Non-steroidal anti-inflammatory drugs (NSAIDs) are a class of drugs universally used to treat both the acute and chronic injuries. However, the chronic nature of overuse injuries requires NSAIDs to be taken orally for an extended period of time. NSAIDs share a common mechanism of action through the inhibition of cyclooxygenase 1 (COX1) and cyclooxygenase 2 (COX2), while available under many brand names and formulations. Ibuprofen is the most widely used and most frequently prescribed NSAID, it has many side effects like gastritis, dyspepsia, heart burn, peptic ulcer/gastrointestinal bleeding, which are dependent on the supplied dose, ibuprofen also inhibits the production of prostaglandins, which relax the bronchial smooth muscle; however, it is difficult to formulate the overcome the limitations associated with oral dose as injectable liquid due to its extremely poor aqueous solubility, Ibuprofen is a broadly NSAIDs used cyclo-oxygenase inhibitor in clinical practice. It has been established by others to have an inhibitory effect on fracture repair in animals. Studies of fracture healing in animals treated with NSAIDs showed that it impairs the bone healing process, the limited clinical data also support the assumption that inhibition of COX-2 by non-selective or COX-2-selective NSAIDs delays fracture healing.
Non-steroidal anti-inflammatory drugs (NSAIDs) are a class of drugs universally used to treat both the acute and chronic injuries. In many parts of the world, NSAIDs can be procured over-the-counter and used without any physician oversight. However, the chronic nature of overuse injuries requires NSAIDs to be taken orally for an extended period of time. Non-steroidal anti-inflammatory drugs (NSAIDs) are among the most commonly suggested drugs in the world. NSAIDs share a common mechanism of action through the inhibition of cyclooxygenase 1 (COX1) and cyclooxygenase 2 (COX2), while available under many brand names and formulations [1]. Several animal models suggest that NSAIDs harmfully affect bone healing and report that the duration, timing, and dosing of NSAIDs are important predictors of fracture healing complications. Conversely, a study investigating a juvenile animal model reported no inhibitory effects on fracture healing from NSAIDs. In addition, studies on adult patients have produced inconsistent results, showing both harmful effects and no effect on fracture healing with both the long-term and short-term use of NSAIDs [2]. Many animal studies show that NSAIDs have an obvious tendency to delay bone healing, although the healing delay was not noticeable when NSAIDs were used for a short period of 7 days [3]. NSAIDs are also associated with risks to the digestive system, as well as cardiovascular and renal complications [4]. Non-steroidal anti-inflammatory drugs (NSAIDs) also can impair or inhibit bone healing process because their influence is critical on the stages of healing including coagulation, inflammation and angiogenesis and finally on the clinical outcome [5].
Ibuprofen
Ibuprofen is the most widely used and most frequently prescribed NSAID, its chemical structure is (2RS)-1[4-(2-methyl propyl) phenyl] propionic acid (fig.1), it was the first member of propionic acid derivatives to be introduced in 1969 as a better alternative to Aspirin. Gastric discomfort, vomiting and nausea, though less than aspirin. It is a non-selective inhibitor of cyclooxygenase-1 (COX-1) and Cyclooxygenase-2 (COX-2) [6]. Its anti- inflammatory properties may be weaker than those of some other NSAIDs, it has a prominent antipyretic and analgesic role. It has inhibitory mechanism on cyclo-oxygenases, which are involved in the synthesis of prostaglandins that have an important role in the production of pain, fever and inflammation [7].
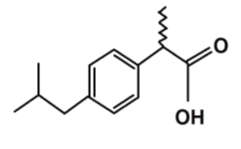
Figure 1: Structural formula of ibuprofen [6].
The common dose of ibuprofen as is 400 to 800 mg three times a day [8]. It is insoluble in water with pKa of 5.3 [9]. Recently this drug is chiefly administered by oral route in the as tablets, capsules, suspensions, and oral solutions. The most common used oral dose is 200-600 mg 6 hourly, it can be increased up to 2.4 g to 3.2 g daily depending on the situation. The required dosage for therapeutic effect in an adult is approximately 20 mg ~ 30 mg, while the supplied dose is more than ten times this amount because of its poor absorption and its first pass metabolism [10, 11]. The main side effects of ibuprofen are gastritis, dyspepsia, heart burn, peptic ulcer/gastrointestinal bleeding, which are dependent on the supplied dose, ibuprofen also inhibits the production of prostaglandins, which relax the bronchial smooth muscle; however, it is difficult to formulate the overcome the limitations associated with oral dose as injectable liquid due to its extremely poor aqueous solubility [12].
Medicinal applications of ibuprofen drug
It is widely used as an analgesic, an anti- inflammatory and an antipyretic drug, a low dose ibuprofen is as effective as paracetamol and aspirin for the indications normally treated [13-15]. The main therapeutic applications of ibuprofen are: Rheumatoid and osteo-arthritis (RA and OA), Patent Ductus arterosus (PDA), Cystic fibrosis (CF), Orthostatic hypotension, Dental pain, Dysmenorrhea, Prophylaxis of Alzheimers disease, Parkinson’s disease (PD), Breast cancer, fever and headache [6]. Racemic ibuprofen and S(+) enantiomer are mostly used in the cure of mild to moderate pain such as headache, migraine, postoperative dental pain, osteoarthritis, rheumatoid arthritis and soft tissue disorder [16].
Ibuprofen's reverse actions
Ibuprofen was a probable cause of GI bleeding [17,18], increasing the risk of gastric ulcers and renal failure [19-22], apoptosis [23], heart failure [24]. Other less commonly adverse effects of ibuprofen includes thrombocytopenia, rashes, headache, dizziness, blurred vision and in rare cases toxic amblyopia, fluid retention and edema [25]. As well as all NSAIDs, ibuprofen has effects on kidney include acute renal failure, interstitial nephritis, and nephritic syndrome, but these very rarely occur [26].
Effect ibuprofen on bone and cartilage
A number of studies have confirmed that nonspecific NSAIDS may inhibits the growth of new bones and interrupt fracture healing [27, 28]. Recently, Goodman and colleagues conducted a study on rabbits comparing a non-specific COX-1 and COX-2 inhibitor, and a specific COX-2 inhibitor to determine differences in the growth of bones and tissue differentiation [29]. The researchers found that when administered naproxen (non-specific COX-1 and COX-2 inhibitor), tissue bone ingrowth decreased by 15.9% versus 18.5% with the rofecoxib (selective COX-2 inhibitor). Although these results were not statistically significant, they concluded that bone ingrowth or fracture curative may be delayed when agents with COX-2 inhibitory properties are used. Because the bone healing of rabbit is very similar to humans, these results can be applicable. The researchers concluded that patients should not take COX-2 inhibitors for at least 6 weeks after a fracture or implant procedure, although the duration of effect is still now under research [30]. Ibuprofen is a broadly NSAIDs used cyclo-oxygenase inhibitor in clinical practice. It has been established by others to have an inhibitory effect on fracture repair in animals [31]. Studies of fracture healing in animals treated with NSAIDs or in mice lacking COX-2 gene demonstrated that inhibition or deficiency of COX-2 impairs the bone healing process, the limited clinical data also support the assumption that inhibition of COX-2 by non-selective or COX-2-selective NSAIDs delays fracture healing [32]. Cartilage damage also can lead to persistent symptoms, including pain, swelling, loss of function, and may eventually progress to symptomatic degeneration of the joint if it is left untreated [33]. O'Connor et a.l, found that ibuprofen treatment delayed healing of the rabbit fibula osteotomies which was evident from the substantial amount of cartilage remaining in the osteotomy callus and the diminished peak torque after 6 weeks of healing (fig. 2& 3). These data support that short-acting NSAID therapy is less deleterious to fracture healing than long-acting NSAID therapy. However, an analysis of plasma ibuprofen levels confirmed that a single oral dose of 50 mg ibuprofen provided significant COX-2 inhibition for approximately 4 h (between 1 and 5 h after dosing; data not shown). Thus, they suspect that the ibuprofen dosing regimen used allowed consistent inhibition of COX-2 for no more than 14 h each day. This would leave a substantial period of at least 10 h when COX-2 function was not impaired [34].
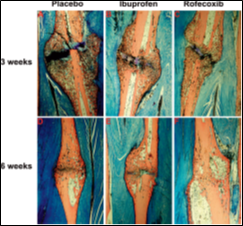
Figure 2: Representative histological sections through the fibula osteotomy callus. (Panels A–C) show sections from rabbit fibulas after 3 weeks of healing that were treated with placebo, ibuprofen, or rofecoxib as indicated. Similarly, (panels D–F) show sections from rabbit fibulas after 6 weeks of healing. Note the shell-like morphology of the osteotomy callus in the rofecoxib-treated rabbit after 6 weeks of healing [34].
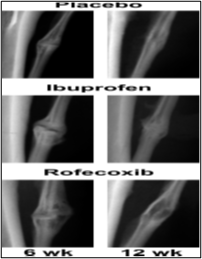
Figure 3: Representative images of the fibula osteotomy calluses: lateral radiographs made from the resected lower hind limbs of each rabbit at (6 and 12 weeks) after osteotomy. In each radiograph, the tibia cortex can be seen on the left margin and each is oriented with the proximal end up. Note the persistence of the osteotomy gap after 6 weeks in the ibuprofen-treated rabbit, suggesting a delay in healing. (After 12) weeks, the osteotomies in the placebo- and ibu¬profen-treated rabbits appear to have healed well. In contrast, significant radiolucent areas are evident in the (6- and 12-week) calluses of the rofe¬coxib-treated rabbits [34].
Abdul-Jabbar and Mohammed (2021) found that regarding the effect of the is decreased in the weights of the bones of frontal and posterior limb, as the relative rate of weight decreased significantly during the two periods of potion. This can be explained by the decrease in the weights of laboratory animals treated with the drug ,which has led to a decrease in the weights of the bones of the limbs and may be due to the explanation of the above to the role of bone metabolism based on pathological and physiological conditions (fig. 5& 6) [35]. Co x-2 inhibitors are likely to play an influential role in bone thickness thus inhibiting bone formation. The low weight of limb bones may be caused by poor vascular formation as inhibition of Cox-2 ring oxidant enzymes is known to reduce prostaglandin throbbing ,which has a role in the formation of blood vessels necessary for ossification within [36] . Inhibition of new bone formation may be due to the effect of the drug on bone-forming cells and may be likely to be affected by cartilage differentiation ,which is important in bone formation led to a decrease in the weights of the bones of the limbs [37].


 Eftikhaar hasan kadhum*
Eftikhaar hasan kadhum*



 10.5281/zenodo.10956922
10.5281/zenodo.10956922