Abstract
Oral mucosal drug delivery is a novel route for systemic drug delivery, which not only has many advantages but also has many potentials. Sublingual, meaning "under the tongue," is the method of drug administration in which the drug substance is placed under the tongue alone for rapid absorption through the vessels beneath the tongue. The sublingual route bypasses hepatic first-pass metabolic processes, which results in better bioavailability, faster onset of action, and greater patient compliance. If sublingual medications are used, the onset of action is faster than that expected for orally administered tablets. Sublingual tablets disintegrate quickly, and very little saliva secretion is usually enough for rapid disintegration along with good dissolution and great bioavailability. The mechanism of action provided by sublingual tablets involves placing a tablet under the tongue, where it rapidly dissolves in saliva and allows the active pharmaceutical ingredients to pass through the sublingual mucosa directly into the systemic circulation. This causes the drug to avoid the gastrointestinal tract and liver first-pass metabolism, resulting in a faster onset of action with more predictable therapeutic effects. Sublingual tablets find their best use in drugs which require instant therapeutic responses such as cardiovascular conditions, pain management, and acute conditions such as angina. Important formulation parameters include drug solubility, stability, taste masking, and the use of excipients to promote their release from the formulation. Developments in science and technology have enhanced the application of this dosage form by introducing innovations such as the fast-dissolving and controlled-release systems.
Keywords
Sublingual tablets, bioavailability, rapid absorption, first-pass metabolism, fast-dissolving systems, patient compliance.
Introduction
Sublingual drug delivery (SL) is a procedure of administering the drug beneath the tongue; the drug passes directly into the bloodstream via the ventral surface of the tongue and the floor of the mouth. The main way by which the drug is absorbed into the oral mucosa is passive diffusion across the lipoidal membranes. Sublingual absorption is 3–10 times more than that via the oral route; it is only exceeded by hypodermic injection. For such purposes, small volumes of saliva are generally sufficient to affect the disintegration of the tablet in the oral cavity [1].
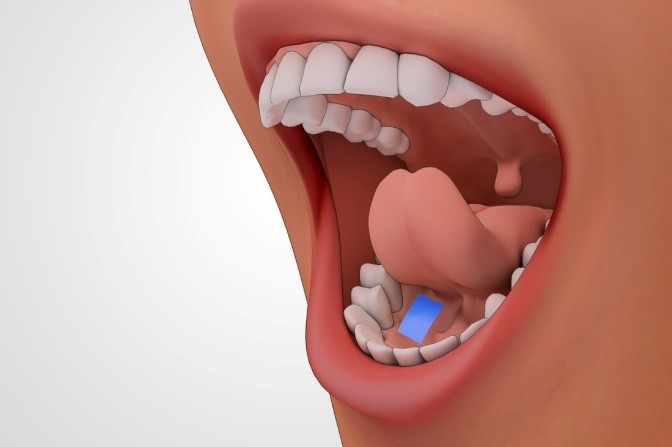
Figure 1. Administration of Tablet
In theory, the sublingual route is preferred for local and systemic drug administration for some agents. This route has specific advantages that are very different from oral drug delivery: The presence of rich supply of blood vessels, rapid onset of action, better bioavailability, avoidance of the hepatic first-pass effect, food effect, increased patient compliance, and easy self-medication. In recent years, a lot of novel delivery systems have been taking advantage of sublingual drug delivery and have been successfully 2 marketed. Sublingual delivery and subsequent absorption of a drug depend on the permeability of the sublingual membrane, physicochemical properties of the drug, and design of the dosage form. In this review, we mainly discuss physicochemical properties and formulation design, as the recognition of these brings about the selection of suitable drug molecules for 3 sublingual delivery and optimization of the final formulation. [2].
The oral mucosa consists of the outermost stratified squamous epithelium, basement membrane, lamina propria, and submucosa as the innermost layer. It is advisable to view the oral mucosa as an intermediate tissue with respect to permeability between the epidermis and the intestinal mucosa. Permeability changes between various regions of the oral cavity due to differing structures and functions of oral mucosa.
The Sublingual Gland:
The sublingual glands are found in the floor of the mouth beneath the tongue. Mucin produced there, produces saliva. The secreted fluid from the glands mixes with food such that the food can be masticated easily. Absorption is defined as the process of transferring an administered drug into systemic circulation; hence one could rightly say that absorption is directly proportional to layer thickness. Hence the order of drug absorption will follow as Sublingual > Buccal > Gingival >Palatal (Figure 2). The sublingual route is thus of high importance because of providing rapid onset of action because of high permeability and rich blood supply and hence is a suitable route for the delivery of drugs having short delivery periods and frequent dosing regimens [3].
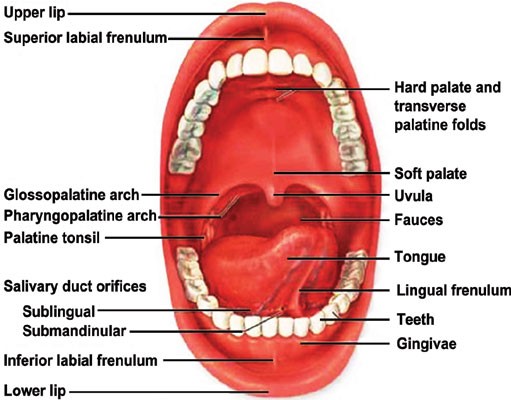
Figure 2: Human Oral Cavity
Sublingual means immediate action by allowing the drug to be absorbed rapidly or directly through the mucosal lining of the mouth under the tongue, with increases in the systemic blood concentration of the drug, thus avoiding first-pass metabolism. After getting absorbed from the stomach, the drug gets into the mesenteric circulation that connects through a portal vein. These sublingual tablets normally appear small and flat and are lightly compressed to keep them soft. The tablets must allow for quick dissolution for drug absorption. They are designed to dissolve in a small volume of saliva and, once the tablet is placed in the mouth beneath the tongue, the patient should refrain from eating, drinking, smoking, and possibly talking so as to allow the tablet to remain in the correct position. Systemic drug delivery via the sublingual route has made a foray mainly to allow an immediate onset of drug action and, therefore, a variety of indications from migraine (for which rapid onset of action is paramount) to mental illness (for which patient compliance is requisite for the treatment of chronic indications like depression and schizophrenia) have been catered to by sublingual products. Sublingual route provides time's greater absorption of the drug in comparison to the oral route and is second only to hypodermic injection. Sublingual route is very much appropriate for short-acting drugs. Most of the drugs administered through sublingual route are absorbed by simple diffusion; here the sublingual area acts like a litmus paper soaking in the substances at a fast pace, however not all substances pass the cut-off and are thus considered permeable and accessible to the oral mucosa. Most of the drugs administered via the sublingual route fall under the category of antianginal drugs. Systemic delivery of drugs through the sublingual route had stemmed from the desire for an immediate drug action [3].
Sublingual Drug Absorption Mechanism:
The permeability of the solution commonly referred to as osmosis, ionization, and the molecular weight of the drug in question determine the absorption of the drug. Endocytosis is the major mechanism by which oral epithelium cells adsorb the drug. In all likelihood, the same mechanism will not be applied throughout the stratified epithelium. These factors are said to cooperate with acidic stimulation of the salivary glands to promote absorption into the circulatory system through causing vasodilatation. The mouth is lined by a mucous membrane that is coated with squamous epithelium containing mucous glands.
The absorption potential of buccal mucosa is determined by several factors: the lipid solubility and related permeability of a solution (osmosis), the ionization (pH), and finally the molecular weight of the substance. In particular, it has been shown that drugs that can be absorbed via the buccal mucosa have increased absorption when the carrier pH is lowered (more acidic), while some drugs have reduced absorption with acidic pH. Other than this, the cells of the oral epithelium and epidermis are able to absorb substances via endocytosis (that is, the uptake of particles by the cell as if wrapping hollowly throughout them. The engulfed particles are usually of a size too large to diffuse through its wall). This pathway is most probably not utilized along the entire stratified epithelium. Also, it is unlikely that any active transport processes are utilized inside the oral mucosa. Yet, acidic stimulation uptake into the circulatory system is considered to take place. The oral cavity is lined by a mucosa consisting generally of squamous epithelium and containing mucous glands. The buccal mucosa resembles the sublingual mucosal tissue (Figure 3). The surface of the sublingual mucosal tissue bears similarities to that of buccal mucosa. Salivary glands consist of lobules of cells secreting saliva through salivary ducts into the mouth. The three major pairs are the parotid, submandibular and sublingual which lies on the floor of the mouth. Sublingual artery travels forward to the sublingual gland. It supplies the gland and gives branches to the neighbouring muscles and mucous membranes of the mouth, tongue, and gums. Two symmetrical branches run behind the jawbone under the tongue to meet and join at its tip. Another branch meets and anastomoses with the sub-mental branches of the facial artery. The sublingual artery stems from the lingual artery - the main blood supply to the tongue and the floor of the mouth, arising from the external carotid artery. Just by its proximity with the internal carotid artery, swift access is gained to its path supplying most of the cerebral hemisphere [4]. Biochemical characteristics of the buccal and sublingual membrane are responsible for the barrier functions and permeability. Drug permeation through the membrane is affected by various factors related to the drug molecule, such as lipid solubility, degree of ionization, pKa of the drug, pH of the drug solution, saliva, membrane characteristics, molecular weight, and the size of the drug. The various physicochemical properties of the formulation and the presence or absence of permeation enhancers would affect the absorption and permeation of the drugs through the oral mucosa.

Figure 3: Sublingual Drug Absorption Mechanism
However, peroral drug administration has its disadvantages such as hepatic first pass metabolism and degradation by the enzymes found in the gastrointestinal tract. Hence, a keen interest is being observed in delivering therapeutic agents via several transmucosal routes to give the therapeutic amount of drug in the correct body site to achieve and then maintain the desired concentration immediately. Rapid absorption and acceptable bioavailability of many drugs are provided by the sublingual route and is considered to be one of the most convenient, easily accessible and well accepted routes. Sublingual mucosa is said to exhibit greater permeability than buccal, depriving it, however, of rapid absorption and good bioavailability. When it comes to device placement, the sublingual mucosa is rather problematic due to the lack of a wide expanse of smooth muscles or immobile mucosa, together with a constant washing with quite an amount of saliva. The absorption of the drug through the highly vascular lining of the mouth takes the drug through sublingual or buccal capillaries and veins to the jugular veins and to the superior vena cava directly to the heart and arterial circulation without passing through the liver, thereby avoiding the hepatic first pass metabolism [5].
Physicochemical Criteria of Drug For Sublingual Drug Delivery:
Table 1.1 Physiochemical Characteristic of Drug for Sublingual Drug [6]
|
Physiochemical Properties of Drug
|
Accepted Range
|
|
Dose
|
<20mg>
|
|
Taste
|
Not intensely bitter
|
|
Stability
|
Good stable in saliva and water
|
|
Molecular weight
|
Small to moderate (163.3-342.3)
|
|
pKa
|
>2 for acidic drug
<10>
|
|
Log P
|
1.6-3.3
|
|
Lipophilicity
|
Lipophilic
|
|
No. of hydrogen bond accepter site
|
1-5 (2.93)
|
|
No. of hydrogen bond donor site
|
0-2.5 (1.26)
|
|
Polar surface area
|
13.0-16.0 (38.1)
|
|
No. of rateable bonds
|
0.5-6 (3.30)
|
Drugs For Sublingual Administration:
The sublingual route is well established in administering cardiovascular drugs, steroids, some barbiturates, and enzymes. This route has been evolving with vitamins and minerals that are found to be absorbed readily and completely. Antianginal drugs, such as nitrites and nitrates, antihypertensives, such as nifedipine, analgesics like morphine, and bronchodilators such as fenoterol are administered via this route. Some other examples are estradiol and oxytocin. Other drugs that can be delivered by this route include fentanyl citrate, prochlorperazine dimaleate, and hydrazine HCL (HYD) [7].
Advantages Of Sublingual Tablets:
- The sublingual tablets are placed under the tongue to act systemically in an immediate fashion allowing for quick or direct absorption of the drug through the volume or mucosa lining of the mouth underneath the tongue.
- Reduction in required dose.
- Very rapid.
- Better bioavailability.
- Reduced side effects.
- Useful in conditions such as nausea, vomiting, migraine, and schizophrenia.
- Water is not needed for tablet administration.
- Provides sustained release.
- Drug administration becomes easy.
- The sublingual area has higher permeability compared to the buccal area.
- Drugs which could be absorbed through the oral administration will be subjected to hepatic first-pass metabolism. It bypasses the GI tract and the hepatic portal system, thus enhancing the bioavailability of orally administered drugs [7,14].
Disadvantages Of Sublingual Tablets:
- Prolonged administration is generally ruled out for the sublingual route, since it interferes with eating, drinking, and talking.
- While not well suited for sustained-delivery systems
- The sublingual route of administration cannot be used when the patient may be uncooperative or is unconscious.
- The patient should not smoke during sublingual medications because smoking constricts blood vessels and decreases the absorption of medication [8,14].
Factors Effecting Sublingual Absorption:
- Solubility In Salivary Secretion
Apart from high lipid solubility, it is important that the drug be soluble in aqueous buccal fluids, thus requiring a biphasic solubility of drug for the absorption process.
- Binding To Oral Mucosa
Their binding to oral mucosa seriously impairs the systemic availability of drugs.
- Salivary pH and PKA
The mean pH of saliva being 6.0 promotes absorption of drugs remaining unionized. More importantly, for absorption across the oral mucosa, the pKa of any drug involved should be greater than 2 for acids and less than 10 for bases.
- Lipophilicity Of Drug
For total absorption to occur through the sublingual route, the drug must possess a little more lipid solubility than that required for absorption along the GI tract in passive permeation.
- Thickness Of Oral Epithelium
So absorption of drugs hastens due to thinner epithelium and also small volume immersion of drug in saliva, as the thickness of the sublingual epithelium ranges from 100-200 ?m, in contrast with buccal thickness [8, 15].
Types Of Sublingual Tablets:
- Fast Disintegrating Sublingual Tablets
In the case of tablets disintegrating or dissolving rapidly in the patient, they prove useful for special situations such as with young children and elderly geriatric patients unable to swallow in instances where drinkable liquids may not be available. FDT is defined as solid dosage forms containing medicinal substances that rapidly disintegrate (within seconds) in the absence of water when administered on the tongue. The dissolved, dispersed, or released drug is swallowed and absorbed across the gastrointestinal tract with the help of saliva. The greatest appeal of FDTs is convenience and occasional preference over conventional solid oral dosage forms. These ODTs may represent a significant improvement over existing treatment options for some patient groups, in this case, paediatric patients. FDT sublingual tablets may display increased oral bioavailability. The ODT tablets are also referred to as orodispersible tablets, quick disintegrating tablets and mouth dissolving tablets, fast disintegrating or fast dissolving porous tablets, rapid dissolving tablet, and rapimelts. Water-wicking and swelling are the 2 most vital mechanisms of disintegrant action for most sublingual tablets.
- Bioadhesive Sublingual Tablets
The new sublingual tablet concept proposed is based on interactive mixtures consisting of a water-soluble carrier covered with fine drug particles, and a bio adhesive component. The idea thus presented causes rapid dissolution to occur and bioadhesive retention within the oral cavity.
- Lipid Matrix Sublingual Tablets
Sublingual tablets prepared from lipid matrices are bioavailability, rapidity, convenience, and consistency of bulk dosage forms for many specially nutraceuticals that are typically orally administered. Through advances in sublingual and liposomal technology, the lipid matrix sublingual tablet investigations have thus evolved into a faster, completely absorbed dosage form than other routes of administration.
- Sublingual Vitamin Tablets
Vitamin B12 only under the sublingual category is offered by doctors. Vitamin B12 boosts our body metabolism. It is proposed to be taken by mouth.
- Sublingual Immunotherapy
Sublingual immunotherapy, or SLIT, is a method of immunotherapy whereby drops of allergen extracts are given under the tongue. SLIT is usually delivered in 1 of 2 ways-drops or tablets of allergen extracts are placed under the tongue, and then swallowed or spat out. This method helps very much in the case of SAC (seasonal allergic conjunctivitis) and PAC (perennial allergic conjunctivitis), which are spreading with great speed among workers in industries, and require longer sublingual immunotherapy, often going on for one year along with mast cell stabilizers, antihistamines, and sometimes local steroids [9].
Marketed Preparations of Sublingual Tablets:
Table 1.2 Marketed Formulation of Sublingual Tablets [10]
|
Brand Name
|
Active Ingredients
|
Category
|
Manufacturer
|
|
Saphris
|
Asenapine
|
Antipsychotic agent
|
Merck
|
|
Ergomes
|
Ergoloid mesylates
|
-
|
Cipla
|
|
Subutex
|
Buprenorphine HCL
|
Narcotic + Opioid Analgesic
|
Sun pharma
|
|
Ergomar
|
Ergotamine tartrate
|
-
|
Rosedale pharmaceuticals
|
|
Abstral
|
Fentanyl citrate
|
Opioid Analgesic
|
Galena Biopharmaceuticals
|
|
Imdur
|
Isosorbide dinitrate
|
Vasodilators
|
Astrazeneca
|
|
Nitrostat
|
Nitroglycerin
|
Antianginal
|
Pfizer
|
|
Intermezzo
|
Zolpidem tartrate
|
Sedatives/Hypnotics
|
Purdue Pharma
|
Excipients In Sublingual Tablets:
Table 1.3 Commonly Used Excipients [11]
|
Excipients
|
Uses
|
|
HPMC
|
Tablet binder, Stabilizing agent.
|
|
Lactose Monohydrate
|
Diluent, Tablet binder
|
|
Crosspovidone
|
Superdisintegrant
|
|
Cross carmellose sodium
|
Superdisintegrant
|
|
Sodium starch glycolate
|
Superdisintegrant
|
Methods Of Preparation of Sublingual Tablets:
- Direct compression
- Freez drying
- Molding
- Sublimation
- Spray drying
- Mass extrusion
- Melt granulation
-
Direct Compression
It is simplest way to make tablets. Direct compression involves conventional equipment, commonly available excipients, a few processing steps, and no more. The mixture to be compressed must flow extremely well and be maintained under pressure; there is no pre-treatment like the wet granulation. Furthermore, as larger drug doses can be accommodated and the final tablet weight may be increased slightly more than that of some other processes, disintegration and solubilization basically depend on tablet size and hardness. Good improvements have been made to excipients like super-disintegrants, including croscarmellose sodium, microcrystalline cellulose, crospovidone, sodium starch glyco, partially substituted hydroxypropyl cellulose, and effervescent agents (citric acid and sodium bicarbonate, etc.). With these improved excipients, this technology can be used in fabricating rapidly dissolving tablets. Sugar excipients (i.e., dextrose, fructose, isomalt, maltose, mannitol, sorbitol, starch hydrolysates, polydextrose, and xylitol) are now also available.
- Freeze Drying/ Lyophilisation
Lyophilization helps in manufacturing tablets with a porous open matrix network that gets readily dispersed in saliva when placed in the mouth. The drug is encapsulated in a quick-dissolving lyophilized water-soluble matrix. Depending on either efficacy or manufacturing performance improvements, the final formulation may also contain other excipients, such as suspending agents, wetting agents, preservatives, antioxidants, colorants, and flavors. The lyophilized formulation is expected to have drug properties such as water insolubilization, low dosage, chemical stability, and small particle size. Although this technology helps improve the absorption and hence bioavailability, freeze-drying is an expensive and time-consuming method of manufacture. Other disadvantages include brittleness, hindering use for adequate conventional packaging, and being less stable during storage and when subjected to unfavorable conditioning.
- Molding
This technology utilizes water-soluble materials to promote quick disintegration and dissolve properties of tablets. The mixture of powders is moistened with a hydroalcoholic solvent and compressed into tablets under lower pressures than conventional tablets. The solvent is then air-dried away. Two commonly encountered problems in this process are lower mechanical strength and taste-masking properties. Binders such as sucrose, acacia, and polyvinylpyrrolidone can be used to improve the mechanical strength of tablets. The heat-moulding process uses an agar solution as a binder and mold to form the tablets.
- Sublimation
Fundamentally, the key principle in producing fast-dissolving tablets with sublimation technology is the addition of a volatile substance to the tableting components, mixing the ingredients to obtain a significantly homogenous mass, heating the volatile salts under vacuum at 80 °C for 30 minutes in a vacuum to remove the volatile components that create pores in the tablet. The removal of the volatile agents from the interior creates voids in the tablet, which facilitates rapid disintegration when saliva is introduced. For instance, camphor, ammonium bicarbonate, naphthalene, and urea are active ingredients used for this purpose.
- Spray drying:
Spray drying makes very porous, spherical particles as the solvent evaporates during the process. The different possible carrier matrices employed in this spraying would include hydrolysed and non-hydrolysed gelatine along with other ingredients, such as mannitol as a bulking agent; sodium starch glycolate and sodium carmellose as disintegrant agents; and acidic substances like citric acid and sodium bicarbonate as alkali-type agents for increasing attenuation and resolution.
- Mass-Extrusion
In this approach, a solvent blend of water-soluble polyethylene glycol is first used to soften an active mixture of methanol, which is then extruded or expelled from an extruder or syringe, and afterwards a heated blade is applied to slice the extrudate into uniform segments to form tablets. The dry cylinders may also be used to coat the bitter granules, thereby masking the taste.
- Melt granulation
Melt granulation is the operation of agglomerating pharmaceutical powders efficiently by means of a binder which is either in a molten liquid state, or solid, or will melt during the process. A high shear mixer is used for this process. The frictional heat generated by melt granulation using heating jackets or impeller blades raises the product temperature above the melting point of the binder. The main advantage this technology holds in comparison to conventional granulation is that water and organic solvents are excluded from use during this process. The other advantages of the process include the elimination of a drying step; use of less energy compared to wet granulation; and saving time in the production schedule by virtue of being quicker [6].
Evaluation Of Tablets:
For pre compression parameters [12]
- Angle of repose
- Density of powder
- Compressibility Index and Hausner’s Ratio
- Angle of Repose
The angle of repose of the powder blend was determined using the funnel method. The accurate mass of the powder blend was allowed to flow through the funnel freely on the surface. The diameter of the powder cone was measured and the angle of repose was calculated using the equation ? = Tan-1 h/r.
- Density of Powder
A powder blend from each formulation was added in a measuring cylinder of glass hold up to 10 ml. Initial volume and weight were taken down. The cylinder was tapped 50 times on a hard surface from a height of about 2.5 cm with an interval of 1 second. After this, tapped volume was noted. From the data obtained, Untapped Bulk Density and Tapped Bulk Density were calculated.
- Compressibility Index and Hausner’s Ratio
To determine the Compressibility Index as well as the Hausner Ratio of the powder blend, the post-tap and pre-tap bulk densities were used.
For Post Compression Parameters [15]
- Hardness
- Friability
- Uniformity of weight
- Wetting time
- Determination of drug content
- Disintegration Test
- In-vitro drug release profile
- In-vitro Drug release kinetics
- Hardness
A diametric compression test was performed based on European Pharmacopoeia method 2.9.8, using the Monsanto hardness tester. According to standard references, sublingual tablet hardness may be acceptable at a value of about 2 kg/cm2.
- Friability
The friability test was performed in compliance with the procedures of the IP. Because the weight of the tablets (125 mg) is always less than 650 mg, whole tablets corresponding to 6.5 g were randomly de-dusted, accurately weighed, and placed in the drum of the Roche Friability tester (Mfg by Koshiash Industries). The drum rotated 100 times before the tablets were removed, dedusted, and weighed accurately. A maximum of 1.0% weight loss is generally considered acceptable.
- Uniformity of weight
Randomly selected 20 tablets from each batch to be tested for uniformity of weight according to IP and average their weights on a digital weighing balance (Essae Teraoka ltd). The percentage weight difference values have been calculated and checked against IP specifications.
- Wetting time
The wetting time of the samples was determined by utilizing two rectangular layers of the absorbent paper (11 cm by 7.5 cm) in a Petri dish, which were once again thoroughly wetted with distilled water. The test tablet was placed at the middle of the plastic dish, and the time taken for the water to diffuse from the wetted absorbent paper into the whole tablet was recorded with the help of a stopwatch.
- Determination of drug content
Twenty tablets are weighed from each of the formulation and powdered. 10 mg of the powder was weighed accurately and dissolved in 100 ml of distilled water. The mixture was sonicated (Equitron) for 180 seconds and filtered through Whatman filter paper No. 40. After that filtrate was diluted further with distilled water and absorbance was measured at 310 nm. By using slope of standard calibration curve the amount of Ondansetron HCl was calculated.
- Disintegration Test
Disintegration testing was performed based on USP NF guidelines. One tablet was placed in each of six tubes, using distilled water maintained at a temperature of 370 C ± 20 C for disintegration. At the end of the time limit of 2 minutes as directed for a sublingual tablet, the basket was lifted from the fluid and the observation was made on the complete disintegration of tablets.
- In-vitro drug release profile
Modified European Pharmacopoeial methods were adopted for in vitro drug release studies using distilled water as the dissolution medium at 37degreeC ± 0.5degreeC with 50 rpm (paddle). Samples were collected at 2, 4, 6, 10, 15 & 20 minutes intervals. Determination of the amount of Ondansetron HCl released was carried out at 310 nm using a UV spectrophotometer (Lab India 3000+). Cumulative percentage of drug release was calculated, and the obtained data was represented in dissolution rate profiles versus time (Table 3). The literature states an amount of drug released from sublingual tablets must be greater than 80% of total content within 15 minutes.
- In-vitro Drug release kinetics
In-vitro drug release kinetic studies were performed for the prepared Ondansetron HCl sublingual tablets. For zero-order kinetics, the data for in vitro drug release studies were plotted as a function of the % cumulative amount of drug released against time, while for first-order kinetics, the remaining amount of drug was plotted against time in terms of log % cumulative, giving a straight line of slope ?nK/2.303. The results thus obtained were compared with goodness-of-fit test linear regression analysis to characterize the drug release kinetics of the developed formulation [10,13].
Future Prospects
Sublingual tablets provide the best routes for the administration of the medications with low bioavailability like proteins and peptides. Because of the absence of injectable formulations or complicated auto-injectors, patients avoid injections. Oral delivery systems enhanced with odt claim potential in delivering other high-molecular-weight proteins and peptides [10].
CONCLUSION
The present study observed that sublingual tablets enhanced the compliance and drug delivery for pediatric and geriatric populations. Sublingual drug delivery is widely used for quickly acting preparations. These tablets are dosed with a purpose of being able to swallow easily; aimed at a target population that seeks quick adminis-tration of tablets without drinking water. The drug in the tablets enters into the bloodstream from the glands in the sublingual cavity. Peak plasma concentrations are normally achieved within 10 to 15 minutes, as opposed to oral administration, which normally achieves peak plasma concentrations within hours. Sublingual absorption is very much effective; therefore, a drug with rapid onset of action can be achieved. Compared to conventional oral dose forms such as tablets and capsules, sublingual absorption is much more efficient and quicker. Various forms of this drug delivery are available in the marketplace, including pills, films, and sprays.
REFERENCES
- Prathusha P, Kamarapu P. A Review on Sublingual Tablets. Journal of Formulation Science & Bioavailability. 2017;1(1):1-2.
- Khan AB, Kingsley T, Caroline P. Sublingual tablets and the benefits of the sublingual route of administration. Journal of Pharmaceutical Research. 2017 Sep 1;16(3):257-67.
- Thulluru A, Mahammed N, Madhavi C, Nandini K, Sirisha S, Spandana D. Sublingual tablets-an updated review. Asian Journal of Pharmaceutical Research. 2019;9(2):97-103.
- Nibha KP, Pancholi SS. An overview on: Sublingual route for systemic drug delivery. Intra-oral Spray Technology. 2012 Apr:3.
- Singh M, Chitranshi N, Singh AP, Arora V, Siddiqi AW. An overview on fast disintegrating sublingual tablets. International journal of drug delivery. 2012 Oct 1;4(4):407.
- PA R, Malode AJ. A REVIEW: ON SUBLINGUAL TABLETS. Ind. J. Res. Methods Pharm. Sci. 2022;1(6):01-11.
- Nayak BS, Sourajit S, Palo M, Behera S. Sublingual drug delivery system: a novel approach. International Journal of Pharmaceutics and Drug Analysis. 2017;5(10):399-405.
- Nikam RS, Borkar SP, D Jadhav PD, D Yadav V, Jadhav AV. Sublingual tablets: An overview. International Journal of Scientific Research in Science and Technology [Internet]. 2020:333-9.
- Vats AK, Shivakumar HG, Chaudhari CA. Sublingual Drug Delivery: An Extensive Review. International Journal for Pharmaceutical Research Scholars. 2016;5(1):9-19.
- Yarmal RV, Basarkar GD. REVIEW ON: SUBLINGUAL DRUG DELIVERY SYSTEM.
- Jaiswani R, Prakash A, Mishra DK, Jain DK. Sublingual tablets: an overview. Journal of Drug Delivery Research. 2014;3(4):10-21.
- Godbole AM, Somnache SN, Thakker SP, Iliger SR, Joshi AS, Patel BV. Formulation and in-vitro evaluation of sublingual tablets of ondansetron hydrochloride using coprocessed excipients. Indian Journal of Pharmaceutical Education and Research. 2014 Oct 1;48:7-17.
- Singh M, Chitranshi N, Singh AP, Arora V, Siddiqi AW. An overview on fast disintegrating sublingual tablets. International journal of drug delivery. 2012 Oct 1;4(4):407.
- Narang N, Sharma J. Sublingual mucosa as a route for systemic drug delivery. Int J Pharm Pharm Sci. 2011;3(Suppl 2):18-22.
- Chotaliya MK. OVERVIEW OF SUBLINGUAL TABLETS. Pharma Science Monitor. 2013 Apr 15;4(3)


 Mukul Malpure*
Mukul Malpure*
 Aniket Gudur
Aniket Gudur



 10.5281/zenodo.14882066
10.5281/zenodo.14882066