JC. Imputability of a teratogenic effect. Therapie 1986;41(3):207-10.
- Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: Application to drug-induced liver injuries. J Clin Epidemiol 1993;46(11):1323-30
- Mashford ML. The Australian method of drug-event assessment. Special workshop – regulatory. Drug Inf J 1984;18(3-4):271-3.
- Joerg H. Basic principles of pharmacovigilance and data sources.
- 26Sachdev Y. pharmacovigilance: safety matters, Indian pharmacology. February 2008; 40
- Hall et al. 1995; Horbuckle et al. 1999; Tuntti and Neuroren 2002.
- Moore N (2001). The role of clinical pharmacologist in management of ADRs. Drug safety, 211(1): 1- 7.
- The importance of pharmacovigilance; safety monitoring of medical products, Geneva, WHO, 2002.
- J.P Ioannidis, J Lau. Completeness of safety reporting in randomized trials: an evaluation of seven medical areas. JAMA, 2001; 285(4): 437-443.
- P Biswas, A. K. Biswas setting standards for proactive pharmacovigilance in India: the way forward. Indian Journal of pharmacology, 2007; 39: 124-128.Ronaldd M, Elizabeth BA.
- Moore N. The role of clinical pharmacologist in the management of ADRs. Drug safety, 2001; 24(1): 1-7.
- Hall M, Mc Cormack P, Arthur N, Feely J. The spontaneous reporting of ADRs by nurses. British journal of clinical pharmacology, 1995; 40: 173-175.
- Hornbuckle K, WUH-H, Fung MC. Evaluation of spontaneous adverse event of reports by primary reporter a 15 year review (1983-1997). Drug information journal of clinical pharmacology, 1995; 40: 173-175.
- Consumer reporting ADRs. WHO drug information, 2000; 14: 211-215
- Egberts GPG Smulderes M, De Konig FHP et al. Can ADRs be detected earlier? A comparision of reports by patients and professionals. British Medical Journal, 1996; 313: 530-531.
- Beninger P. Pharmacovigilance: an overview. Clinical therapeutics. 2018 Dec 1;40(12):1991-2004.
- Maqbool M, Dar MA, Rasool S, Bhat AU, Geer MI. Drug safety and Pharmacovigilance: An overview. Journal of Drug Delivery and Therapeutics. 2019 Apr 15;9(2-s):543-8


 Bhagyshri Pagare*
Bhagyshri Pagare*
 Vaishnavi Jadhav
Vaishnavi Jadhav






 10.5281/zenodo.14584390
10.5281/zenodo.14584390