Abstract
Transferring technology across nations and organizations with varying degrees of technical proficiency results in barriers and issues for the less developed party."Technology transfer" is the term used to describe the process in the pharmaceutical industry of going from drug discovery to development, clinical trials, and ultimately full-scale commercialization.The conclusion of improving a quality drug item's definition greatly depends on adaptability and successful innovation. Technology transfer design incorporates the improvements in process understanding and improvement through the use of techniques like Six Sigma and Lean (Snee and Hoerl 2003, 2005) that have been made over the past 25 years in technology transfer into an iterative process for technology transfer for individual products.It is crucial for government laboratories to ensure that technology created in state laboratories is accessible to the general public.
Keywords
Pharmaceutical, Production, Technology, Reasearch and Development
Introduction
Various definitions and notions of technology transfer have been explored, with discussions varying depending on the study fields and objectives. Technology transfer, according to Gibson and Smilor (1991), is frequently a chaotic, disorganized process involving groups and individuals who may have differing opinions about the usefulness and possible applications of the technology. They contend that technology frequently has no clear purpose or significance. Users, developers, and researchers are likely to view the technology differently. Technology transfer is a complicated and challenging process, even when it happens between different departments within a single product division of a single organization, according to a survey of the literature on the subject.1 In the pharmaceutical industry, the process of moving from medication discovery to development, clinical trials, and eventually full-scale commercialization is referred to as "technology transfer." It is a crucial stage that creates a link between the discovery of new drugs and the creation of novel medicinal products. "A logical procedure that controls the transfer of any method in conjunction with its documentation and skilled experience between development and manufacture or between manufacture sites" is how the World Health Organization defines technology transfer. It can be described as the transfer of technology from the creator or transferrer to the transferee in order to generate advertising profit when passing through mutual love. It also entails applying scientific discoveries that are transferred from one institution to another for.2 The manufacturing process, control strategy, process validation approach, and continuing continuous improvement are all built on this expertise. In order to accomplish product realization, technology transfer activities aim to transmit knowledge about products and processes from development to manufacture, as well as within or across manufacturing sites. By analyzing technology transfer based on the following concepts, this review article also aims to suggest some regulations to realize technology transfer required for high quality and steady manufacture of developed products and current products.6 "A logical procedure that controls the transfer of any process together with its documentation and professional expertise between development and manufacture or between manufacture sites" is how the World Health Organization defines technology transfer. It is a methodical process designed to transfer recorded knowledge and expertise obtained during development and/or commercialization to a suitable, accountable, and approved entity. Technology transfer includes both the documentation transfer and the receiving unit's (RU) proven capacity to carry out the essential functions of the transferred technology to the satisfaction of all involved parties and relevant regulatory agencies. The pharmaceutical business in developing nations mostly relies on the importation of pharmaceutical products and the emulation of rich nation technology. According to a 1983 report by the United Nations Centre on Transnational Corporations, pharmaceutical businesses in developing nations depended on technology brought in from overseas by multinational corporations (MNCs) that conducted business there.Pharmaceutical production and manufacturing were carried out by a transnational corporation or by a local company licensing a multinational corporation. As a result, Thai pharmaceutical companies, like those in other developing nations, imported cutting-edge technology from industrialized nations through foreign direct investment (FDI) under the umbrella of multinational corporations through licenses, joint ventures, or wholly owned subsidiaries. An MNC-supervised production process is thus one avenue through which a Thai company can obtain.12 The outcome of the definition improvement of a quality drug item exceptionally relies upon the versatility and productive innovation move. Very much arranged and reported approaches ought to be attempted and rehearsed all through the cluster amplification and innovation move stage. Distinguishing basic parts during item and cycle advancement is moving and needs exceptional thoughtfulness regarding stay away from item disappointment in business bunches. Different subtleties like hardware and their capability, scientific strategy advancement and move, process approval, and different administrative necessities ought to be painstakingly checked and utilized to accomplish the objective of effective innovation move. This part manages administrative rules (WHO) for innovation move (TT) and examines different prerequisites from documentation perspectives for executing the innovation move exercises.14 In the non-industrial nations, the drug business relies generally upon the importation of drug items and impersonation of innovation from created nations. The Unified Countries Place on Transnational Partnerships (1983 )reported that the drug organizations in the non-industrial nations depended on innovation presented from abroad through worldwide corporations(MNC) working business in their countries. The fabricating cycle and drug creation was under a transnational organization or under the permitting of a global organization by a neighborhood firm. As needs be, trend setting innovation in Thai drug industry, like other emerging nations, was imported from created nations through unfamiliar direct speculation (FDI) under the global companies through an entirely owned subsidiary, a joint-adventure or a permitting. The assembling system regulated by a MNC is, thusly, one channel for a Thai organization to get an innovation move from created nations. The decrease in imported innovation, the expansion in the productivity of innovation work, technological competence and mechanical seriousness of Thai drug industry, particularly among the ASEAN nations rely upon a capable Thai drug industry and qualified personnel.they ought to have the option to adsorb, learn, apply and create or fit for getting innovation and expanding their innovative capacity.17 In the drug business innovation move alludes to the necessary cycles for effective advancement from drug disclosure to item improvement, to clinical preliminaries to full scale commercialization or it is the interaction by which a designer of innovation makes its innovation accessible to business accomplice that will take advantage of innovation. In drug industry readiness of dose structure needs increase in/at a few phases, for example, limited scope research center improvement from 0.5-2kg clump can be increased to 5-10 kg and afterward to 20-100 kg on a pilot scale. Creation scale can normally go from 200 kg to more prominent than 1000 kg. Innovation move includes fabricating drug item with expanding clump sizes on bigger gear or utilizing constant handling on pilot scale hardware. For the most part increase includes the exchange of innovation and the exchange of information that has been amassed during the limited scale improvement of item and cycles. It is vital to understand that great correspondence is basic for plan and cycle move to find success. It is fundamental for a scientist or engineer of innovation to make accessible this innovation to someone else's to take advantage of for the advancement of improvement of innovation and for double-dealing of an innovation in various fields of utilizations and to make is use with another association that might have better assembling capacity, promoting capacity and business capacity .18
DEFINATION OF TECHNOLOGY TRANSFER :
WHAT IS TECHNOLOGY TRANSFER (TT)?
Technological transfer refers to the transfer of manufacturing process of a new pharmaceutical drug substance (DS) or drug product (DP) from a transferring site (R and D site) to a receiving site (commercial manufacturing site). This includes all knowledge, information, and skills needed to produce the DS or DP at a receiving site.7
"Innovation move" in drug industry alludes to the necessary cycles for fruitful advancement from drug disclosure to item improvement to clinical preliminaries to full-scale commercialization or it is the cycle by which a designer of innovation makes its innovation accessible to business accomplice that will take advantage of the innovation.8
TECHNOLOGY AND TECHNOLOGY TRANSFER:
Technology is defined differently. Sociologists, Economists, Management Scientists, and other faculties, have their own definitions of technology. Apparently there are professional definitions for this word, but all have common aspects. There are different aspects for technology (Figure 1):
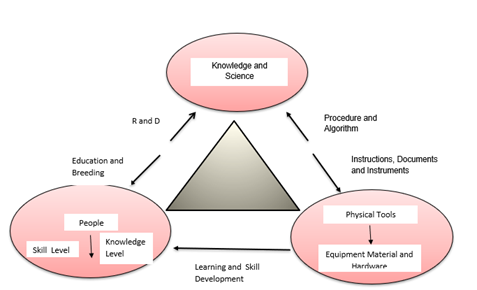
Fig.1:Technology Triangle
FACETS OF TECHNOLOGY TRANSFER:
The transfer of technology could happen in any of following ways;
- State laboratories for enterprises of the private sector.
- Between companies of the private sector of the same country.
- Among companies of the private sector of various countries.
- From universities to private sector companies.
- Academia, industry collaboration and government.3
GOVERNMENT LABS TO PRIVATE SECTOR FIRMS:
Government laboratories are an important area for intellectual activity. With state funding, it is very important that the technology developed in state laboratories reach the masses, and the promotion of cooperation between state and private sectors is an important part of technology transfer. Here, government laboratories are technology developers, while private sector companies are technology absorbers. The main advantage of this type of technology transfer is that state laboratories would receive additional financial support, and the flow of money to state-funded institutions would reduce the financial burden on states. On the other hand, a private sector company could use the technology at a relatively lower cost and be able to make the technology available to the masses.9
TECHNOLOGY TRANSFER PROCESS :
Technology transfer in different countries and organizations with different levels of technical expertise creates limitations and problems for the less developed recipient. Technology transfer is a complex and difficult process that requires thorough and exhaustive research. If one ignores various aspects of technology transfer; this can lead to weaknesses in national technology. The technology transfer process includes some preventive scales that must be considered before choosing a technology transfer method. These factors (Malekifar, 1999) include:.
- Awareness of the fundamental and important factors required for technology transfer.
- Awareness of the failure factors of technology transfer.
- Effort to acquire the appropriate technology to achieve the appropriate status of the organization.
- Consideration of existing and legacy technologies.10
PURPOSE OF TECHNOLOGY TRANSFER :
- Inadequacy of manufacturing extent:
The technology introducer might have its own manufacturing equipment that is applicable to small scale operation and large-scale manufacturing operations.
- Shortage of resources to discharge product commercially:
For conducting the early stage analysis like animal studies and toxicology studies the inventor must-have resources. But for performing clinical studies the resources are not available.
- Deficiency of distribution and marketing competence :
The inventor would completely establish a technology and obtained product registrations and regulatory approvals.
- Application in various fields :
To create an extra income the inventor may transfer the technology to someone else to use in another field that varies from the already applied field.4
IMPORTANCE OF TECHNOLOGY TRANSFER:
- To elucidate necessary information to transfer technology from R&D to actual manufacturing by sorting out various information obtained during R&D.
- Demonstration of necessary information to technology transfer from research and development to actual manufacturing.
- To elucidate necessary information to transfer technology of existing products between various manufacturing places.
- To exemplify specific procedures and points of concern for smooth technology transfer. For the smooth manufacturing of commercialized products.5
EFFECTIVE FACTORS IN TECHNOLOGY TRANSFER:
Eight factors are essential to create favorable conditions for the transfer of medical technology :
- Visible and easily accessible local markets;
- Political stability, good financial management;
- Clear development priorities;
- Effective regulation;
- Availability of skilled labor;
- Adequate capital markets;
- Strong intellectual property rights and effective enforcement;
- The quality of the relationship between industry and government and the extent to which they are able to work together effectively in the long term.9
VARIOUS METHODS OF TECHNOLOGY TRANSFER :
- By selling or transferring technology
- By licensing technology
- By pooling capital, management and know-how
- By using technological information such as plans, microfilms , etc.
- technical staff as a tool.8
TECHNOLOGY TRANSFER ORGANIZATION WORLD INTELLECTUAL PROPERTY ORGANIZATION :
- National Institutes of Health
- Technology Transfer Focal Points
- Biotechnology Industry Organization.
PREVENTIVE FACTORS OF THE TECHNOLOGY TRANSFER PROCESS :
- Must be aware of the basic and important factors of the technology transfer process.
- Factors of failure of the technology transfer process.
- Must increase efforts to get the appropriate technology to achieve. the appropriate organizational performance position.
- The overview of existing, active and old technologies.11
WHY DOES A TECHNOLOGY TRANSFER DEVELOPER IN THE PHARMACEUTICAL INDUSTRY :
make their technology available to a business partner who exploits the technology. In the pharmaceutical industry, the production of dosage forms requires scaling up in several stages, for example, small-scale laboratory development of a batch of 0.5-2 kg can be scaled up to 5-10 kg and then on a pilot scale of 20-100 kg .Production volume can usually vary from 200 kg to more than 1000 kg. Technological transfer involves the production of medicine by increasing sets of larger equipment or by continuous processing in an experiment. In general, technology and knowledge accumulated in connection with product and process development are implemented on a large scale. It is important to understand that good communication is essential to the success of design and process transfer. It is important for a scientist or technology developer to make that technology available to another person to advance technology development and technology. using the technology in different application areas and using it with another organization that may have better manufacturing, marketing and commercial capabilities.1 For both full cooperation and non-cooperation, this dissertation empirically evaluates four main theories in the discussion of why technology transfer is difficult for industrial firms in developing countries:
- Technical Knowledge;
- Reception Characteristics;
- Characteristics Of The Donor; And
- The Economic Environment.15
Technology transfer design takes the advances made in technology transfer over the past 25 years in understanding and improving processes using methods such as Six Sigma and Lean and integrates them into an iterative approach to technology transfer for individual products or to many at once. Depending on the type of transfer, the number of steps in the process can vary, but for a typical move from one place to another, there are about ten steps to create a transfer process and investigate potential problems:
- Set the scope. , risks and strategy. Determine the number of facilities and products involved, the markets the products serve, the regulatory documents required, and the personnel and skill requirements—information that must be documented and approved by senior management. Assess regulatory, financial and market risks. If the transfer involves the closure of the factory, PR risks must also be considered. Once you understand the scope and risks of the project, you can determine the optimal migration strategy - where, when and to whom (CMO or back office) the migration will take place. These analyzes should always be based on the result of unwavering concentration.
- Indicate common openings. Perform management operations, supply chain, quality, regulatory, marketing, technology / Rand D and all other functions related to the movement. Transfer support personnel should be assigned to these departments and mentors selected to advise them during the process.
- Define communication and reporting channels. Make reporting channels clear within each site and between sites. Include senior management reporting structure, frequency of communication, methods of communication and approach to remove language barriers.
- Define measures. Appropriate measures should be selected to measure the progress and success of technology transfer. These metrics may include: budget execution, schedule adherence, validation speed and success rate, regulatory compliance acceptance rate, man-hours by task title, labor costs (internal and external), and opportunity costs due to sales delays. 5. Develop and train transition teams. Designate transfer teams at headquarters, the sending plant and, in the case of an internal transfer, the receiving plant. Ideally, both the sending and receiving manufacturing teams will have senior people from manufacturing, quality, technical services, engineering and supply chain. Coach teams in troubleshooting, problem solving, communication and time management.16
Reasons For Technology Transfer:
There may be many reasons why a technology developer might consider offering their technology to another person rather than exploiting it themselves.Some of them include:Alliances with partners who can advance the development of the technology to bring it to market The technology developer can be has resources to export the technology to a certain stage of development, such as animal testing and toxicology studies, but it does not have the resources to take the technology through the clinical and regulatory stages and must work with another organization to use it. through these stages and into the market.Forming alliances with partners who have manufacturing capabilities. A technology developer may have developed a technology to the point where it is almost market-ready, but does not have the capability or resources to manufacture the product in. clean room and must collaborate with another organization that has this opportunity.Form alliances with partners that have marketing and distribution capabilities. A technology developer may have fully developed the technology and even obtained regulatory approvals and product registrations for a marketable product, but does not have the marketing and distribution channels to enable it to be labeled, and they must work with another organization that has this capability.Use in another application area. the technology may be capable of exploiting the technology itself in diagnostic applications, and it may authorize the commercial partner to exploit therapeutic applications. By transferring the technology to another person for use in another field of application, the developer of the technology creates a new revenue stream from the use in this other field.No business opportunity The technology developer may be a university research institution that does not. has a commercial use opportunity. completely and must work with another organization that has this ability. In the disposal of pharmaceutical products, technology transfer is common in the industry in collaboration with this method to bring the medicinal product to market.18
TECHNOLOGY TRANSFER OVERVIEW :
The main goal of any technology transfer is to get the majority of consumers using the technology to adopt it. However, as each organization has its own goals and culture, there is no one-size-fits-all transfer process for all organizations and events. Rather, most technology transfer processes have several key steps or activities, and each process is tailored to the needs of the organization.19
Important perspectives from the technology transfer forum technology transfer:
This forum included a roundtable discussion with technology transfer experts representing research universities, federal laboratories, the US government, and various industries. A significant part of the agenda was dedicated to an open forum where participants could comment publicly. The open discussion period also provided an opportunity for additional comments. Many people who were unable to attend the forum were able to submit comments using an online survey and comment form.19
GENERAL VIEWS OF TECHNOLOGY TRANSFER:
-
- Panelists and participants noted that technology transfer should be viewed more broadly. A framework that included federal investment, legislation, and commercialization appeared to be beneficial.
- Many forum members discussed technology transfer in a global context. American competitiveness and an increasingly global economy contributed to these comments. The industry's partnership with foreign research institutes was also a frequent topic of discussion.
- Successful commercialization requires much more than a good idea or new technology. Developing a successful product requires, among other things, effective management, strategy, timing and marketing. Coordination between many organizations, some with very different missions, is a major challenge.19
WHEN DOES TECHNOLOGY TRANSFER OCCUR:
- Development Lab to Kilo Lab
- Discovery Lab to Development Lab
- Idea to Discovery Lab
- Pilot Plant to Semi-works (other pilot plant)
- Pilot Plant/ Semi-works to Manufacturing
- Lab to Pilot Plant
- Manufacturing to Manufacturing
STEPS INVOLVED IN THE TECHNOLOGY TRANSFER PROCESS :
-
- Development of technology by R&D. (Research Phase) –
Procedure design and excipient selection by R&D - Innovative product qualities are the basis for R&D's process design and material selection. (b) R&D determines quality and specifications: A product's quality should match that of an innovative product.
-
- Technology transfer from R&D to production (Development Phase) –
The product development laboratory receives a technology transfer dossier (TTD) document from R&D that includes all of the following formulation and drug product information: The product name, strength, generic name, MFC number, page number, effective date, shelf life, and market are all listed on the Master Formula Card (MFC). (b) Master Packing Card: Provides details on the type of packaging, the material used to package, the stability profile, and the packaging's shelf life. (c) Master Formula: Contains production guidelines and the order of formulation. (The order of the processes and the surroundings.) (d) Standard Test Procedures (STP) and Specifications: These provide information on the profile of active components and excipients, in-process parameters, product release specifications, and specifics about the final product.
3. Optimization and Production. (Production Phase)
a. Validation Studies –
Production is implemented after validation studies that can verify that process can stabilize the product based on transferred manufacturing formula. The manufacturing department accepting technology is responsible for validation and the R&D department transferring technology should take responsibility for validation such as performance qualification, cleaning, and process validation.. (b) Scale-up for production –Scale up production Involves the transfer of technology during process and scale up development. It is essential to consider the production environment and system during the development of a process. Operators should concentrate on keeping their segment of the production process running smoothly.
b. Technology Transfer Documentation –
generally understood to be a document outlining the technology transfer's contents for the parties transferring and being transferred. Every stage of the technology transfer process, from R&D to production, needs to be well-documented. Task assignments and duties should also be made clear, as should the acceptance standards for each technology that is to be transferred. All technology transfer processes require the documentation to be reviewed and approved by the Quality Assurance department.
c. Exhibit –
After taking procedure of scale-up batches of the product, manufacturing of exhibit batches takes place. In the case of an exhibit, batch sizes are increased along with equipment and their processes. This is done for filling purposes in regulatory agencies. 20
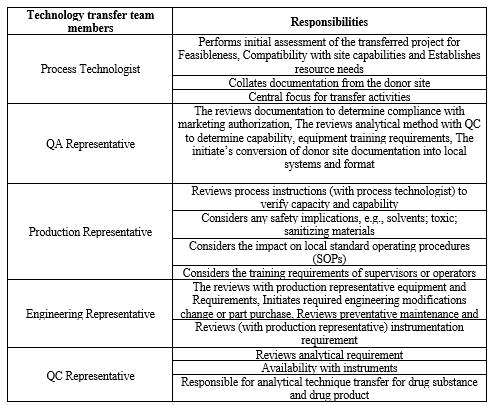
TECHNOLOGY TRANSFER TEAM:
Various persons involved in the process technology transfer are:
- Researchers
- Production
- Quality Assurance
- Quality Control
- Engineering22
Table 1- Technology Transfer Team Members And Responsibilities
ORAL DRUG TECHNOLOGY TRANSFER
Every step of the oral drug manufacturing process, including pre-IND, IND, NDA, ANDA, NADA, ANADA, Phase I, II, III, IV (CTM manufacture), and commercialization, is best handled by a CDMO (CONTRACT DEVELOPMENT MANUFACTURING ORGANIZATION) with many years of experience. Different manufacturing scales, regulatory requirements, and budgetary priorities apply to each phase. A staff member with experience in every stage will be more qualified to handle any bumps in the road during and after the transfer. A CDMO needs to be well-versed in all pertinent approaches used in the majority of tech transfers in order for an oral drug tech transfer to be successful. Examples of liquid-based systems include lipid-based systems/self-microemulsifying drug delivery systems (SMEDDS), nano-suspension, inclusion complexes, and the improvement of solubility and bioavailability for poorly soluble drugs by solubilization using GRAS solvents for the intended drug load. Spray drying, roller compaction, tableting/encapsulation, and other methods are examples of solid dosage forms. A CDMO for oral medications ought to have clean rooms, cGMP and FDA-certified facilities, and machinery for making tablets, capsules, gelatin capsules, and non-sterile liquids and suspensions. If your CDMO uses hormones, cytotoxic materials, highly potent compounds, or DEA-controlled drugs I–V, containment facilities will be required. Bonus points if your CDMO can find premium raw ingredients and excipients at competitive prices. A CDMO ought to be able to provide project schedules and cost estimates in less than a week after the technical evaluation starts. The CDMO needs to always be cognizant of the budget and have a track record of reducing costs for sponsors without sacrificing the caliber of the output. Verify that your CDMO has a history of bringing medications to market promptly and effectively, from preclinical to commercialization.
Though hundreds of innovative delivery platforms are under development and complicated items are becoming more and more popular, only a small number of CDMOs are equipped to manage them. A CDMO with experience in complicated dosage forms, small and large molecular compounds, niche technology, NCEs, and NDAs is required for the tech transfer if your oral dose product is formulated complexly. Complex processing issues including extrusion, spray drying, particle/bead coating, and milling/particle engineering should be handled by your potential CDMO.23
CANADIAN TECHNOLOGY TRANSFER:
The term "technology transfer" refers to transactions in which knowledge developed in academia is transferred to private sector firms for industrial and commercial purposes. As such, technology transfer is a subcategory of "information transfer".Knowledge can be transferred in a number of ways, including research collaboration, publication, consulting, standardization, employment of graduates, etc., the use of which depends on the circumstances, available resources and the goals of the stakeholders.Technology transfer involves Through various formal and informal means, this report specifically focuses on patenting, channels related to licensing and intellectual property law and policy. With that in mind, technology transfer begins when research conducted at a postsecondary institution (PSI) leads to a potential new product or service that is then marketed by a "spin-off" company or an existing company.Technology transfer is often referred to. to as a "technology transfer office" (TTO). TTO staff housed in PSIs bring together business, legal and technical expertise to advance and commercialize the fruits of academic knowledge. Technology transfer is described as a highly collaborative and mutually beneficial process that allows private companies to take advantage of academic opportunities.20
CONCLUSION:
- Improved documentation and communication by the technology transfer team can lead to appropriate efficiency in technology transfer from development to commercialization. A team works together achieves more successful first and repeat runs, which leads to an earlier licensing, an earlier launch, and a larger market share
- A specialized technology transfer company is established to oversee and carry out the procedure. Technology transfer is deemed successful when a sending unit, a development unit, and a receiving unit can consistently replicate the transferred product, process, or method in accordance with a predetermined set of specifications.
- Pharmaceutical companies will be able to fully profit from the latest advancements in new drug discovery and perform more successfully in a market that is changing quickly through the use of enhanced methodologies such technology transfer to the development and start-up of new production systems.
- In the pharmaceutical sector, licensing has become a vital technology transfer strategy that allows pharmaceutical companies to support research and development. Since technology transfer is a complicated topic, it should be approached from all angles.
REFERENCE :
- Reddy NM, Zhao L. International technology transfer: A review. Research policy. 1990 Aug 1;19(4):285-307.
- Pandey K, Joshi H, Paliwal S, Pawar S, Kumar N. Technology transfer: An overview of process transfer from development to commercialization. International Journal of Current Research and Review. 2020 Oct;12(19):188-92.
- Waghmare YS, Mahaparale SP. The important role of technology transfer in pharmaceutical industry-a review. World Journal of Pharmaceutics Research. 2017 Jun 28;6(9):310-29.
- Pavithra GM, Manoranjith SN, Nagalakshmi S. An overview of technology transfer as a regulatory aspect. International Journal of Applied Pharmaceutics. 2021;13(2):14-9.
- John RM. Technology transfer in pharmaceutical industry. The Pharma Innovation. 2017 Mar 1;6(3, Part D):235.
- Alam MS, Ahmad J. Pharmaceutical technology transfer: An overview. International Journal of Pharmaceutical Sciences and Research. 2013 Jul 1;4(7):2441.
- Popat B. Mohite, Sachin V. Sangle. Technology transfer in pharmaceutical industry- A Review. International Journal of Advances in Pharmaceutics.2017;06(01):01:07
- Amrita K, Shrikhande S, Prudhvi RP, Biswas UK, Bajaj A. Technology Transfer: A New Buzz Word in Pharmaceutical Industry. Journal of Advanced Scientific Research. 2013 Feb 10;4(01):16.
- Janodia MD, Sreedhar D, Ligade VS, Pise A. Facets of technology transfer: A perspective of pharmaceutical industry.
- Mahboudi M, Ananthan BR. Effective Factors in Technology Transfer in the Pharmaceutical Industries of Iran: A Case Study. IUP Journal of Knowledge Management. 2010 Jan 1;8.
- Amrita K, Shrikhande S, Prudhvi RP, Biswas UK, Bajaj A. Technology Transfer: A New Buzz Word in Pharmaceutical Industry. Journal of Advanced Scientific Research. 2013 Feb 10;4(01):1-6.
- Toyama O, Kongmuang S, Toyama M. Technology transfer and technological capability: A case study in manufacturing process in Thai pharmaceutical industry. Science, Engineering and Health Studies. 2014 May 27:51-61.
- Amanjeet Singh* And Geeta Aggarwal Technology Transfer In Pharmaceutical Industry: A Discussion. International Journal Of Pharma And Bio Sciences.2010 Sep
- Kaity S. Technology development and transfer in pharmaceutical product development. InDosage Forms, Formulation Developments and Regulations 2024 Jan 1 (pp. 471-491). Academic Press.
- Mourshed M. Technology transfer dynamics: Lessons from the Egyptian and Indian pharmaceutical industries (Doctoral dissertation, Massachusetts Institute of Technology).
- Snee RD, Reilly WJ, Meyers CA. International technology transfer by design. Int. Pharm. Ind. 2008 Jan;1(1):4-10.
- Onoomar Toyama*, Somlak Kongmuamg and Manoon Toyama -Technology transfer and technological capability: A case study in manufacturing process in thai Pharmaceutical industry, Silpakorn U Science & Tech J 8(2): 51-61, 2014
- Manral MS, Prashar B, Sheikh Y. Technology transfer in pharmaceutical industry: facts and steps involved. Am. J. PharmTech Res. 2012 Jun 14;2(4):73-82.
- Wang M, Pfleeger S, Adamson DM, Bloom G, Butz W, Fossum D, Gross M, Kelley C, Kelly T, Kofner A, Rippen H. Technology transfer of federally funded R&D. InConference Proceedings: Perspectives from a
- Canada. Parliament. House of Commons. Standing Committee on Industry, Science and Technology, Ruimy D. Intellectual Property and Technology Transfer: Promoting Best Practices. House of Commons; 2017 Nov. Medical
- Rajesh Dumpala, Chirag Patil-Review on Technology transfer in Pharmaceutical Industry Facts & steps Involved,Europian Journal of Pharmaceutical & Medical Research,2020.
- Sagar Pagar*, Akshay Khivansara, Pankaj Pagar, Review article on Technology transfer, Department of Quality Assurance techniques, M.V.P College of Pharmacy, Nashik-422002, Maharashtra, India.
- Koshy George, Dylan Amig and Sundeep Sethia, Tech transfer for oral pharmaceutical products,Pharmaceutics International Inc.Aug 2021.


 Mayuri bhokre*
Mayuri bhokre*
 Pratiksha Ashok Udawant
Pratiksha Ashok Udawant


 10.5281/zenodo.11129183
10.5281/zenodo.11129183