Abstract
Many benefits, including avoiding hepatic first-pass metabolism, maintaining a constant plasma concentration, safety, and compliance over oral or parenteral methods, come with drug delivery via the skin. The largest obstacle to transdermal distribution, however, is the fact that only a small number of strong medications with optimal physicochemical characteristics can pass through skin barriers, intercellularly penetrate, and attain therapeutic concentration through this mechanism. Considerable work has gone into creating strategies to improve the medications' transdermal penetration. One of the microscale physical enhancement techniques that significantly broadens the range of medications available for transdermal and intradermal administration is the use of microneedles. The length of microneedles is usually between 0.1 and 1 mm. Materials for microneedles, types of microneedles, manufacturing processes, and transdermal delivery uses are covered in this review. A wide range of materials, including silicon, polymers, and stainless steel, have been utilized to create solid, coated, hollow, and dissolvable microneedles. Numerous discussions have been held regarding their implications for transdermal drug delivery. Ongoing delivery, effectiveness, affordable fabrication, and large-scale manufacture are still problems, nevertheless. This review covers the various techniques of microneedle patches, types, materials, advantages, applications ,vaccine delivery and drug delivery.
Keywords
Microneedle patches, laser ablation, drug delivery, vaccine delivery
Introduction
To improve the quality of health and extend human life drugs have been delivered in a variety of pathways. Drug delivery have seen improvements from chewing of therapeutic leaves to capsules, pills, injectables, and implantable devices[1]. To relieve distressing symptoms for patients, higher adsorption and transport of the drugs can be achieved. Over the years, the therapeutic efficacy of the drugs has been enhanced by targeting the localized aliment region while reducing its toxic effect to healthy cells[2]. Different routes of drug delivery into the human body, includes transdermal, parenteral, oral, and inhalation routes for the delivery of the drug to the targeted sites to shows its action[3]. Route of administration of the drug selection depends on 3 factors, namely 1.desired effect, 2.Type of the disease, 3.Type of the product. For conventional and novel drug delivery oral route has been used and it is the oldest route. This route is convenient for the patients because ease of administration and highly preferred for the long-term medications. And also oral route of administration having the side effects which impacts the liver and kidney[3], [4]. Parenteral route is the administration of the drug into the patient is other than the oral route, those routes includes intravenous, intramuscular, intra-arterial and subcutaneous route etc. Parenteral route of administration is rapid delivery method, and it is less painful so it is not preferred that much by the patients[5]. Whereas inhalation route is less painful compared to parenteral route and comfortable. Drug delivers directly into the lungs. This route increases the bioavailability of the drug. But the risk associated with this route is overdosing by self-administration requires multiple doses each day[6][3], [7][8], [9].
Finally for the administrating of the drugs through the layers of the skin is focused by the transdermal route. For example in neonates and geriatric patient population they are unable to swallow the oral drugs so, TDD route is used as the alternative route to the oral route to deliver the drugs. And also provide alternative for macromolecules/ protein / peptides to bypass the digestive track and provide the good bioavailability[10]. This route will transfer the active ingredients directly into systemic circulation without gastrointestinal and liver metabolism[11][12][13].
Transcutaneous delivery is ideal for delivering the vaccines, and therapeutic reagents into the skin but pain and fear accompany injection with syringe, that is replaced with painless, less fear methods like transdermal patches. An intriguing solution is a device bearing arrays of microneedles (a microneedle patch) that is coated with or encapsulates bio-active molecular that can be delivered into skin by intradermal administration. Because of the tiny size, microneedle patches could eventually be widely used for dermal drug delivery or vaccine delivery[14],[15].
Transdermal Drug Delivery:
Transdermal drug delivery applying the drug directly to the skin, and the drug penetrates into the skin through the stratum corneum and then passes through epidermis and dermis. When the drug reaches the dermal layer and it is available for absorption[10],[16]. Different transdermal drug delivery are represented in the figure 1.

Figure 1. Different transdermal drug delivery
Transdermal drug delivery history into four generations are divided by prausnitz and langer. By using natural diffusion in patch-based technologies providing low drug load in first generation, whereas second generation is focused on using the chemical precursors to actuate drug delivery. Some technologies are involved in third generation such as electroporation, thermal ablation and microneedles, which can target the drug upon entry into the stratum corneum. Finally fourth generation involves the combination of sensing modalities along with drug delivery microneedles to control the release of pharmaceutical agents with high precision.
Transdermal drug delivery has many advantages over the delivery methods. some of the advantages like reduction of the side effects of drugs by preventing the drugs passing through the critical organs such as liver and kidney. TDD has the ability to deliver the drug directly into the blood with desired dosage in a sustained and controlled manner[17]. Drugs with high doses and molecular weights are not suitable for the passive transdermal route, an active penetration enhancer is required[18]. Major barrier to limit drug flux into the skin is stratum corneum and it is the outermost layer having both hydrophobic and hydrophilic regions of biphasic skin layer ranging from 10-20 micrometers [19].
Drugs can diffuse through the skin when they are come in contact with the interstitial fluids, once the stratum corneum is breached. And it is possible to deliver the hydrophilic drugs through this method are it may utilize the sweat glands as an other method[20].
Materials Used In Microneedle Patches
The principal factor promoting MN manufacture is their unbreakable and pliable skin penetration. The MN manufacturing difficulty has been addressed by taking into account a number of elements, including material, manufacturing process, and design. Different kinds of MNs have been created using a range of materials seen in figure 2. Polymers, metals, ceramics, and silicon are a few examples of these materials[21], [22], [23], [24].

Figure 2. Different materials used in microneedle
Silicon
The first MN was created using silicon material in the 1990s. Among its many benefits over other materials is silicon's inherent flexibility, which makes it simple to manufacture MNs in the desired sizes and shapes. MNs that are coated, hollow, and solid have all been created using silicon. However, there are drawbacks to silicon use as well, including labour-intensive manufacture, expensive pricing, and the potential to cause skin fractures[25], [26], [27], [28].
Ceramic
Ceramic materials like alumina, with their exceptional chemical characteristics and resilience to compression, have been employed to create MNs. On the other hand, alumina's tensile strength is lower than that of other material[13]. Two more types of ceramics that are used in the creation of MNs are calcium phosphate dihydrate and calcium sulfate dihydrate. Ceramic material can be utilized to construct an MN utilizing a micro-mold process. This method provides low-cost, scaled-up production[29]. According to a study by Bystrova et al., MNs made of alumina shattered when applied to the skin by hand.
Metal
Because of their superior mechanical and biocompatibility, metals are used in the production of MNs[30]. Metals have high values for yield strength and fracture toughness[31]. Metals are stronger and more difficult to shatter than silicon[13]. Titanium was the next metal used in the construction of an MN after stainless steel[32] . Even while metal MN can penetrate skin, using it topically may result in an allergic reaction[33].
Polymer
For MN, polymers present a possible material substitute. They are inexpensive, highly biocompatible, and toxic-free[34]. Nevertheless, their strength is inferior than that of silicon and metals[31]. Polymers are typically used to create solid, coated, hollow, and hydrogel-forming MN arrays as well as dissolvable and hydrogel-forming MN arrays[35],[26]. Biodegradable MNs have been used to apply a variety of medications to the skin[36]. Poly (methyl methacrylate) (PMMA), polylactic acid (PLA), poly (carbonate), polystyrene, and SU-8 photoresist were among the polymer types employed in the creation of MNs[13].
Types Of Microneedle Patches
To create solid, coated, hollow, or dissolvable microneedles seen in figure 3, a range of materials have been employed, including silicon, polymers, sugar, and stainless steel. Every variety of microneedle has distinct qualities, benefits, drawbacks, uses, and material types.

Figure 3. Different types of microneedle patches
Solid microneedle patch
The purpose of this solid microneedle creation is to promote drug administration to the dermis, hence enhancing bioavailability and kinetic transport across the skin, by penetrating the stratum corneum[37], [38]. In comparison to intramuscular delivery, the solid microneedle is suitable for delivery of vaccines as it lasts longer and possesses a more robust antibody response[39]. Compared to hollow microneedles, solid microneedles are easier to make, have better mechanical qualities, and have sharper points[40]. Furthermore, a solid microneedle can be made of a variety of materials, including silicon, metals, and polymers[25].
Hollow microneedle patch
The hollow microneedle is made up of a hollow or empty core or chamber that can be used to inject or store medication. The hollow microneedle has a higher dose/amount of medication solution handling capacity than the solid microneedle[41]. Moreover, a hollow microneedle can introduce the medication into the dermis or viable epidermis, which is appropriate for high molecular weight chemicals. Furthermore, it regulates the drug release over time, making it appropriate for use with formulations of liquid vaccines[42], [43]. Hollow microneedles are an active drug delivery method that forms a path for drug diffusion into the dermis based on a non-pressurized drug reservoir, in contrast to solid microneedles, which largely elute pharmaceuticals based on the osmotic gradient. To allow for adjustable release kinetics, hollow microneedles' material composition and manufacturing characteristics can be combined. Depending on the purpose of the application, pharmaceuticals at higher concentrations may produce pharmacological profiles with burst release, whereas medications put into a matrix may allow for a steady-state drug release that lasts days or weeks[44]. Hollow microneedles are similar to hypodermic needles in that they can be made to allow for pressure and flow rate adjustment. Process variables that can be adjusted for quick release, gradual infusion, or variable delivery rate over time include the microneedle aspect ratio (height to base diameter ratio). The hollow microneedle has been effectively used for a number of vaccinations and vaccines over the years. Nevertheless, because hollow microneedles are comparatively weaker than solid microneedles and necessitate meticulous attention to needle design and insertion technique, they have garnered less attention than solid microneedles. In addition, there are technical issues with the hollow microneedle that include leaking and blockage when injecting[45], [46].
Coated microneedle patches
The solid-type MN coated with a medication solution is the coated microneedle. Generally speaking, the thickness of the coating layer determines how much of the medication is carried[47] .The capacity to consistently coat a regulated drug layer onto MNs is critical to the efficacy of drug delivery employing coated MNs. Proteins and DNA can be delivered using a coated MN in a minimally invasive way. A coated MN has the benefit of quickly delivering the medication to the skin; nevertheless, any remaining medication at the needle's tip could potentially infect further patients Lastly, the[48], [49], [50]. outcomes of administering the vaccine via coated MN resembled those of vaccines administered via intradermal and intramuscular methods[51].
Dissolving microneedle patch
Based on its features, the dissolvable MN method, first debuted in 2005, shows promise. Among these qualities are those that promote the quick release of macromolecules and a single-step medication application process that makes drug administration simple[52], [53]. The dissolvable MN tip can be loaded promptly via a two-step casting method; upon insertion of the dissolvable MN into the skin, the drug-load releases and diffuses easily by dissolving the needle tip. Water-soluble materials are most appropriate for the manufacture of the dissolvable MN. Improvements in applying dissolvable MNs after "poke-and-release" have led to the conclusion that this approach is superior to other approaches[54], [55].Similarly, the best fabrication technique for the dissolvable MN is the micro-mold approach[42]. Because of their long-term replacement inside the epidermal layer, detachable (separable) MNs, which belong to dissolving MNs, are known to avoid issues related to the potential immune response following needle disintegration, as shown in dissolving MNs. These microneedle forms have a rapid rate of delivering a suitable dosage of targeted medications to the intended locations. While the arrowhead points of detachable MNs are constructed from biodegradable scaffolds, the solid core of the structures is composed of non-biodegradable materials[56].
Swellable microneedle patches
Certain types of MNs known as "swellable MNs" enlarge after coming into contact with the cutaneous interstitial fluid. Swellable MNs offer skin microchannels for the introduction of additional drugs, just like other MN kinds. Hydrophilic polymers with a high swell capacity, such as acrylate derivatives and polyethylene glycol, are the building blocks of swellable MNs. It appears that adding more methods can enhance MNs' hydrophilicity[56], [57], [58]. For example, Chew et al. used UV radiation and hyaluronic acid cross-linking to increase the hydrophilicity of PEGDA polymer. The coating using photo-curable compounds improved the MN attachment performance as well. In a brief period of around 10 minutes, the manufactured matrix inflated rapidly, releasing 90% of the loaded medication into the aqueous phase. In this case, following the MN fabrication procedure, this platform offers high concentrate drug loading and an effective delivery[59].
Smart microneedle patches
The need for precise control release, high agent absorption efficacy, and accurate elective cell distribution with significantly fewer side effects became crucial for professional self-health administration ways as the MN delivery system developed. Consequently, smart MNs were presented as responsive drug delivery systems that could adjust the rate, quantity, and timing of transdermal cutaneous medication delivery in response to external, endogenous, or multiple events. Different responsive materials are used in the design of smart MNs in this regard. Three phases make up the smart MN synthesis process, which results in selective drug release and an MN matrix phase transition or shape-shifting (leaking, swelling, and shrinking)[60], [61]. The process involves three steps: (I) alterations in pH, temperature, or osmotic pressure caused by the environment; (II) interactions between biomolecules based on the interactions between enzymes and substrates, or between antibodies and antigens, or between hosts and guests, which can be triggered by internal modification rates of glucose, nitric oxide, thrombin, ROS, etc.; and (III) various exogenous stimuli, including UV, NIR, electricity, magnetic fields, and ultrasound, which cause dynamic bond cleavage, isomerization, reduction, or ring-opening between the structural materials used in MNs[62].
METHODS OF MICRONEEDLE PATCHES
There are multiple ways to fabricate Microneedle arrays. Laser ablation, micro-molding, chemical isotropic etching, injection molding, additive manufacturing, surface/bulk micromachining, and lithography-electroforming-replication are the most widely used techniques.
Micro-Molding
The technique of micro-molding entails copying the master mold. A solution including a polymer and active medicinal ingredients is used to cast the mold figure 4. Micro-molding is a mass production technique that is thought to be economical[63]. For the manufacture of MN, micro-molding is frequently employed with polymer material[64]. When it comes to micro-molding processes, the PDMS has a number of benefits, including low cost, convenience of usage, low surface energy, and thermal stability[65], [66]. The challenges in regulating the drug load capacity, mechanical behaviour of the polymer, and penetration depth are the constraints of this approach [64].
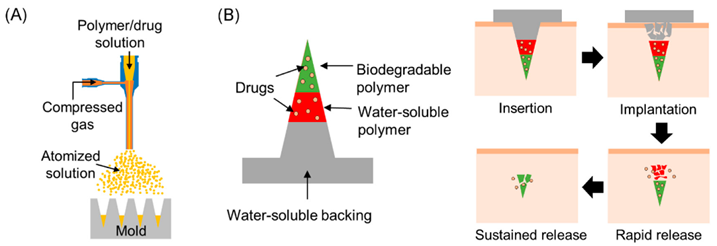
Figure 4.Micro-molding technique of microneedle patch
In order to produce MN arrays, laser ablation uses a concentrated optical light beam seen in figure 5, to remove material from a substrate. For a variety of purposes, lasers have been utilized to treat materials at the micro- and nanoscale[67], [68], [69]. Different kinds of lasers have been investigated for the production of MN arrays. These consist of femtosecond laser machines, CO2, and UV excimers[70], [71]. The laser ablation technique is thought to be a quick and efficient way to create MNs. It takes the laser beam between 10 and 100 nanoseconds to get close to the material sheet's burn point. Any metal could be shaped with a laser as well. The MN structure and mechanical properties are altered by this process due to heat impacts at the cutting surface[72]. In MNs, this could result in unfavourable outcomes like fatigue resistance or cracking. The substrate is exposed to minimal heat loads during the non-contact laser ablation process[73]. But when compared to other kinds of technology, the laser is more expensive. Large-scale manufacturing is not a good fit for the laser ablation technique [72].

Figure 5. Laser ablation technique of microneedle patch
The geometric shape master pattern is transferred onto a substrate's surface using the lithography technique[74], [75]. Because photolithography has so many applications in the microelectronics area, it is mostly employed for pattern transfer. Lithography serves as the initial stage in the fabrication of an MN in other techniques, such as microelectronic and micromachining. The photoresist must be precisely processed for lithography. This process adds between thirty and thirty-three percent to the cost of producing integrated circuits[76] Glass, metal, ceramics, and plastics are just a few of the materials that can be produced using lithography. Moreover, it creates smooth vertical sidewalls and exact geometries figure 6. Nevertheless, this method necessitates a sophisticated space (cleanroom) and more time for production [71], [77].

Figure 6. Lithography technique of microneedle patch
Microneedle Patches For Transdermal Drug Delivery
The goal of Microneedle (MN) technology is to replace traditional syringe injections with an active transdermal drug delivery method. The medication is delivered with a minimally invasive effect by penetrating the stratum corneum via the microneedle array[33]. These arrays are made up of tiny needles that range in height from 25 to 2000 ?m[78]. MNs have been employed in a variety of fields, including cosmetics, illness diagnostics, and the administration of medications and vaccines. The structural configurations, forms, materials, and manufacturing processes of MN are diverse, and this review study provides additional examples. According to Donnelly et al., investigations using microneedles accounted for thirty percent of the most recent scholarly literature on "transdermal delivery technology." External variables include skin physiology, physiochemical characteristics, and environmental conditions can affect the MN drug delivery route[79]. These include the temperature and relative humidity close to the application region. The release of medications into the skin layers will be delayed by too sparse (low humidity); conversely, excessive humidity (sweat) might alter the osmotic gradient for transdermal drug delivery by causing excess water and other salts to interfere with drug release kinetics. Moreover, excessive sweating can hinder the microneedle patch's adherence to the skin, which would further delay the release of medications through the skin. Likewise, drugs may be less permeable into the stratus corneum and beyond in the vicinity of the skin at extremely high or low pH ranges[80]. Increasing skin temperature can improve drug penetration because it increases skin vascular diffusivity and vasodilation[81]. Proper microneedle measuring and dosage loading are crucial when delivering delicate medications like insulin and chemotherapy. Because digestion and first-pass metabolism are avoided, microneedle patches usually require a lower dosage to provide equal therapeutic efficacies than oral consumption. When compared to the oral route, the pharmacokinetics of microneedles demonstrate a rapid absorption in the bloodstream, which can be useful for treating localized disorders with considerably lower drug loading. Compared to solid microneedles, hollow microneedles may hold larger dosages and function as drug reservoirs. Using inkjet and spray atomization processes, solid microneedles composed of ceramic or metal materials can be coated with very accurate medication compositions[82].The most frequent ways to administer therapeutic medications are by oral administration and parenteral injection, yet both has a number of drawbacks. Acids and enzymes in the digestive tract significantly limit the bioavailability of medications taken orally. Additionally, needle injections may result in needle phobias or the development of infectious diseases that compromise the effectiveness of medications. systemic processes as well as certain physiological elements. Drug delivery instruments such as microneedle patches can be used to get around these benefits. Microneedle patches were first developed in 1990 to explore drug delivery, and a wide variety of pharmaceuticals were studied. For instance, Comier et al. used transdermal administration to coat a microneedle patch with desmopressin, a synthetic peptide hormone, to treat enuresis. The outcomes demonstrated that this was a child-safe and effective approach that may replace the existing ways, which included injection and intranasal and oral administration[83]. Patients with type 1 diabetes must administer insulin on a daily basis in order to improve their quality of life. However, administering insulin by injection every day can be extremely painful and traumatizing to the skin, making it difficult for patients to maintain adequate compliance. Therefore, a more comfortable method of administering insulin ought to be explored. Microneedle patches for insulin delivery have been thoroughly studied since the advent of nanotechnology[84]. Additionally, advancements have been achieved in the use of microneedle patches for insulin delivery. Qiu et al. delivered lyophilized hydrogel insulin using microneedle patches[85]. Dissolving microneedle patches and its variations are used in insulin delivery to increase the effectiveness of the drug delivery process. These patches have the advantages of quick diffusion, regulated release, and strong mechanical strength. Microneedle patches have been researched for self-administration in cosmetic application to broaden the scope of use.
Microneedle patches for Vaccine Delivery
One popular kind of MN used for vaccine administration is a dissolvable MN. In place of the conventional hypodermic injection needles used to provide vaccinations, the dissolvable MNs were employed. Dissolvable MNs are resilient, biocompatible, scalable, and do not produce biohazardous waste, in contrast to other types of MNs[86]. Vaccines against polio, HIV, Hepatitis B, influenza, malaria, and diphtheria were administered using soluble molecular nanoparticles (MNs)[87], [88], [89].
Coated MNs arrays have been successfully employed for vaccination purposes, while dissolvable MNs are the most often used form of MNs administration. Pig’s immune systems were strengthened in a trial by using a coated MN and the Bacillus Calmette-Guérin (BCG) vaccine, which was easy, safe, and compliant to give. Hepatitis C virus protein was effectively encoded in a DNA vaccine coated on a microneedle in another investigation. In mice, the microneedle was successfully primed for certain cytotoxic T lymphocytes (CTLs). Additionally, a coated microneedle with influenza viral antigen was used to vaccinate mice.
Rather than administering the anthrax recombinant protective antigen vaccination by injection, hollow MNs have been used to do so. In a mouse model, a hollow microneedle was tested as a vaccine against plaque. Comparing an intramuscular injection to a hollow microneedle used in a human clinical trial for influenza immunization, the immune system responded similarly [90].
ADVANTAGES
A microneedle is regarded as one of the best transdermal drug delivery methods. Because pharmaceuticals delivered via this approach avoid important human organs like the liver[25]. Moreover, it provides a painless experience, which removes the discomfort connected with IV injections. It is therefore regarded as the greatest option for those with trypanophobia (needle phobia). The ease of use of microneedle transdermal drug administration is facilitated by the lack of training requirements for staff[91]. Any medicinal agent's molecules are blocked from passing through the stratum corneum and into the dermis or epidermal layer of the skin. Without inflicting any pain, a microneedle can transport the medication into the top dermis or epidermis layer by avoiding the stratum corneum barrier[92].
DISADVANTAGES
The use of a microneedle for transdermal medication delivery comes with many drawbacks, including the necessity for a good biocompatible material, multiple patches in a given area, and an extended application time. According to Rzhevskiy et al., the MN patch route's difficulties in obtaining relevant pharmacokinetic data can affect the dose settings and perhaps cause unfavourable side effects[93]. Bariya et al. claimed that microneedle depth design should also be strongly regarded while contemplating the variances in the thickness of the stratum corneum and other layers of the skin from a various patient populations[94]. It is also necessary to put the MN device perpendicular to the skin's surface in order to achieve optimal drug delivery and penetration kinetics. There is a possibility that the drug dose may escape, or the needles may struggle to penetrate the skin at non-conformal angles. Moreover, repetitive applications of microneedles may result in scarring of the skin surface. There may also be certain drawbacks with respect to the shapes and conformation of needle structures, thus affecting their efficacy. For hollow MNs, for example, their micropores can sometimes get blocked due to compressed tissue for certain skin types, thus affecting their delivery kinetics and penetrability. Drawbacks of using TTD technologies at the applications include swelling, infection, irritation, redness, pain etc[95], [96].
APPLICATIONS
MNs have drawn wide interest by academics, scientists, and industry participants. Several studies have proved the potential and capacity to administrate MN in different sectors. These include drug administration, vaccine delivery, illness diagnostic, and cosmetics application. Dissolvable MNs were utilized to give vaccinations for malaria, diphtheria, influenza, Hepatitis B, HIV, and polio[86], [87], [88], [89]. Rather than giving a conventional injection, a rabbit received the anthrax recombinant protective antigen vaccination using hollow MNs[90]. microneedle technology offers bioassays solution with painless experience and simple implementation[97]. Microneedles containing nanoparticles were able to recognize the biomarkers in the early stage of osteoarthritis. An enzyme based on microneedles was functionalized to monitor alcohol in artificial interstitial fluid. MNs have been widely used in cosmetic applications, including skin treatment and hair growth. Kim et al. developed a dissolvable MN patch based on hyaluronic acid for the intradermal delivery of ascorbic acid and retinyl retinoate. Kumar et al. demonstrated the improvement of local delivery of eflornithine (used to reduce facial hirsutism) both in vitro and in vivo through the use of a solid MN. Additionally, MN technology was able to treat two patients with alopecia areata disease, and these patients experienced hair growth following treatment. Metal microneedles may not operate as easily as other types of microneedles because longer microneedles typically more than 1 mm are required to avoid problems related to the porosity of electroplated metals[98] .
CONCLUSION
We described the development and present uses of microneedle patches as medication and vaccine delivery vehicles in this review. Compared to conventional distribution methods, the transdermal route offers a convenient, painless, and non-invasive approach of administering reagents. More significantly, and this is a big plus for the healthcare sector, microneedle patches don't need to be stored or transported via a cold chain in the future. Moreover, its implementation ought to reduce medical waste from sharps, injuries caused by needles, and the spread of blood-borne infections in rural areas. Microneedle patches are a potential reagent delivery technology that improve medication delivery efficiency and adsorption through controlled release into bloodstream and circumvention of liver metabolism. Furthermore, because the skin is the largest organ and contains a multitude of immune cells in both the dermis and epidermis, intracutaneous delivery of vaccine antigen-coated microneedle patches may enhance humoral immunity and T cell response. Despite significant advancements in research, the microneedle patch is still not suitable for clinical use. To enhance this delivery platform, more research in a variety of disciplines, including immunology, materials science, and vaccination, should be conducted. Since microneedle patches are developing so quickly, some researchers have already studied primates. Microneedle patches are anticipated to be extensively used in clinical treatment and immunization in the near future due to their likely dose-sparing ability, safety, and ease of treatment compliance.
REFERENCE
- V. V. Ranade, M. A. Hollinger, and J. B. Cannon, “Drug Delivery Systems,” Aug. 2003, doi: 10.1201/9781420040142.
- “Sci-Hub | Drug delivery systems: An updated review. International Journal of Pharmaceutical Investigation, 2(1), 2 | 10.4103/2230-973X.96920.” Accessed: Jun. 20, 2024. [Online]. Available: https://sci-hub.se/10.4103/2230-973X.96920
- B. Abdul Rasool Hassan, “Overview on Drug Delivery System,” 2012, doi: 10.4172/2153-2435.1000e137.
- G. Robbie, T. C. Wu, and W. L. Chiou, “Poor and unusually prolonged oral absorption of amphotericin B in rats,” Pharm Res, vol. 16, no. 3, pp. 455–458, 1999, doi: 10.1023/A:1011961322883.
- A. A. Date and M. S. Nagarsenker, “Parenteral microemulsions: an overview,” Int J Pharm, vol. 355, no. 1–2, pp. 19–30, May 2008, doi: 10.1016/J.IJPHARM.2008.01.004.
- J. L. Rau, “The inhalation of drugs: advantages and problems,” Respir Care, vol. 50, no. 3, pp. 367–382, Mar. 2005, Accessed: Jun. 22, 2024. [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/15737247/
- X. M. Zeng, G. P. Martin, and C. Marriott, “The controlled delivery of drugs to the lung,” Int J Pharm, vol. 124, no. 2, pp. 149–164, Oct. 1995, doi: 10.1016/0378-5173(95)00104-Q.
- M. H. El-Newehy, M. E. El-Naggar, S. Alotaiby, H. El-Hamshary, M. Moydeen, and S. Al-Deyab, “Green Electrospining of Hydroxypropyl Cellulose Nanofibres for Drug Delivery Applications,” J Nanosci Nanotechnol, vol. 18, no. 2, pp. 805–814, Sep. 2018, doi: 10.1166/JNN.2018.13852.
- H. Om et al., “Combating atherosclerosis with targeted Diosmin nanoparticles-treated experimental diabetes,” Invest New Drugs, vol. 38, no. 5, pp. 1303–1315, Oct. 2020, doi: 10.1007/S10637-020-00905-6.
- A. Z. Alkilani, M. T. C. McCrudden, and R. F. Donnelly, “Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the stratum corneum,” Pharmaceutics, vol. 7, no. 4, p. 438, Oct. 2015, doi: 10.3390/PHARMACEUTICS7040438.
- M. Bok, Z. J. Zhao, S. Jeon, J. H. Jeong, and E. Lim, “Ultrasonically and Iontophoretically Enhanced Drug-Delivery System Based on Dissolving Microneedle Patches,” Sci Rep, vol. 10, no. 1, Dec. 2020, doi: 10.1038/S41598-020-58822-W.
- M. Bok, Z. J. Zhao, S. Jeon, J. H. Jeong, and E. Lim, “Ultrasonically and Iontophoretically Enhanced Drug-Delivery System Based on Dissolving Microneedle Patches,” Sci Rep, vol. 10, no. 1, Dec. 2020, doi: 10.1038/S41598-020-58822-W.
- T. Waghule et al., “Microneedles: A smart approach and increasing potential for transdermal drug delivery system,” Biomed Pharmacother, vol. 109, pp. 1249–1258, Jan. 2019, doi: 10.1016/J.BIOPHA.2018.10.078.
- M. R. Prausnitz, “Engineering Microneedle Patches for Vaccination and Drug Delivery to Skin,” Annu Rev Chem Biomol Eng, vol. 8, pp. 177–200, Jun. 2017, doi: 10.1146/ANNUREV-CHEMBIOENG-060816-101514.
- “Sci-Hub | Microneedle Patches as Drug and Vaccine Delivery Platform. Current Medicinal Chemistry, 24(22) | 10.2174/0929867324666170526124053.” Accessed: Jul. 05, 2024. [Online]. Available: https://sci-hub.se/10.2174/0929867324666170526124053
- T. M. Tuan-Mahmood et al., “Microneedles for intradermal and transdermal delivery,” Eur J Pharm Sci, vol. 50, no. 5, p. 623, Dec. 2013, doi: 10.1016/J.EJPS.2013.05.005
- V. Rastogi and P. Yadav, “Transdermal drug delivery system: An overview,” Asian J Pharm, vol. 6, no. 3, pp. 161–170, Jul. 2012, doi: 10.4103/0973-8398.104828.
- F. Erdo, N. Hashimoto, G. Karvaly, N. Nakamichi, and Y. Kato, “Critical evaluation and methodological positioning of the transdermal microdialysis technique. A review,” J Control Release, vol. 233, pp. 147–161, Jul. 2016, doi: 10.1016/J.JCONREL.2016.05.035.
- P. M. Elias, “Stratum Corneum Defensive Functions: An Integrated View,” J Gen Intern Med, vol. 20, no. 5, pp. 183–200, May 2005, doi: 10.1111/J.0022-202X.2005.23668.X.
- J. Seok, J. Y. Hong, S. Y. Choi, K. Y. Park, and B. J. Kim, “A potential relationship between skin hydration and stamp-type microneedle intradermal hyaluronic acid injection in middle-aged male face,” J Cosmet Dermatol, vol. 15, no. 4, pp. 578–582, Dec. 2016, doi: 10.1111/JOCD.12244.
- S. Desai, B. Bidanda, and P. J. Bártolo, “Emerging Trends in the Applications of Metallic and Ceramic Biomaterials,” Bio-Materials and Prototyping Applications in Medicine: Second Edition, pp. 1–17, Jan. 2020, doi: 10.1007/978-3-030-35876-1_1.
- X. Zhao, X. Li, P. Zhang, J. Du, and Y. Wang, “Tip-loaded fast-dissolving microneedle patches for photodynamic therapy of subcutaneous tumor,” J Control Release, vol. 286, pp. 201–209, Sep. 2018, doi: 10.1016/J.JCONREL.2018.07.038.
- S. Desai and M. R. Shankar, “Emerging Trends in Polymers, Composites, and Nano Biomaterial Applications,” Bio-Materials and Prototyping Applications in Medicine: Second Edition, pp. 19–34, Jan. 2020, doi: 10.1007/978-3-030-35876-1_2.
- S. Desai and M. R. Shankar, “Polymers, composites and nano biomaterials: Current and future developments,” Bio-Materials and Prototyping Applications in Medicine, pp. 15–26, 2008, doi: 10.1007/978-0-387-47683-4_2.
- R. Donnelly and D. Douroumis, “Microneedles for drug and vaccine delivery and patient monitoring,” Drug Deliv Transl Res, vol. 5, no. 4, pp. 311–312, Aug. 2015, doi: 10.1007/S13346-015-0250-2.
- E. Larrañeta, R. E. M. Lutton, A. D. Woolfson, and R. F. Donnelly, “Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development,” Materials Science and Engineering: R: Reports, vol. 104, pp. 1–32, Jun. 2016, doi: 10.1016/J.MSER.2016.03.001.
- D. Sharma, “Microneedles: An Approach in Transdermal Drug Delivery: A Review,” Pharmatutor, vol. 6, no. 1, p. 07, Jan. 2018, doi: 10.29161/PT.V6.I1.2018.7.
- C. O’Mahony, “Structural characterization and in-vivo reliability evaluation of silicon microneedles,” Biomed Microdevices, vol. 16, no. 3, pp. 333–343, 2014, doi: 10.1007/S10544-014-9836-6.
- S. Indermun et al., “Current advances in the fabrication of microneedles for transdermal delivery,” J Control Release, vol. 185, no. 1, pp. 130–138, Jul. 2014, doi: 10.1016/J.JCONREL.2014.04.052.
- M. Niinomi and M. Nakai, “Titanium-Based Biomaterials for Preventing Stress Shielding between Implant Devices and Bone,” Int J Biomater, vol. 2011, 2011, doi: 10.1155/2011/836587.
- N. A. Monteiro-Riviere, “Toxicology of the Skin,” Feb. 2010, doi: 10.3109/9781420079180.
- F. J. Verbaan et al., “Assembled microneedle arrays enhance the transport of compounds varying over a large range of molecular weight across human dermatomed skin,” J Control Release, vol. 117, no. 2, pp. 238–245, Feb. 2007, doi: 10.1016/J.JCONREL.2006.11.009.
- “Microneedle-mediated Transdermal and Intradermal Drug Delivery - Ryan F. Donnelly, Thakur Raghu Raj Singh, Desmond I. J. Morrow, A. David Woolfson - Google Books.” Accessed: Jul. 13, 2024. [Online]. Available: https://books.google.co.in/books?hl=en&lr=&id=sM6mY3XS1-UC&oi=fnd&pg=PA27&ots=tqwk_PqfrO&sig=-qkXtQW0hTV_MoNsCZGCK_0d3ew&redir_esc=y#v=onepage&q&f=false
- Cecile. Jeggy and Universite? catholique de Louvain (1970- ), “Micro-injection moulding from process to modelling,” p. 272, 2004, Accessed: Jul. 13, 2024. [Online]. Available: https://books.google.com/books/about/Micro_Injection_Moulding.html?id=ryxnprMTukgC
- R. F. Donnelly et al., “Hydrogel-forming and dissolving microneedles for enhanced delivery of photosensitizers and precursors,” Photochem Photobiol, vol. 90, no. 3, pp. 641–647, 2014, doi: 10.1111/PHP.12209.
- X. Hong et al., “Dissolving and biodegradable microneedle technologies for transdermal sustained delivery of drug and vaccine,” Drug Des Devel Ther, vol. 7, pp. 945–952, Sep. 2013, doi: 10.2147/DDDT.S44401.
- “Comprehensive Biotechnology - Google Books.” Accessed: Jul. 13, 2024. [Online]. Available: https://books.google.co.in/books?hl=en&lr=&id=uyWqDwAAQBAJ&oi=fnd&pg=PP1&ots=nlxeRYUVv5&sig=7sW8kwajZ5So1Es3fihlyn4Rv2I&redir_esc=y#v=onepage&q&f=false
- J. Gupta, H. S. Gill, S. N. Andrews, and M. R. Prausnitz, “Kinetics of Skin Resealing After Insertion of Microneedles in Human Subjects,” J Control Release, vol. 154, no. 2, p. 148, Sep. 2011, doi: 10.1016/J.JCONREL.2011.05.021.
- E. Jacoby, C. Jarrahian, H. F. Hull, and D. Zehrung, “Opportunities and challenges in delivering influenza vaccine by microneedle patch,” Vaccine, vol. 33, no. 37, pp. 4699–4704, Sep. 2015, doi: 10.1016/J.VACCINE.2015.03.062.
- K. Nair et al., “Investigation of Plasma Treatment on Micro-Injection Moulded Microneedle for Drug Delivery,” Pharmaceutics 2015, Vol. 7, Pages 471-485, vol. 7, no. 4, pp. 471–485, Oct. 2015, doi: 10.3390/PHARMACEUTICS7040471.
- K. Cheung and D. B. Das, “Microneedles for drug delivery: trends and progress,” Drug Deliv, vol. 23, no. 7, pp. 2338–2354, Sep. 2016, doi: 10.3109/10717544.2014.986309.
- K. Ita, “Transdermal Delivery of Drugs with Microneedles-Potential and Challenges,” Pharmaceutics, vol. 7, no. 3, pp. 90–105, Jun. 2015, doi: 10.3390/PHARMACEUTICS7030090.
- S. T. Sanjay, M. Dou, G. Fu, F. Xu, and X. Li, “Controlled Drug Delivery Using Microdevices,” Curr Pharm Biotechnol, vol. 17, no. 9, p. 772, Jan. 2016, doi: 10.2174/1389201017666160127110440.
- R. F. Donnelly et al., “Hydrogel-forming and dissolving microneedles for enhanced delivery of photosensitisers and precursors,” Photochem Photobiol, vol. 90, no. 3, p. 641, 2014, doi: 10.1111/PHP.12209.
- R. Mishra and T. K. Bhattacharyya, “MEMS-based hollow microneedles for transdermal drug delivery,” Drug Delivery Devices and Therapeutic Systems, pp. 325–344, Jan. 2021, doi: 10.1016/B978-0-12-819838-4.00007-9.
- P. Zhang, C. Dalton, and G. A. Jullien, “Design and fabrication of MEMS-based microneedle arrays for medical applications,” Microsystem Technologies, vol. 15, no. 7, pp. 1073–1082, Jul. 2009, doi: 10.1007/S00542-009-0883-5/FIGURES/8.
- J. Li, M. Zeng, H. Shan, and C. Tong, “Microneedle Patches as Drug and Vaccine Delivery Platform,” Curr Med Chem, vol. 24, no. 22, May 2017, doi: 10.2174/0929867324666170526124053.
- H. S. Gill and M. R. Prausnitz, “Coated microneedles for transdermal delivery,” J Control Release, vol. 117, no. 2, p. 227, Feb. 2007, doi: 10.1016/J.JCONREL.2006.10.017.
- H. T. T. Duong et al., “Microneedle arrays coated with charge reversal pH-sensitive copolymers improve antigen presenting cells-homing DNA vaccine delivery and immune responses,” J Control Release, vol. 269, pp. 225–234, Jan. 2018, doi: 10.1016/J.JCONREL.2017.11.025.
- K. M. Kwon et al., “Microneedles: quick and easy delivery methods of vaccines,” Clin Exp Vaccine Res, vol. 6, no. 2, p. 156, 2017, doi: 10.7774/CEVR.2017.6.2.156.
- “Micro- and Nanotechnology in Vaccine Development - Google Books.” Accessed: Jul. 13, 2024. [Online]. Available: https://books.google.co.in/books?hl=en&lr=&id=WxWKCgAAQBAJ&oi=fnd&pg=PP1&ots=A4CXCSk8rC&sig=NrMdxwKYj5NAbG01DdWj2IgilF0&redir_esc=y#v=onepage&q&f=false
- Y. K. Demir, Z. Akan, and O. Kerimoglu, “Characterization of Polymeric Microneedle Arrays for Transdermal Drug Delivery,” PLoS One, vol. 8, no. 10, p. 77289, Oct. 2013, doi: 10.1371/JOURNAL.PONE.0077289.
- A. M. Rodgers, A. S. Cordeiro, and R. F. Donnelly, “Technology update: dissolvable microneedle patches for vaccine delivery,” Med Devices (Auckl), vol. 12, p. 379, 2019, doi: 10.2147/MDER.S198220.
- A. J. Guillot, A. S. Cordeiro, R. F. Donnelly, M. C. Montesinos, T. M. Garrigues, and A. Melero, “Microneedle-Based Delivery: An Overview of Current Applications and Trends,” Pharmaceutics, vol. 12, no. 6, pp. 1–28, Jun. 2020, doi: 10.3390/PHARMACEUTICS12060569.
- P. González-Vázquez et al., “Transdermal delivery of gentamicin using dissolving microneedle arrays for potential treatment of neonatal sepsis,” Journal of Controlled Release, vol. 265, p. 30, Nov. 2017, doi: 10.1016/J.JCONREL.2017.07.032.
- N. W. Kang et al., “Microneedles for drug delivery: recent advances in materials and geometry for preclinical and clinical studies,” Expert Opin Drug Deliv, vol. 18, no. 7, pp. 929–947, 2021, doi: 10.1080/17425247.2021.1828860.
- R. Nagarkar, M. Singh, H. X. Nguyen, and S. Jonnalagadda, “A review of recent advances in microneedle technology for transdermal drug delivery,” J Drug Deliv Sci Technol, vol. 59, p. 101923, Oct. 2020, doi: 10.1016/J.JDDST.2020.101923.
- E. McAlister et al., “Directly Compressed Tablets: A Novel Drug-Containing Reservoir Combined with Hydrogel-Forming Microneedle Arrays for Transdermal Drug Delivery,” Adv Healthc Mater, vol. 10, no. 3, Feb. 2021, doi: 10.1002/ADHM.202001256.
- S. W. T. Chew et al., “A self?adhesive microneedle patch with drug loading capability through swelling effect,” Bioeng Transl Med, vol. 5, no. 2, May 2020, doi: 10.1002/BTM2.10157.
- C. A. Dreiss, “Hydrogel design strategies for drug delivery,” Curr Opin Colloid Interface Sci, vol. 48, pp. 1–17, Aug. 2020, doi: 10.1016/J.COCIS.2020.02.001.
- Y. Zhang, J. Yu, H. N. Bomba, Y. Zhu, and Z. Gu, “Mechanical Force-Triggered Drug Delivery,” Chem Rev, vol. 116, no. 19, pp. 12536–12563, Oct. 2016, doi: 10.1021/ACS.CHEMREV.6B00369.
- A. Raza, T. Rasheed, F. Nabeel, U. Hayat, M. Bilal, and H. M. N. Iqbal, “Endogenous and Exogenous Stimuli-Responsive Drug Delivery Systems for Programmed Site-Specific Release,” Molecules, vol. 24, no. 6, Mar. 2019, doi: 10.3390/MOLECULES24061117.
- “Micro injection moulding?: tooling and process factors - ProQuest.” Accessed: Jul. 13, 2024. [Online]. Available: https://www.proquest.com/openview/da2e431fa872071a2225e1f05290258c/1?pq-origsite=gscholar&cbl=2026366
- M. J. Kim et al., “Fabrication of Circular Obelisk-Type Multilayer Microneedles Using Micro-Milling and Spray Deposition,” Front Bioeng Biotechnol, vol. 6, no. MAY, May 2018, doi: 10.3389/FBIOE.2018.00054.
- D. Armani, C. Liu, and N. Aluru, “Re-configurable fluid circuits by PDMS elastomer micromachining,” Proceedings of the IEEE Micro Electro Mechanical Systems (MEMS), pp. 222–227, 1999, doi: 10.1109/MEMSYS.1999.746817.
- J. H. Park, M. G. Allen, and M. R. Prausnitz, “Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery,” J Control Release, vol. 104, no. 1, pp. 51–66, May 2005, doi: 10.1016/J.JCONREL.2005.02.002.
- M. Yang, Z. Xu, S. Desai, D. Kumar, and J. Sankar, “Fabrication of Micro Single Chamber Solid Oxide Fuel Cell Using Photolithography and Pulsed Laser Deposition,” J Fuel Cell Sci Technol, vol. 12, no. 2, Apr. 2015, doi: 10.1115/1.4029094/371816.
- S. Desai, M. Craps, and T. Esho, “Direct writing of nanomaterials for flexible thin-film transistors (fTFTs),” International Journal of Advanced Manufacturing Technology, vol. 64, no. 1–4, pp. 537–543, Jan. 2013, doi: 10.1007/S00170-012-4425-4/METRICS.
- M. K. Ahmed, M. E. El-Naggar, A. Aldalbahi, M. H. El-Newehy, and A. A. Menazea, “Methylene blue degradation under visible light of metallic nanoparticles scattered into graphene oxide using laser ablation technique in aqueous solutions,” J Mol Liq, vol. 315, p. 113794, Oct. 2020, doi: 10.1016/J.MOLLIQ.2020.113794.
- K. T. Tu and C. K. Chung, “Fabrication of biodegradable polymer microneedle array via CO2 laser ablation,” 2015 IEEE 10th International Conference on Nano/Micro Engineered and Molecular Systems, NEMS 2015, pp. 494–497, Jul. 2015, doi: 10.1109/NEMS.2015.7147476.
- H. R. Nejad, A. Sadeqi, G. Kiaee, and S. Sonkusale, “Low-cost and cleanroom-free fabrication of microneedles,” Microsystems & Nanoengineering 2018 4:1, vol. 4, no. 1, pp. 1–7, Jan. 2018, doi: 10.1038/micronano.2017.73.
- R. E. M. Lutton, E. Larrañeta, M. C. Kearney, P. Boyd, A. D. Woolfson, and R. F. Donnelly, “A novel scalable manufacturing process for the production of hydrogel-forming microneedle arrays,” Int J Pharm, vol. 494, no. 1, p. 417, Oct. 2015, doi: 10.1016/J.IJPHARM.2015.08.049.
- J. Nold, P. St. J. Russell, W. Chang, J. C. Travers, and N. Y. Joly, “Ultrafast nonlinear optics in gas-filled hollow-core photonic crystal fibers [Invited],” JOSA B, Vol. 28, Issue 12, pp. A11-A26, vol. 28, no. 12, pp. A11–A26, Dec. 2011, doi: 10.1364/JOSAB.28.000A11.
- R. F. Donnelly, T. R. Raj Singh, and A. D. Woolfson, “Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety,” Drug Deliv, vol. 17, no. 4, p. 187, May 2010, doi: 10.3109/10717541003667798.
- A. Gaikwad and S. Desai, “Molecular Dynamics Investigation of the Deformation Mechanism of Gold with Variations in Mold Profiles during Nanoimprinting,” Materials, vol. 14, no. 10, May 2021, doi: 10.3390/MA14102548.
- J. D. Plummer, M. D. Deal, and P. B. Griffin, “Silicon VLSI technology?: fundamentals, practice and modeling,” p. 817, Accessed: Jul. 13, 2024. [Online]. Available: https://books.google.com/books/about/Silicon_VLSI_Technology.html?id=U97p5phY-h4C
- K. T. M. Tran and T. D. Nguyen, “Lithography-based methods to manufacture biomaterials at small scales,” Journal of Science: Advanced Materials and Devices, vol. 2, no. 1, pp. 1–14, Mar. 2017, doi: 10.1016/J.JSAMD.2016.12.001.
- T. R. R. Singh, H. Mcmillan, K. Mooney, A. Z. Alkilani, and R. F. Donnelly, “Microneedles for drug delivery and monitoring,” Microfluidic Devices for Biomedical Applications, pp. 185–230, Jan. 2013, doi: 10.1533/9780857097040.2.185.
- B. W. Barry, “Novel mechanisms and devices to enable successful transdermal drug delivery,” European Journal of Pharmaceutical Sciences, vol. 14, no. 2, pp. 101–114, 2001, doi: 10.1016/S0928-0987(01)00167-1.
- P. Ghosh, N. K. Brogden, and A. L. Stinchcomb, “Effect of formulation pH on transport of naltrexone species and pore closure in microneedle-enhanced transdermal drug delivery,” Mol Pharm, vol. 10, no. 6, p. 2331, Jun. 2013, doi: 10.1021/MP3007083.
- A. Arora, M. R. Prausnitz, and S. Mitragotri, “Micro-scale Devices for Transdermal Drug Delivery,” Int J Pharm, vol. 364, no. 2, p. 227, Dec. 2008, doi: 10.1016/J.IJPHARM.2008.08.032.
- A. Tröls, M. A. Hintermüller, M. Saeedipour, S. Pirker, and B. Jakoby, “Drug dosage for microneedle-based transdermal drug delivery systems utilizing evaporation-induced droplet transport,” Microfluid Nanofluidics, vol. 23, no. 7, pp. 1–5, Jul. 2019, doi: 10.1007/S10404-019-2257-3/FIGURES/6.
- M. Cormier et al., “Transdermal delivery of desmopressin using a coated microneedle array patch system,” Journal of Controlled Release, vol. 97, no. 3, pp. 503–511, Jul. 2004, doi: 10.1016/j.jconrel.2004.04.003.
- L. Nordquist, N. Roxhed, P. Griss, and G. Stemme, “Novel microneedle patches for active insulin delivery are efficient in maintaining glycaemic control: an initial comparison with subcutaneous administration,” Pharm Res, vol. 24, no. 7, pp. 1381–1388, Jul. 2007, doi: 10.1007/S11095-007-9256-X.
- Y. Qiu, G. Qin, S. Zhang, Y. Wu, B. Xu, and Y. Gao, “Novel lyophilized hydrogel patches for convenient and effective administration of microneedle-mediated insulin delivery,” Int J Pharm, vol. 437, no. 1–2, pp. 51–56, Nov. 2012, doi: 10.1016/J.IJPHARM.2012.07.035.
- S. Marshall, L. J. Sahm, and A. C. Moore, “The success of microneedle-mediated vaccine delivery into skin,” Hum Vaccin Immunother, vol. 12, no. 11, p. 2975, Nov. 2016, doi: 10.1080/21645515.2016.1171440.
- K. Matsuo et al., “Transcutaneous immunization using a dissolving microneedle array protects against tetanus, diphtheria, malaria, and influenza,” J Control Release, vol. 160, no. 3, pp. 495–501, Jun. 2012, doi: 10.1016/J.JCONREL.2012.04.001.
- D. Poirier et al., “Hepatitis B surface antigen incorporated in dissolvable microneedle array patch is antigenic and thermostable,” Biomaterials, vol. 145, pp. 256–265, Nov. 2017, doi: 10.1016/J.BIOMATERIALS.2017.08.038.
- C. Edens, N. C. Dybdahl-Sissoko, W. C. Weldon, M. S. Oberste, and M. R. Prausnitz, “Inactivated polio vaccination using a microneedle patch is immunogenic in the rhesus macaque,” Vaccine, vol. 33, no. 37, p. 4683, Sep. 2015, doi: 10.1016/J.VACCINE.2015.01.089.
- J. A. Mikszta et al., “Microneedle-Based Intradermal Delivery of the Anthrax Recombinant Protective Antigen Vaccine,” Infect Immun, vol. 74, no. 12, p. 6806, Dec. 2006, doi: 10.1128/IAI.01210-06.
- E. L. Giudice and J. D. Campbell, “Needle-free vaccine delivery,” Adv Drug Deliv Rev, vol. 58, no. 1, pp. 68–89, Apr. 2006, doi: 10.1016/J.ADDR.2005.12.003.
- A. C. Williams and B. W. Barry, “Penetration enhancers,” Adv Drug Deliv Rev, vol. 56, no. 5, pp. 603–618, Mar. 2004, doi: 10.1016/j.addr.2003.10.025.
- A. S. Rzhevskiy, T. R. R. Singh, R. F. Donnelly, and Y. G. Anissimov, “Microneedles as the technique of drug delivery enhancement in diverse organs and tissues,” J Control Release, vol. 270, pp. 184–202, Jan. 2018, doi: 10.1016/J.JCONREL.2017.11.048.
- S. H. Bariya, M. C. Gohel, T. A. Mehta, and O. P. Sharma, “Microneedles: an emerging transdermal drug delivery system,” J Pharm Pharmacol, vol. 64, no. 1, pp. 11–29, Jan. 2012, doi: 10.1111/J.2042-7158.2011.01369.X.
- D. Ramadon, M. T. C. McCrudden, A. J. Courtenay, and R. F. Donnelly, “Enhancement strategies for transdermal drug delivery systems: current trends and applications,” Drug Deliv Transl Res, vol. 12, no. 4, p. 758, Apr. 2022, doi: 10.1007/S13346-021-00909-6.
- K. Kawahara and K. Tojo, “Skin irritation in transdermal drug delivery systems: a strategy for its reduction,” Pharm Res, vol. 24, no. 2, pp. 399–408, Feb. 2007, doi: 10.1007/S11095-006-9165-4.
- J. Zhu, X. Zhou, A. Libanori, and W. Sun, “Microneedle-based bioassays,” Nanoscale Adv, vol. 2, no. 10, p. 4295, Oct. 2020, doi: 10.1039/D0NA00543F.
- H. S. Gill, D. D. Denson, B. A. Burris, and M. R. Prausnitz, “Effect of microneedle design on pain in human volunteers,” Clin J Pain, vol. 24, no. 7, pp. 585–594, 2008, doi: 10.1097/AJP.0B013E31816778F9.


 Prasanth Yerramsetti*
Prasanth Yerramsetti*
 Jyothi Munjavarapu
Jyothi Munjavarapu






 10.5281/zenodo.13176052
10.5281/zenodo.13176052