The use of Artificial Intelligence (AI) in discovering new medicines is like a big change in how we create treatments. This overview talks about how AI is used in different parts of making a new drug. It starts with finding and checking the targets for medicines, then goes into testing different compounds, making predictions, finding special markers, and other ways AI is used in this process. There are some problems like making sure the data is good, understanding the results, and following the rules. Working together is important to solve these problems and make new ideas. The rules we have for making medicines are also looked at closely, showing how AI fits into them. Looking ahead, we see a future were using technology, doing the right thing, and working together can bring us new medicines that are not only possible but also ethical, clear, and available to everyone.
Target identification, compound screening, predictive modeling, biomarker discovery, challenges
targets with a level of precision and speed unattainable through traditional methods. Moreover, the ability of AI to analyze complex biological interactions facilitates the validation of these targets, ensuring a more robust foundation for subsequent drug development endeavors.(3)
Compound Screening and Design:
One of the hallmark applications of AI in drug discovery lies in virtual screening and de novo drug design. AI-driven computational models analyze chemical databases, predict the binding affinity of molecules to specific targets, and propose novel compounds with optimized pharmacological properties. This not only expedites the identification of lead compounds but also enables the exploration of chemical space in a more targeted and resource-efficient manner.(4)
Predictive Modeling and Machine Learning:(5) Machine learning algorithms, a subset of AI, contribute significantly to predictive modeling in drug discovery. These algorithms analyze diverse datasets to predict drug-drug interactions, assess potential toxicities, and optimize pharmacokinetic profiles. (6)The integration of AI in these areas not only enhances the accuracy of predictions but also facilitates data-driven decision-making, thereby streamlining the drug development trajectory. While the integration of AI in drug discovery holds immense promise, it is not without its challenges. Issues related to data quality, interpretability of AI models, and the need for robust validation frameworks necessitate careful consideration.(7) In the subsequent sections of this review, we delve into these challenges, exploring the ethical dimensions of AI implementation and navigating case studies that exemplify both the successes and hurdles encountered on the path to leveraging artificial intelligence for drug discovery. As we traverse this intricate landscape, we aim to provide a comprehensive understanding of the transformative potential and inherent complexities associated with the integration of AI in the pharmaceutical research ecosystem. (8)
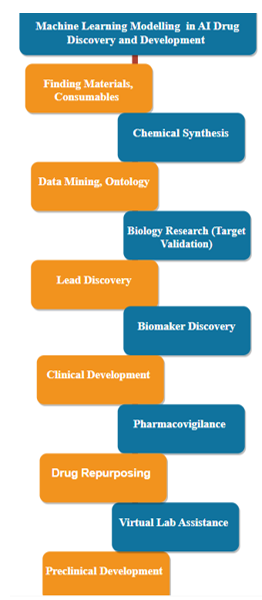
Figure 1: Machine learning model in for AI in Drug Discovery and SDevelopment(9)
Integration of AI in Drug Discovery:
The integration of Artificial Intelligence (AI) into the drug discovery process marks a paradigm shift, introducing computational methodologies that augment and expedite traditional approaches. AI technologies, including machine learning, predictive modeling, and data analytics, have demonstrated unparalleled efficacy in navigating the intricate landscape of drug development.(10) The following sections delve into how AI is seamlessly integrated into key stages of drug discovery. (11)
- Target Identification and Validation
- AI Algorithms in Genomic Analysis
- Utilizing vast datasets from genomics, AI algorithms discern patterns and correlations indicative of potential drug targets.(12)
- Machine learning models prioritize targets based on their relevance to disease pathways, streamlining the identification process.(13)
- Predictive Models for Target Validation
- AI contributes to experimental design and validation by predicting the likelihood of a target's involvement in disease mechanisms.(14)
- Computational models analyze complex biological interactions, providing researchers with insights into the therapeutic potential of identified targets.(15)
- Compound Screening and Design:
- Virtual Screening with AI:
- AI expedites the screening of chemical libraries through virtual screening, predicting the affinity of molecules to specific targets.(16)
- Machine learning models analyze structural and pharmacological data to identify lead compounds with higher chances of success.(17)
- De Novo Drug Design:
- AI facilitates de novo drug design by generating novel chemical structures with optimized pharmacological properties(18).
- Computational models explore chemical space, proposing potential drug candidates that may not be evident through traditional approaches.
- Predictive Modeling and Machine Learning:
- Optimizing Pharmacokinetics and Toxicity:(19)
- AI contributes to predictive modeling by assessing pharmacokinetics and predicting potential toxicities in silico.
- Machine learning models analyze diverse datasets to make informed predictions, guiding researchers in prioritizing compounds with favorable safety profiles.(20)
- Data-Driven Decision-Making:
- Machine learning aids in data-driven decision-making throughout the drug development process.
- Analyzing large datasets, AI models extract meaningful insights, enabling researchers to make informed choices regarding lead compounds, trial design, and progression to clinical phases.(21)
- AI in Biomarker Discovery:(22)
- Precision Medicine and Biomarkers:
- AI plays a pivotal role in identifying biomarkers associated with specific diseases.(23)
- This enables the development of targeted therapies and facilitates the stratification of patient populations for more effective and personalized treatment approaches.(24)
- Challenges and Considerations:
- Data Quality and Bias:
Addressing challenges related to data quality is crucial for the reliability of AI models.(25)
Ensuring representativeness and minimizing bias in training data is a continuous area of focus.
- Interpretability and Explainability:(26)
The interpretability of AI models is a challenge, especially in complex decision-making processes.
Researchers are actively working on developing methods to enhance the interpretability and explainability of AI-driven insights.(27)
Challenges and Limitations in the Integration of AI in Drug Discovery:
Data Quality and Bias:(28)
One of the primary challenges lies in the quality of data utilized to train AI models. The reliance on biased or incomplete datasets can compromise the robustness of AI predictions, introducing skewed outcomes. Addressing this challenge necessitates meticulous data curation and validation processes.(29) Ongoing efforts are crucial to identify and mitigate biases, promoting the development of more accurate and unbiased AI models.
Interpretability and Explainability:
The complexity of AI models, particularly deep learning structures, presents a challenge in terms of interpretability and explainability. The often-opaque nature of these models raises concerns about the ability to understand their decision-making processes. Research is actively underway to enhance interpretability through Explainable AI (XAI) methods. These approaches aim to shed light on the black-box nature of AI, providing researchers and clinicians with insights into the rationale behind AI-driven recommendations.(30)
Lack of Standardization:
The absence of standardized protocols and benchmarks poses a challenge in evaluating the performance of various AI models. Without consistent reporting methods and metrics, comparing results between studies becomes challenging.(31) Addressing this challenge requires collaborative efforts to establish standardized protocols for data collection, model training, and evaluation. Shared benchmarks and datasets can contribute to the development of more reliable and reproducible AI models.(32)
Scarcity of High-Quality Training Data:
Generating high-quality, labeled training data, especially for rare diseases or novel targets, remains a significant challenge. The scarcity of data can limit the generalizability of AI models. To overcome this hurdle, collaborative initiatives and data-sharing platforms are crucial. Pooling resources and facilitating access to diverse datasets can address the challenges associated with the scarcity of high-quality training data.(33)
Regulatory and Ethical Considerations:
The rapid evolution of AI technologies has outpaced the development of regulatory frameworks, introducing challenges in ensuring ethical use. Considerations such as data privacy, informed consent, and transparency require careful attention. (34)Collaborative efforts between researchers, industry stakeholders, and regulatory bodies are essential to establish guidelines and standards for the ethical use of AI in drug discovery. Transparency in AI models and adherence to ethical principles will foster trust within the scientific and regulatory communities.
Integration with Traditional Methods:
Integrating AI into existing drug discovery workflows may encounter resistance and skepticism. Harmonizing AI technologies with traditional methods poses organizational and cultural challenges. Comprehensive training programs and educational initiatives can play a pivotal role in familiarizing researchers and industry professionals with AI methodologies. Promoting a collaborative environment that values the strengths of both AI and traditional approaches can enhance the integration process.(35)
Computational Resource Requirements:
The computational demands of complex AI models, particularly deep learning architectures, present challenges in terms of resource requirements. Access to high-performance computing infrastructure may be a limiting factor for some researchers and institutions. Addressing this challenge involves leveraging cloud computing services and collaborative platforms to provide accessible computational resources. Ongoing research in optimization techniques and the development of lightweight models aims to alleviate computational resource constraints.(36)
Ethical Considerations of AI in Drug Discovery:
The integration of Artificial Intelligence (AI) into drug discovery is accompanied by a host of ethical considerations that necessitate careful examination. As we delve into the transformative potential of AI in shaping the landscape of pharmaceutical research, it is imperative to navigate the ethical dimensions inherent in this technological frontier.(37)
Data Privacy and Informed Consent:
One of the paramount ethical considerations revolves around the protection of individuals' data privacy and ensuring informed consent. AI in drug discovery relies heavily on large datasets, often sourced from electronic health records, genomics, and other personal health information. Safeguarding the privacy of individuals from whom the data originates is paramount. Researchers must adhere to stringent ethical standards to de-identify data, implement robust security measures, and obtain informed consent from patients contributing to these datasets.
Transparency and Explainability:
The inherent complexity of AI algorithms, particularly in deep learning models, poses challenges related to transparency and explainability. Ensuring that AI-driven insights are understandable and interpretable is essential for fostering trust among researchers, clinicians, and the broader public. Efforts in developing Explainable AI (XAI) methodologies are underway, aiming to demystify the decision-making processes of AI models. Transparent models contribute to accountability, allowing stakeholders to comprehend and scrutinize the rationale behind AI-generated recommendations.(37)
Equitable Access and Bias Mitigation:
Addressing bias in AI models is a critical ethical consideration. Biases present in training data can perpetuate disparities in healthcare outcomes. It is crucial to implement measures that mitigate biases and ensure the equitable representation of diverse populations in training datasets. Striving for inclusivity in data collection and analysis helps avoid perpetuating or exacerbating existing disparities in healthcare.
Responsible Innovation and Accountability:
The rapid evolution of AI technologies demands a commitment to responsible innovation and accountability. Researchers and industry stakeholders must be vigilant in anticipating and addressing potential ethical challenges associated with AI in drug discovery. This includes adherence to ethical guidelines, regular assessments of the social impact of AI applications, and a commitment to correcting and learning from any unintended consequences that may arise during the development and deployment of AI systems.(38)
Regulatory Compliance:
Navigating the evolving regulatory landscape is another ethical consideration in the integration of AI in drug discovery. Regulatory bodies are working to adapt to the rapid advancements in AI technologies, and researchers must ensure compliance with existing and emerging regulations. Ethical conduct extends to transparency in reporting methodologies, accurately representing the capabilities and limitations of AI models, and actively engaging with regulatory bodies to contribute to the development of ethical guidelines.
Collaboration and Partnerships in AI-Driven Drug Discovery:
The integration of Artificial Intelligence (AI) into the field of drug discovery has transformed a paradigm shift, resulting in a landscape characterized by collaboration. Recognizing the intricate interplay between AI technologies, traditional drug development practices, and academic expertise, collaborations and partnerships have become integral components of the pharmaceutical industry's strategy to expedite the discovery and development of novel therapeutics. Pharmaceutical companies are actively engaging in partnerships with specialized AI firms, capitalizing on the technological prowess of these entities. These collaborations involve the infusion of AI technologies into various stages of drug discovery, from target identification to compound screening. For instance, AstraZeneca's collaboration with Benevolent AI exemplifies the marriage of pharmaceutical domain knowledge with AI algorithms to streamline the identification of novel drug candidates for diseases such as chronic kidney disease and idiopathic pulmonary fibrosis.
The collaborative spirit extends to partnerships between academic institutions and pharmaceutical giants. These collaborations leverage the deep expertise of academic researchers in disease biology, genetics, and target identification, complemented by the computational power of AI. In the collaborative venture between Pfizer and the University of California, San Francisco (UCSF), the convergence of academic insight with industrial capabilities is aimed at accelerating the development of cancer therapeutics through AI-enhanced drug discovery workflows.(37)
Government initiatives further fuel collaboration by providing a supportive framework for joint efforts between industry and academia. Examples like the Innovative Medicines Initiative (IMI) in Europe showcase how collaborative projects, integrating AI approaches, can address specific challenges in drug discovery, from improving drug safety to advancing personalized medicine approaches.(38) AI consortia and knowledge-sharing platforms, such as the Pistoia Alliance's Center of Excellence for Artificial Intelligence in Life Sciences, have emerged as crucibles of collaboration. By bringing together diverse stakeholders, including pharmaceutical companies, technology providers, and research organizations, these platforms facilitate the sharing of data, development of common standards, and a culture of open innovation in the AI-driven drug discovery landscape.(39)
Regulatory Landscape in AI-Driven Drug Discovery:
Artificial Intelligence (AI) and drug discovery have transformed the regulatory landscape, as agencies worldwide grapple with the challenges and opportunities presented by these transformative technologies. Regulatory bodies, including the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), are actively engaged in adapting their frameworks to ensure the safe, effective, and ethical integration of AI into the drug development process. (40)As AI technologies become integral to medical decision-making, regulatory adaptation is a key theme. The dynamic nature of AI algorithms, particularly in machine learning and deep learning, necessitates flexible guidelines that can accommodate rapid advancements. Regulatory agencies are working to strike a balance, fostering an environment that encourages innovation while upholding rigorous standards for safety and efficacy. The classification of AI-driven tools and algorithms as medical devices has prompted regulatory scrutiny. Frameworks such as the FDA's Software as a Medical Device (SaMD) provide guidance on the classification and oversight of these AI applications based on their intended use and risk profile. This classification is pivotal in determining the regulatory pathway these technologies must navigate for approval and deployment in clinical settings. Transparency and explainability are paramount considerations in the regulatory evaluation of AI applications. Regulators emphasize the need for transparency to ensure accountability in decision-making processes. The interpretability of AI-driven insights by clinicians and researchers is crucial for regulatory approval and acceptance within the medical community. The incorporation of real-world evidence (RWE) in the validation of AI applications reflects a shift in regulatory perspectives. Recognizing the limitations of controlled clinical trial data, regulators are exploring ways to leverage broader, more representative datasets to validate the performance and generalizability of AI models.(41) Ethical and privacy considerations loom large in regulatory discussions. Protecting patient data, ensuring informed consent, and upholding ethical principles are at the forefront of regulatory priorities. Striking a balance between leveraging the potential of AI and safeguarding individual privacy remains a delicate yet crucial aspect of regulatory decision-making. Internationally, collaboration between regulatory bodies is gaining prominence. Initiatives like the collaboration between the FDA and the EMA aim to harmonize standards for the evaluation and approval of AI applications. (41)This collaborative approach acknowledges the global nature of pharmaceutical research and seeks to create a cohesive regulatory environment for AI-driven technologies. Adaptive regulation, emphasizing post-market surveillance and continuous monitoring, is emerging as a responsive strategy. The iterative nature of AI model updates and improvements necessitates ongoing scrutiny to ensure that these technologies maintain their safety and efficacy profiles in real-world settings.(42)
Applications of AI in Drug Discovery:

Table 1: Table of an overview of AI in drug discovery(41)

Figure 2: Application of AI in Drug Discovery and Development(42)
CONCLUSION


 Atish B. Sarode* 1
Atish B. Sarode* 1



 10.5281/zenodo.10894618
10.5281/zenodo.10894618