Abstract
Background:
Periodontitis is inflammation of the supporting tissues of the teeth caused by specific microorganisms. Intra-periodontal pocket, mucoadhesive drug delivery systems have been shown to be clinically effective in the treatment of periodontitis. The aim of this study was to formulate a mucoadhesive oral gel from the Ketorolac Tromethamine for the treatment of periodontitis.
Materials and Methods:
The semisolid concentrated extracts were incorporated in gel base. mucoadhesive oral gel were prepared using Carbopol 934, sodium carboxymethylcellulose (sodium CMC) and hydroxypropyl methylcellulose K100M (HPMC) as bio adhesive polymers. Physicochemical tests, mucoadhesive strength measurement and in vitro drug release study were carried out. There were total 8 batches of different polymers with different concentration are prepared. Out of these F3 batch containing drug and polymer Carbapol 934, chitosan and HPMC (1:2:4) were found to be an optimized batch. It showed satisfactory percent drug diffuse that is 94.95 % and also better result of other evaluation parameter like Drug content .
Results:
The main aim of the presence study was to formulate and evaluate oral mucoadhesive gel by using Ketorolac tromethamine. Total eight formulations containing different concentration of mucoadhesive polymer, secondary gelling agent and penetration enhancer were prepared. All the prepared batches were evaluated for pH, Spreadibility, Viscosity, diffusion study, drug content and stability studies. All the formulation found to be clear and slightly yellowish appearance with no phase separation. Out of all the batches, Batch F3 was optimized and it shows drug diffusion 94.95 % and it satisfied all the required parameters. So it was concluded that mucoadhesive oral gel of Ketorolac tromethamine was successfully Formulated & its shows better therapeutic efficacy over conventional gel.
Conclusion:
The ideal formulation for the treatment of periodontitis should exhibit high value of Mucoadhesion, show controlled release of drug and be easily delivered into the periodontal pocket preferably using a syringe. Based on in vitro release and mucoadhesion studies, F3 was selected as the best formulation.
Keywords
Mucoadhesive oral gel, Ketorolac tromethamine, Carbopol 934, sodium carboxymethylcellulose (sodium CMC)
Introduction
Mucoadhesive drug delivery systems are delivery systems which utilize the property of bio adhesion of certain polymers which become adhesive on hydration and hence can be used for targeting a drug to a particular region of the body for extended periods of time. Bioadhesion is an interfacial phenomenon in which two materials, at least one of which is biological, are held together by means of interfacial forces. The attachment could be between an artificial material and biological substrate, such as adhesion between a polymer and a biological membrane. In the case of polymer attached to the mucin layer of a mucosal tissue, the term “mucoadhesion” is used. Scaling and root planning is not always adequately effective. This has led to the adjunctive use of antibiotics, usually in the form of a local delivery system. Because periodontal infections may contain a wide variety of bacteria, no single antibiotic is effective against all putative pathogens. Now, an ideal antibiotic for the treatment of periodontal disease does not exist. In periodontitis, antioxidant activities of saliva and crevicular fluid decrease. Therefore, the use of antioxidants may inhibit periodontal disease development. Intra-periodontal pocket, mucoadhesive drug delivery systems have been shown to be clinically effective in the treatment of periodontitis. Periodontal pocket is able to retain a delivery system for a desired period of time. Natural herbs can help stop or reverse the development of periodontitis. Plants are cost-effective and have fewer side-effects. It would be expected that local delivery of an herbal product into periodontal pocket as an adjunct to scaling and root planning, which has antibacterial, antioxidant activities, anti-inflammatory and analgesic effects and also can control bleeding on probing would be advantageous. A significant characteristic of the oral gel is mucoadhesive strength for adhesion to the mucosa in the dental pocket. Good gel adhesion to the mucosal surface results in prolonged residence time and contact time and better clinical efficacy. Beside increase the residence time and contact time of gel with the mucosa, drug release from the gel must be controlled. Gels are able to control drug release. Ketorolac inhibits the enzyme cyclooxygenase that converts arachidonic acid to thromboxane, prostacyclin, and prostaglandins. Prostaglandins secreted at the site of injury/inflammation augment the sensitization of afferent nerve endings.1 However, there are some grounds to confirm that NSAIDs may have a central mechanism of action due to its effect on the hypothalamic prostaglandin system. In addition, central (serotoninergic, beta-endorphin, and monoaminergic) pathways involved in nociception may also be affected by the NSAIDs. This confirms the limited entry of ketorolac and its active metabolites into CSF, indicating that the central prostaglandin synthase inhibition is not significant. However, there still remains the possibility of a central mechanism of action due to lack of data regarding the sensitivity and amount of drug required for inhibition of the central prostaglandin system and central pathways of nociception.
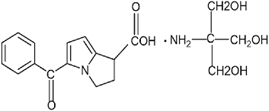
Fig. 1: Structure of Ketorolac Tromethamine
The aim of this study was to formulate a mucoadhesive oral gel from the Ketorolac Tromethamine been adjunct to scaling and root planning for the treatment of periodontitis, which has antiseptic, anti-inflammatory and analgesic effects and for the control of bleeding on probing.
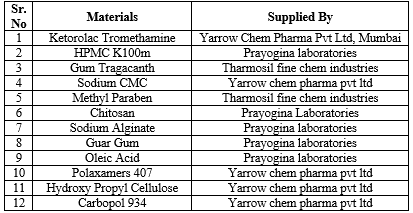
Table no 1: List of drug and chemical used and their manufacturers

EQUIPMENTS USED:
Table No 2: List of the instrument and their manufactures
EXPERIMENTAL WORK:
PREFORMULATION STUDIES:
Preformulation testing is an investigation of physical and chemical properties of drug substances alone and when combined with pharmaceutical excipients. It is the first step in the rational development of dosage form.
Physical characterization of drug sample
Ketorolac Tromethamine was supplied as a gift sample by Yarrow Chem Pharma Pvt Ltd, Mumbai and was characterized for its identification and authenticity. The drug was physically characterized according to following methods-
Experimental work
The received sample of Ketorolac Tromethamine was subjected to the following tests for its characterization:
- Color of the drug sample:
A white or almost white, crystalline powder
- Melting point of the drug sample:
The melting point is one of the important methods for the identification of the drug sample. The melting point of the given drug sample was carried out using Melting Point Apparatus. Melting point of the drug was determined by the capillary method using melting point apparatus. It can be performed by filling of the drug in capillary tube by pressing the open end gently into pure drug sample and packed by tapping the bottom of the capillary on a hard surface so that the drug pack down into the bottom of the tube. The apparatus was started and dip the thermometer in this liquid paraffin and note the point which drug started melting in the capillary.
- Solubility
The solubility of the drug sample was carried out in different solvents( organic) according to the Indian Pharmacopoeia 1996. The results are then compared with those given in the official books and L.P. 1996.
Drug Identification
Experimental work
- By Ultraviolet absorption spectroscopy
A solution of 2 µg/ml concentration containing ketorolac tromethamine was prepared in Phosphate buffer of pH 6.8 and was scanned between 322nm for getting the absorb and in.
- Drug Excipient Compatibility Studies
FT-IR Spectroscopy
It's important to check any kind of interaction between drug candidate and polymer. The polymers which are to be incorporated into formulation should be compatible with the drug This compatibility study or interaction study was done using Fourier Transformed Infrared Spectroscopy.
IR Spectra of pure Ketorolac Tromethamine and Polymer Viz. Carbapol 934, Chitosan and HMPC K100M were taken separately. Then to know if there is any interaction between and polymer. IR spectra of Ketorolac Tromethamine and polymers were taken in combination. The results shows that there was seen between drug Ketorolac Tromethamine and polymers as there was no significant change in the pattern of peaks of optimized batch of mucoadhesive oral gel with pure drug.
C. Preparation of standard calibration curve of ketorolac tromethamine
- Calibration curve of ketorolac tromethamine in Phosphate buffer of pH 6.8 :
- Preparation of Phosphate buffer of pH 6.8
- Preparation of 0.2M sodium hydroxide:
Dissolve about 8 g of sodium hydroxide in sufficient quantity of distilled water and made up to 1000ml with distilled water.
- Preparation of 0.2M potassium dihydrogen phosphate:
Dissolve potassium phosphate about 27.218g in sufficient quantity of distilled water and made up to 1000ml with distilled.
- Preparation of Phosphate buffer of pH 6.8:
Take about 50ml of potassium dihydrogen phosphate in a 200ml volumetric flask and add 22.4ml of 0.2M Sodium hydroxide and made up to 200ml with distilled water. Check the pH of resulting solution and adjust to pH 6.8 by using 0.2M sodium hydroxide solution.
- Calibration curve of Ketorolac Tromethamine in Phosphate buffer of pH 6.8:
10mg drug Ketorolac Tromethamine was dissolved in phosphate buffer of pH 6.8 and volume was make up to 100 ml to make stock solution of concentration 100µg/ml. Then 2ml of Rock solution was taken and diluted upto 100ml with the buffer of pH 6.8 to get concentration of 2 µg/ml and in similar way dilutions were made as 2, 4, 6, 8, 10 µg/ml respectively and absorbance was measured at 322 nm by UV visible spectrophotometer. The absorbance values were plotted against concentration (µg/ml) to obtain the standard calibration curve.
COMPOSITION OF ORAL MUCOADHESIVE GEL:
Formulation Batches:
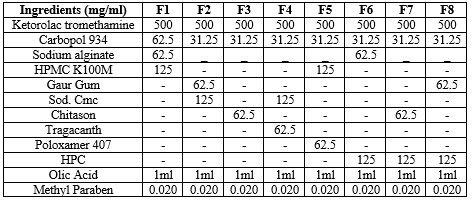
Table No 3: Different batches of oral Mucoadhesive Gel
METHOD OF PREPARATION OF MUCOADHESIVE ORAL GEL :
The required amount of carbopol-934P were weighed and and dispersed slowly to a beaker containing 12.5 ml distilled com water with continuous stirring at 400-600 rpm for 1h until it pota forms a clear solution. Care was taken to avoid a indispensable lumps of the polymers. Accurately weighed Ketorolac Tromethamine were was added to the carbopol solution by stirring for 3-4 h to obtain a homogeneous dispersion". Then, 6.25ml distilled water and the gelling agent were added slowly under continuous magnetic stirring at 100 rpm. The final quantity was made up to 25 g with distilled water. Care was taken to avoid the formation of air bubbles. The mixture was allowed to stand for air bubbles to separate. The pH was further adjusted to 6.75 ± 0.05 with 10% w/v sodium hydroxide solution as a simulation of oral pH12. The prepared gel was kept for 24 hr for complete polymer desolation.
EVALUATION OF ORAL MUCOADHESIVE GEL:
Spreadibility test:
Spreadibility test was carried out for all the formulations. The Spreadibility indicates that the mucoadhesive oral gel is easily spreadable by small amount of shear. Spreadibility of the mucoadhesive oral gel decreases with the increase in the concentration of the polymer. The spreadability is very much important as it shows the behavior of mucoadhesive oral gel when it comes out from the tube.
pH:
pH of prepared formulations were measured by using digital pH meter. The solution of mucoadhesive oral gel, was prepared by using 100ml of distilled water and set aside for 2 hrs. pH was determined in triplicate for the solution and average value was calculated.
In-Vitro Diffusion study:
The in vitro drug diffusion studies of the mucoadhesive oral gel were carried out in modified Diffusion sell using Dialysis membrane (dry, unwashed, open ended, flat width: 28.46 mm; inflated diameter: 17,5 mm: Length: 1 m). The membrane was soaked in phosphate buffer pH 6.8 for 9. 12 h was clamped carefully to one end of the hollow glass tube of dialysis cell (23 cm diameter, +16 cm2 area). Then mucoadhesive oral gel was spread uniformly on the dialysis membrane. 25 ml of phosphate buffer was taken in a beaker, which was used as receptor compartment. The donor compartment was kept in contact with receptor compartment. This whole assembly was kept on a magnetic stirrer and the solution on the receptor side was stirred continuously using a magnetic bead and temperature of the cell was maintained at 37°C. A similar blank set was run simultaneously as a control. Sample (2 ml) was withdrawn at suitable time intervals and replaced with equal amounts of fresh dissolution media. The Samples were analyzed UV- spectrophotometrically at 322 nm and the cumulative percent drug release was calculated. The difference between the readings of drug release and control was used as the actual reading in each case.

Fig 2: Franz Diffusion cell
Viscosity Determination:
The measurement of viscosity of the prepared mucoadhesive oral gel was completed by using Brookfield Viscometer. The mucoadhesive oral gel was rotated at 20 and 30 rpm using spindle no.3 (LV) at each speed and the corresponding dial reading was noted.
Stability study:
The mucoadhesive oral gel formulations were submitted to one months for stability testing at 40°C and 75% RH. At one month’s interval, parameter such as appearance, pH, drug content, in- vitro drug release were evaluated as per the ICH guidelines Q1C.
Drug Content:
Drug content was studied by an accurately weighing 100 mg of mucoadhesive oral gel and was dissolved in 100 ml of phosphate buffer (pH 6.8). Then the solution was stirred continuously for 24 hours on a magnetic stirrer. Then the whole solution was sonicated. After sonication and subsequent filtration, the drug in solution was estimated UV- spectrophotometrically by appropriate dilution.
RESULTS AND DISCUSSION:
PREFORMULATION STUDY:
Preformulation studies are an investigation of physical & chemical properties of drug substance alone and when combined with excipient. Preformulation involves the application of biopharmaceutical principles to the physicochemical parameters of drug substance are characterized with the goal of designing optimum drug delivery system. In these prior studies taken before the formulations of various dosage forms.
Physical Characterization of Drug Sample:
The results of physical characterization of the drug candidate are as follows-
The received sample of Ketorolac Tromethamine was found to show the following characteristics.
Description:
A white or almost white, crystalline powder
1670 C
Soluble in organic solvent such as ethanol , DMOS & dimethyl formamide (DMF) and phosphate buffer 6.8
- Identification of drug:
- Determination of max by UV-Vis Spectrophotometer:
- The absorption maxima of Ketorolac Tromethamine were determined by scanning the sample drug solution concentration in double beam UV spectrophotometer for range of 322 nm and standard specification given in Indian pharmacopoeia or literature
FTIR study of Ketorolac tromethamine
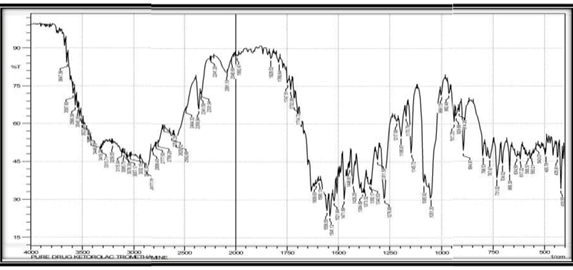
Table No 4: FTIR study of Ketorolac tromethamine
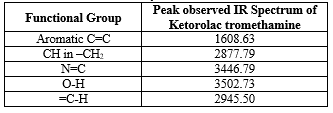
FTIR study of Ketorolac Tromethamine with excipient
Fig 4: FTIR study of Ketorolac Tromethamine with excipient
Table No 5: FTIR study of Ketorolac Tromethamine with excipient

Calibration curve of Ketorolac Tromethamine buffer pH 6.8
The calibration curve for Ketorolac Tromethamine in pH 6.8 in concentration range of 2 -10 ug/ml was found to pass through the origin and was a straight line. The results are show in below table No.6
Table No 6: Calibration curve of Ketorolac Tromethamine buffer pH 6.8
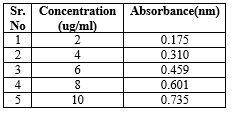

Fig No 5: Calibration curve of Ketorolac Tromethamine buffer pH 6.8
CHARACTERIZATION OF MUCOADHESIVE ORAL GEL:
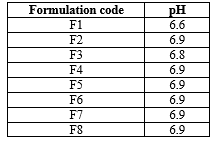
pH of the Formulation:
It is known that the normal physiological pH of oral mucosa is 6.8-7.4
Table No 7: pH prepared formulation
Physical Character of Formulated batches of Mucoadhesive Oral Gel :
Table no 8: Physical Character of Formulated batches of Mucoadhesive Oral Gel

STABILITY STUDY:
Stability study was performed on F3 formulations. The preparations were packed in glass ware container (20 g) and subjected to stability studies at 40°C / 75 % RH, for a period of 1 month. Samples were withdrawn at interval of 30 days and were evaluated for physical appearance, rheological properties, and drug content. All the test results were found to be in limits. Hence the formulations were stable under stated storage condition.
Drug Content Determination:
The drug content of mucoadhesive oral gel was determined by UV-Spectrophotometer at 322 nm. Drug concentration range from 10.57 to 88 % Formulation batch F3 shows highest drug content.
Table No 10: Drug content

Calculation:
Drug Content for Formulation Batch F3,
Y= mx + c
Y= 0.0706 x + 0.0327
x= 0.835 – 0.00327
0.0706
x= 0.8023
0.0706
x=11.36 µg/ml
x=1136 µg/100ml
x=1.136 mg/100ml
100 mg = 100 X 1
1.13
= 88 %
Spreadibility:
When 1gm of mucoadhesive oral gel applied on the skin then(f3) formulation easily spread on the skin
In-Vitro diffusion study:
In-vitro diffusion study was carried out for 8 hours. Table no.24 shows the % drug diffusion. The highest % drug diffusion release was achieved formulation by batch F3 (94.95 %)
Table No. 11: In-Vitro diffusion study
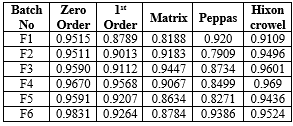

Fig 6: % Cumulative Drug of Formulation F1 to F4
Fig 7: % Cumulative Drug of Formulation F5 to F6
Drug release Kinetics:
In-vitro drug release for all formulations of the Ketorolac Tromethamine mucoadhesive oral gel was fixed into different equations and kinetic models to explain the release kinetics. Calculated regression coefficient Ketorolac Tromethamine mucoadhesive oral gel (R) of formulations for different kinetics models were shown in following tables. drug release from optimized batch F3 mucoadhesive oral gel formulation followed Hixon crowel.
Table No 12: Drug release Kinetics


Fig 8: Drug release Kinetics
SELECTION OF OPTIMISED FORMULA:
There was total 8 batches of different polymers with different concentration are prepared. Out of these F3 batch containing drug and polymer carbapol934, chitosan and HPMC (1:2:4) were found to be an optimized batch. It showed satisfactory percent drug diffuse that is 94.95 % and also better result of other evaluation parameter like Drug content was 88it will follow Hixon crowel order release kinetic model Hence on the basis of these evaluation parameter batch F3 was optimized.
SUMMARY AND CONCLUSION:
Oral mucoadhesive gel have emerged as one of the most interesting mucoadhesive oral drug delivery system, it prolongs the residence time of the dosage form at the site of absorption hence increase the bioavailability. It shows the excellent accessibility rapid onset of action and drug rapid absorption because of enormous blood supply and good flow rate because of drug is protected from degradation in the acidic environment in the git and improved the patient compliance.
The main aim of the presence study was to formulate and evaluate oral mucoadhesive gel by using Ketorolac Tromethamine. Total eight formulations containing different concentration of mucoadhesive polymer, secondary gelling agent and penetration enhancer were prepared. All the prepared batches were evaluated for pH, Spreadibility, Viscosity, diffusion study, drug content and stability studies. All the formulation found to be clear and slightly yellowish appearance with no phase separation. Out of all the batches, Batch F3 was optimized and it shows drug diffusion 94.95 % and it satisfied all the required parameters. So it was concluded that oral mucoadhesive gel of Ketorolac tromethamine was successfully Formulated & its shows better therapeutic efficacy over conventional gel.
REFERENCES
- Priya Mahajan, Amanpreet Kaur, Geeta Aggarwal, S.L. Harikumar, Mucoadhesive Drug Delivery System: A Review, International Journal of Drug Development & Research January-March 2013,Vol. 5.
- Ms. Chavan Mayuri, Dr. Pradum Ige Mucoadhesive Drug Delivery Systems An Overview Journal of Pharmaceutics and Drug Development 2023 Volume 10 Issue 1.
- Rahamatullah Shaikh, Thakur Raghu Raj Singh, Martin James Garland, A David Woolfson, J Pharm Bio allied Sci. Jan-Mar 2011Volume 3 Issue 1
- Shiva Golshani, Alireza Vatanara, Mohsen Amin, Recent Advances in Oral Mucoadhesive Drug Delivery, J Pharm Pharm Sci, 2022.
- Pranshu Tangri, N.V. Satheesh Madhav, Oral Mucoadhesive Drug Delivery Systems A Review International Journal Of Biopharmaceutics,2011 Volume 2 Issue 1.
- B. Saraswathi, Anna Balaji, M.S. Umashankar Polymers in Mucoadhesive Drug Delivery System-Latest Updates International Journal Of Pharmacy And Pharmaceutical Sciences, 2013 Vol 5, Supp 3.
- Fabrizio Ricci, Giuseppe Francesco Racaniello, Nunzio Denora, Thermo responsive mucoadhesive hydrogel based on Pluronic F127/thiolated glycol chitosan for intravesical administration of celecoxib/gemcitabine, Journal of Drug Delivery Science and Technology, September 2023, Volume 86.
- Aya A. Mabrouk, Nesrine S. El-Mezayen, Novel mucoadhesive celecoxib-loaded cubosomal sponges: Anticancer potential and regulation of myeloid-derived suppressor cells in oral squamous cell carcinoma, European Journal of Pharmaceutics and Biopharmaceutics, January 2023, Volume 182.
- Santoshi T. Chavan, Madhavi M. Namanwar, Satish B. Kosalge, Shubham V. Jadhav, Formulation and evaluation of herbal oral gel, Journal of Emerging Technologies and Innovative Research, January 2023, Volume 10, Issue 1.
- Mana Heidari & Mohsen Salmanpour, In-situ fast-prepared mucoadhesive oral gel for palliative treatment of chemotherapy-induced mucositis: preparation, characterizations and pre-post study, Journal of Sol-Gel Science and Technology, 2023, Volume 108.
- Manisha Pandey, Hira Choudhury, Mucoadhesive nanocarriers as a promising strategy to enhance intracellular delivery against oral cavity carcinoma, Pharmaceutics 2022.
- S. Bhattacharyya, R. Reddy HV, In vitro evaluation of mucoadhesive in situ nanogel of celecoxib for buccal delivery, Annales Pharmaceutiques Françaises, 2021.
- Bo?ena Grimling, Formulation and characterization of mucoadhesive dental applications containing benzylamine hydrochloride, Department of Drugs Form Technology. Faculty of Pharmacy. Wroclaw Medical University.
- Kanchan Upadhye, Kirti Charde, Gouri Dixit, Suparna Bakhle, Formulation and evaluation of herbal gel for management of mouth ulcers, Indian Journal of Pharmacy and Pharmacology 2021;8(3).
- Fatmanur Tugcu-Demiroz, Melike Ongun, Emre Tuncel, Esra Kodan, Fahriye Figen Tirnaksiz, Development and characterization of mucoadhesive-thermosensitive buccal gel containing metronidazole for the treatment of oral mucositis, J. Fac. Pharm. Ankara / Ankara Ecz. Fak. Derg., 2020, 44(3).
- Mohammadi Samani S.1, Karimaddini S.1, Sobhani Z.2, Ahmadi F, Preparation and evaluation of an oral mucoadhesive gel containing nystatin-loaded alginate microparticles, Er. Pharm. J. 2020, 67(2).
- Richa Sing, Sagar Bansal and Manoj Kumar Mishra, Formulation and Evaluation of Herbal Oral Gel Containing Extracts of Powdered Psidium guajava Linn Leaves with Curcuma longa Linn Rhizomes to Treat Mouth Ulcer, International Journal of Drug Development and Research, 2020, Vol.12 No.2.
- Hiroomi Sakurai, Yuri Ikeuchi-Takahashi, Ayaka Kobayashi, Formulation Development of Mucoadhesive Microparticle-Laden Gels for Oral Mucositis: An In Vitro and In Vivo Study, Pharmaceutics 2020, Department of Pharmacy.
- Roaa A. Nief, Manar Adnan Tamer, Shaimaa Nazar Abd Alhammid, Mucoadhesive oral in situ gel of itraconazole using pH-sensitive polymers: Preparation, and in vitro characterization, release and rheology study, Drug invention today, Vol 11, 2019.
- S. Maru1, D.S.B. Ongarora and R.W. Njoroge, Formulation and in vitro evaluation of a mucoadhesive metronidazole dental gel for oral application, east and central African journal of pharmaceutical sciences Vol. 2019.
- Hemant K. Jain, Prerana N. Swami, K. N. Gujar, Formulation and evaluation of an antimicrobial mucoadhesive dental gel of azadirachta indica and glycyrrhiza glabra, International Journal of applied Pharmaceutics, 2019 Vol 11, Issue 2.
- Maria Teresa Junqueira Garcia, Cristhiane de Paula Freitas, Chitosan-based mucoadhesive gel for oral mucosal toluidine blue O delivery: The influence of a non-ionic surfactant, Photodiagnosis and Photodynamic Therapy, December 2017, Volume 20.
- Abolfazl Aslani, Alireza Ghannadi1, Hajar Najafi, Design, formulation and evaluation of a mucoadhesive gel from quercus brantii l. and Coriandrum sativum L. as periodontal drug delivery, Advanced Biomedical Research, April - June 2013, Vol 2.
- Shrinath Shah, Sulekha Bhadra, Mucoadhesive in-situ gel for transmucosal delivery of celecoxib, Int J Pharm Pharm Sci, 2014, Vol 6, Issue 10.
- Yara Peluso Cid, Vinícius Pedrazzi, In vitro characterization of chitosan gels for buccal delivery of celecoxib: influence of a penetration enhancer, AAPS Pharm SciTech, Vol. 13, No. 1, March 2012.
- Halah T. Sulayman, some variable affecting formulation of tinidazole mucoadhesive oral gel, AJPS, 2011, Vol. 10, No.2.
- N. M. Harish, P. Prabhu, R. N. Charyulu, M. A. Gulzar and E. V. S. Subrahmanyam, Formulation and evaluation of in situ gels containing clotrimazole for oral candidiasis, Indian J Pharm Sci, 2009, 71 (4).
- Indian Pharmacopeia 2018 volume 2.
- National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 3825, Ketoprofen. Retrieved January 12, 2024 from https://pubchem.ncbi.nlm.nih.gov/compound/Ketoprofen.
- Raymond, C. R. P. J. S. M. E. Q., et al. Handbook of pharmaceutical excipients. Pharmaceutical press, 2005.


 Gokarna Ingale*
Gokarna Ingale*

















 10.5281/zenodo.12807647
10.5281/zenodo.12807647