Abstract
Over the past three decades, formulation technology has significantly advanced, particularly in drug delivery systems. Innovations include novel dosage forms and new uses for existing drugs, offering benefits like improved patient compliance, sustained drug concentration, reduced dosing frequency, targeted delivery, and minimized side effects. Transdermal drug delivery systems (TDDS) are key developments, allowing controlled, continuous medication administration through the skin, bypassing gastrointestinal degradation and hepatic first-pass metabolism, and enhancing bioavailability and patient compliance. The FDA approves roughly one transdermal product every 2.2 years, with the first patch approved four decades ago. This research examines the skin's role as a barrier, clinical trials, patents, commercialization, and the benefits and limitations of TDDS. Various TDDS methods are reviewed, highlighting their advantages, disadvantages, and potential applications. Recent advancements demonstrate TDDS's effectiveness and potential across diverse sectors, emphasizing their transformative impact on drug delivery and therapeutic practices.
Keywords
Transdermal drug delivery systems, Anatomy of skin, Improvement of transdermal administration, Drug delivery systems
Introduction
Drug delivery systems (DDS) encompass a range of physicochemical technologies designed to regulate the delivery and release of pharmacologically active substances within cells, tissues, and organs, thereby optimizing their therapeutic effects. Put simply, DDS includes various administration routes and drug formulations that efficiently deliver medication to maximize therapeutic benefits while minimizing any potential side effects. Depending on the method of delivery, there exist multiple administration modalities, such as oral administration, transdermal administration, lung inhalation, mucosal administration, and intravenous injection. Of these, the transdermal drug delivery system (TDDS) stands out as a particularly appealing approach.[1] TDDS has emerged as a prominent avenue for noninvasive drug delivery through the skin, distinct from the traditional reliance on needle-based injections. Its impact has been profound in facilitating the delivery of various therapeutic agents, particularly in the realms of pain management, hormonal therapy, and the treatment of cardiovascular and central nervous system disorders. Notably, TDDS bypasses the gastrointestinal tract, eliminating first-pass metabolism-related losses, and mitigating concerns regarding pH, enzymes, and intestinal bacteria. Moreover, TDDS enables the regulation of drug release based on usage requirements, contributing to its sustained effectiveness. Crucially, its noninvasive nature translates to minimal discomfort and reduced burden for patients, allowing safe and convenient drug administration, even among pediatric and geriatric populations.[2] Despite its potential, the effectiveness of TDDS remains hindered by the intrinsic skin barrier. Serving as the body's outermost protective layer, the skin boasts a complex, multilayered structure, shielding the body from various external threats, including chemicals, heat, and toxins. This skin structure comprises the epidermis, primarily responsible for protection, and the dermis, which houses blood vessels and facilitates the production of skin cells. Both layers contain components that pose obstacles to transdermal delivery.[3] Transdermal therapeutic systems are discrete, self-contained dosage forms that, upon application to unbroken skin, steadily deliver prescribed medication to the bloodstream. The inaugural Transdermal drug delivery (TDD) system, Transderm-Scop, was created in 1980, primarily featuring Scopolamine for motion sickness management. Operating as a membrane-controlled system, the Transdermal device employs a microporous polypropylene film as the membrane. The drug reservoir contains the drug dissolved in a blend of mineral oil and polyisobutylene, facilitating consistent drug release over a 72-hour period.[4] Achieving the best therapeutic results relies not only on selecting the right medication but also on ensuring efficient drug administration. In recent decades, the advancement of controlled drug delivery has gained significant importance within the pharmaceutical industry. The human skin serves as a convenient surface for drug delivery, covering an average adult body surface area of approximately 2 m?2; and receiving around one-third of the body's circulating blood.On average, the human skin surface comprises 10-70 hair follicles and 200-250 sweat ducts per square centimeter, making it one of the most accessible organs in the human body. The skin holds substantial promise as a site for drug application, both for local and systemic effects. However, the stratum corneum, particularly, poses a significant barrier to drug penetration, thereby limiting topical and transdermal bioavailability.[5]
ADVANTAGES
- Frequency of dosing can be minimized.
- Enhanced bioavailability can lead to decreased drug concentration.
- Bypassing first-pass metabolism in the liver is achievable.
- Gastrointestinal issues related to drug absorption, influenced by stomach pH, enzymatic activity, and potential drug interactions with food or other orally administered pharmaceuticals, can be averted.
- Lower plasma concentration levels of drugs result in reduced side effects.
- Non-invasive administration prevents the complexities associated with parenteral therapy.
- Enhanced compliance compared to earlier dosage forms is achieved as longer therapy is made possible with a single application.
- Swift termination of drug therapy is feasible by removing the application from the skin surface.
- These systems facilitate self-administration.
- They mitigate systemic drug interactions.
- Prolonged duration of action is offered.
DISADVANTAGES
- Transdermal delivery is only suitable for potent drugs.
- Application site irritation may occur in certain patients.
- The system can be financially impractical.
- Potential binding of the drug to the skin might lead to dose dumping.
- It is viable primarily for chronic conditions, not for acute ones, as chronic conditions often necessitate prolonged drug therapy, for instance, in cases of hypertension, angina, and diabetes.
- Cutaneous metabolism can influence the therapeutic efficacy of the medication.
- Ionic drugs are not conducive to transdermal therapy.
- Transdermal therapy is appropriate for drugs with a lower molecular weight, typically less than 500 Daltons.
ANATOMY AND PHYSIOLOGY OF SKIN
As the body's largest organ, the skin spans approximately 20 square feet. It serves as a protective barrier against microbes and environmental factors, aids in maintaining body temperature, and enables the perception of tactile sensations such as touch, heat, and cold.[6]’

The human skin comprises three distinct yet interdependent tissues:
- The stratified, vascular, cellular epidermis
- The underlying dermis of connective tissues
- The hypodermis.
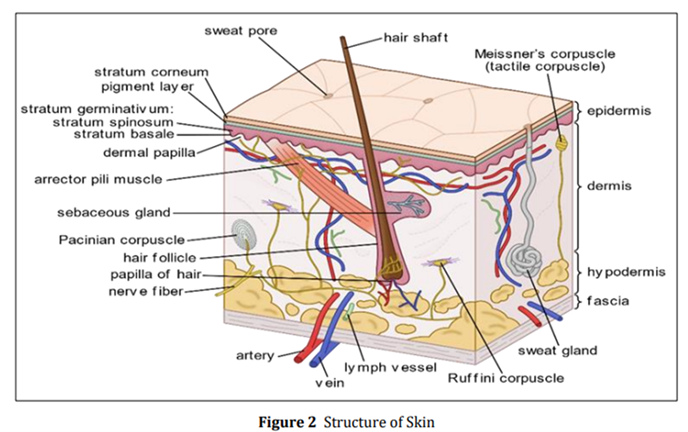
Variances in cell size and the number of cell layers in the epidermis lead to differences in thickness, ranging from 0.06 mm on the eyelids to 0.8 mm on the palms and soles. This outer layer comprises the stratum corneum and the viable epidermis. [6]
1.a: STRATUM CORNEUM
The outermost layer of the skin, known as the stratum corneum or the horny layer, measures approximately 10 µm in thickness when dry, but can expand to multiple times this thickness when adequately hydrated. Comprising 10 to 30 layers of lifeless, keratinized cells known as corneocytes. Drug compounds can traverse the stratum corneum by utilizing one of three pathways. Depending on the physicochemical attributes of the medication, the skin can absorb compounds with both hydrophilic and lipophilic traits through various routes:
- Transcellular pathway
- Intercellular pathway
- Transfollicular pathway
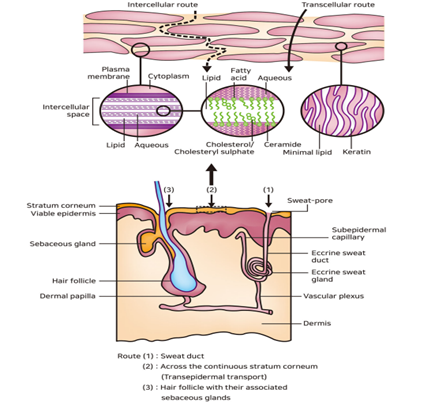
Figure:3
Found beneath the outermost layer, displays varying thickness, ranging from 0.06 mm on the eyelids to 0.8 mm on the palms. Progressing inward, it is composed of several layers, including the stratum granulosum, stratum lucidum, stratum spinosum, and the stratum basal. Within the basal layer, cell divisions through mitosis continually regenerate the epidermis, effectively offsetting the loss of dead horny cells from the skin's surface. [6]
2.THE DERMIS
The dermis with a thickness ranging from 3 to 5 mm, consists of a network of connective tissue containing blood vessels, lymph vessels, and nerves. The cutaneous blood supply plays a significant role in regulating body temperature. Apart from eliminating pollutants and waste, it also supplies the skin with essential nutrients and oxygen. Most molecules that breach the skin barrier diffuse into capillaries located 0.2 mm below the skin's surface. Consequently, the dermal concentration of a permeant remains notably low, emphasizing the pivotal role of the concentration gradient across the epidermis in facilitating transdermal penetration. [6]
3.THE HYPODERMIS
Supporting the dermis and epidermis is the hypodermis, also referred to as subcutaneous fat tissue, which serves as a storage site for fat. Apart from providing mechanical protection and assisting in temperature regulation, this layer offers essential nutritional support. It serves as a connection between the body's major blood vessels and nerves to the skin and may include organs responsible for sensing pressure. [6]
IMPROVEMENT OF TRANSDERMAL ADMINISTRATION
The primary challenge faced by transdermal drug delivery systems (TDDS) revolves around overcoming the barrier properties of the stratum corneum, ensuring effective drug penetration into the skin tissue, and successful traversal through cellular and vascular structures to reach the intended target area. The major issue lies in the limited capacity of the skin tissue to absorb the drug.To address this issue, a multitude of innovative TDDS techniques have been extensively researched and developed, emerging as promising administration approaches. Moreover, these advancements could offer a competitive edge over alternative drug delivery methods by enhancing the delivered dose and optimizing cost-effectiveness. [6] Concerning chemical factors, the extent of ionization of a drug substantially influences its absorption through the skin. Specifically, non-ionized compounds may exhibit superior drug permeation in comparison to ionizable drugs, owing to their hydrophobic resemblance to the stratum corneum (SC). Additionally, the melting point of a drug can impact its permeation into the skin. A drug with a lower melting point could display heightened solubility in the SC, thereby potentially leading to increased drug penetration into the skin. [6] Considering these factors, researchers have devised various approaches to improve drug absorption through the skin. Figure.4 provides an overview of the strategies used for enhancing transdermal drug delivery systems. This paper organizes the enhancement strategies by aligning the previously reported method types by Barry and Morrow et al. with their respective generations, as classified by Prausnitz and Langer.

Figure:4
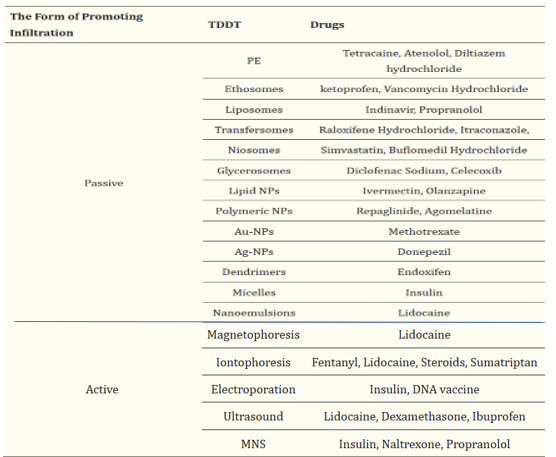
The Initial generation of transdermal drug delivery doesn't involve a patch system; instead, the drug is formulated into traditional liquid sprays, gels, creams, or other topical formulations. These formulations are applied to the skin without the use of sophisticated systems or platforms . This approach relies on passive diffusion for skin absorption, necessitating that the incorporated drug be of low molecular mass (<?600 Da), exhibit sufficient hydrophobicity, and be effective in low-dose administration.However, the efficacy of drug delivery using this method is quite limited, prompting the subsequent development of more advanced enhancement methods. Barry and Morrow et al. have classified the approaches for enhancing transdermal drug delivery into five methods, as depicted in the purple-shaded semi-circle in Figure 4. To exert its effects, the drug must traverse the epidermis to reach the dermis. Research has identified three potential pathways for this: the transcellular pathway, intercellular pathway, and appendage pathway (such as hair follicles, sebaceous glands, and sweat glands). The primary focus of transdermal drug delivery technology (TDDT) research is to enhance drug permeability in the skin. Presently, numerous studies on TDDT utilize diverse approaches to augment skin permeability for effective drug transportation into the dermis. These methods are broadly classified into passive enhancement and active enhancement. We provide a detailed summary of the primary components of these two methods. Table 1 outlines several drugs delivered using various transdermal delivery techniques. [6]
EXAMPLES OF DRUGS ADMINISTERED BY DIFFERENT TRANSDERMAL DELIVERY TECHNIQUES:
Table 1
APPLICATIONS
Transdermal Drug Delivery Systems (TDDS) encompass a wide array of therapeutic areas and have seen significant success in various medical applications:
- Nicotine patches for smoking cessation, widely used in the United States and Europe.
- Fentanyl CII (Duragesic) and buprenorphine CIII (BuTrans) patches for managing severe pain.
- Hormonal patches including estrogen patches for menopausal symptoms and hormone replacement therapy, contraceptive patches (Ortho Evra or Evra), and testosterone patches for both men (Androderm) and women (Intrinsa).
- Nitroglycerin patches for the treatment of angina.
- Transdermal scopolamine for motion sickness.
- Clonidine patches for hypertension.
- Emsam, a transdermal form of the MAOI selegiline, for antidepressant therapy.
- Daytrana, the first methylphenidate transdermal delivery system for ADHD treatment.
- Secuado, a transdermal form of the atypical antipsychotic asenapine.
- Vitamin B12 and 5-Hydroxytryptophan (5-HTP) patches for transdermal administration.
- Rivastigmine patches for Alzheimer's treatment.
- Quantum dot dye technology developed by Robert S. Langer and his team for the subcutaneous storage of medical information, especially beneficial in developing nations.
- Caffeine patches designed for transdermal delivery of caffeine.
These examples underscore the diverse and expanding applications of TDDS in the realm of modern medicine, catering to a range of therapeutic needs and patient requirements.[7]
ADVERSE EVENTS
Certain transdermal drug delivery systems have been reported over the years, leading to safety concerns and subsequent regulatory actions:
- In 2005, the FDA launched an investigation into reports of fatalities and other serious adverse events associated with narcotic overdose in patients using Duragesic, the fentanyl transdermal patch for pain management. As a result, the Duragesic product label was updated in June 2005 to include additional safety information.
- In 2007, manufacturers Shire and Noven Pharmaceuticals voluntarily recalled specific batches of the Daytrana ADHD patch due to issues related to the separation of the patch from its protective release liner. No further complications with the patch or its protective packaging were reported subsequently.
- In 2008, two manufacturers of the fentanyl patch, ALZA Pharmaceuticals (a division of Johnson & Johnson) and Sandoz, recalled their versions of the patch due to a manufacturing defect leading to the rapid leakage of the gel containing the medication. This defect raised the risk of potential overdose and subsequent fatalities. Sandoz, now manufactured by ALZA, ceased using gel in its transdermal fentanyl patch, employing a matrix/adhesive suspension instead, similar to other fentanyl patch manufacturers such as Mylan and Janssen.
- In 2009, the FDA issued a public health advisory highlighting the risk of burns during MRI scans associated with transdermal drug patches containing metallic backings. Patients were advised to remove any medicated patch before undergoing an MRI scan and replace it with a new patch after the scan.
- In 2009, an article in the Europace journal documented cases of skin burns resulting from transdermal patches containing metal, commonly used as a backing material. These burns were attributed to shock therapy from external as well as internal cardioverter defibrillators (ICD).[9]
CONCLUTION AND FUTURE ASPECTS
The advancement of transdermal drug delivery systems (TDDS) technology has been widely acknowledged as a prominent means of drug administration, particularly for its ability to bypass first-pass metabolism and circumvent sensitivities associated with alternative delivery routes. TDDS facilitates reliable and safe systemic drug delivery through the skin, ensuring the stability of drugs and safeguarding them from biochemical alterations until they reach the intended target tissue. Notably, TDDS is characterized by its noninvasive, nonallergenic nature, predetermined duration, and controlled dose delivery mechanism, ensuring uniform drug distribution at prescribed rates.This technology has rapidly gained ground in the pharmaceutical domain, proving instrumental in enhancing the bioavailability of poorly absorbable drugs through convenient administration routes, thereby enabling the extended release of substantial doses over extended periods. However, despite extensive research over the years, the success of passive methods such as chemical enhancers in augmenting transdermal transport of small molecules remains limited, especially in the context of enhancing the transport of macromolecules under clinically acceptable conditions. In recent times, the transdermal drug delivery system (TDDS) has witnessed a substantial expansion within both domestic and international drug delivery markets. This growth is evidenced by a surge in research studies, patents, and the availability of commercial products from various companies and research institutions. Particularly, the emergence of microneedles has garnered significant attention within the realm of TDDS, addressing the limitations of conventional simple application and patch-type needles and amalgamating the advantages of microneedles to achieve enhanced treatment efficiency and efficacy. To this end, manufacturing and commercialization methods are actively being developed, incorporating cutting-edge technologies such as 3D bioprinting. Progress in these TDDSs holds the potential to serve as a catalyst in curbing the prevalence of various diseases, including those related to the cardiovascular and central nervous systems, diabetes, neuromuscular conditions, genetic disorders, and infectious diseases, both general and localized. Additionally, these advancements are poised to revolutionize vaccination practices and cater to patient preferences for self-administered long-term treatment solutions. Looking ahead, the continued evolution of these methodologies, along with ongoing technological innovations, is expected to open new avenues for enhanced drug delivery. The integration of these techniques with cutting-edge nanotechnology and biopharmaceutical research holds the potential to revolutionize the field, facilitating the development of personalized and patient-centric transdermal therapies. Additionally, the exploration of novel biocompatible materials and the implementation of innovative delivery strategies are poised to further optimize transdermal drug delivery systems, ensuring their continued significance in the realm of modern medicine.
REFERENCES
- Jeong, W.Y., Kwon, M., Choi, H.E. et al. Recent advances in transdermal drug delivery systems: a review. Biomater Res 25, 24 (2021). https://doi.org/10.1186/s40824-021-00226-6
- Alkilani, A. Z., Nasereddin, J., Hamed, R., Nimrawi, S., Hussein, G., Abo-Zour, H., & Donnelly, R. F. (2022). Beneath the Skin: A Review of Current Trends and Future Prospects of Transdermal Drug Delivery Systems. Pharmaceutics, 14(6), 1152. https://doi.org/10.3390/pharmaceutics14061152. PMCID: PMC9231212. PMID: 35745725.
- Leong, M. Y., Kong, Y. L., Burgess, K., Wong, W. F., Sethi, G., & Looi, C. Y. (2023). Recent Development of Nanomaterials for Transdermal Drug Delivery. Biomedicines, 11(4), 1124. https://doi.org/10.3390/biomedicines11041124. PMCID: PMC10136181. PMID: 37189742.
- Halder, J., Gupta, S., Kumari, R., et al. (2021). Microneedle Array: Applications, Recent Advances, and Clinical Pertinence in Transdermal Drug Delivery. Journal of Pharmaceutical Innovation, 16, 558–565. https://doi.org/10.1007/s12247-020-09460-2.
- Sabbagh, F., & Kim, B. S. (2022). Recent advances in polymeric transdermal drug delivery systems. Journal of Controlled Release, 341, 132-146. https://doi.org/10.1016/j.jconrel.2021.11.025.
- Ramadon, D., McCrudden, M. T. C., Courtenay, A. J., & Donnelly, R. F. (2022). Enhancement strategies for transdermal drug delivery systems: current trends and applications. Drug Delivery and Translational Research, 12(4), 758-791. https://doi.org/10.1007/s13346-021-00909-6. PMCID: PMC7817074. PMID: 33474709.
- Chen, X., Xiao, H., Shi, X., Zhao, Q., Xu, X., & Fan, P. (2023). Bibliometric analysis and visualization of transdermal drug delivery research in the last decade: global research trends and hotspots. Frontiers in Pharmacology, 14, 1173251. https://doi.org/10.3389/fphar.2023.1173251. PMCID: PMC10313210. PMID: 37397493
- Wong, W. F., Ang, K. P., Sethi, G., & Looi, C. Y. (2023). Recent Advancement of Medical Patch for Transdermal Drug Delivery. Medicina (Kaunas), 59(4), 778. https://doi.org/10.3390/medicina59040778. PMCID: PMC10142343. PMID: 37109736.
- He, J., Zhang, Y., Yu, X., & Xu, C. (2023). Wearable patches for transdermal drug delivery. Acta Pharmaceutica Sinica B. Retrieved from www.sciencedirect.com.
- Raviraj, V., Pham, B.T.T., Kim, B.J. et al. Non-invasive transdermal delivery of chemotherapeutic molecules in vivo using superparamagnetic iron oxide nanoparticles. Cancer Nano 12, 6 (2021). https://doi.org/10.1186/s12645-021-00079-7
- Li, Z., Fang, X., & Yu, D. (2021). Transdermal Drug Delivery Systems and Their Use in Obesity Treatment. International Journal of Molecular Sciences, 22(23), 12754. https://doi.org/10.3390/ijms222312754. PMCID: PMC8657870. PMID: 34884558.
- Hmingthansanga, V., Singh, N., Banerjee, S., Manickam, S., Velayutham, R., & Natesan, S. (2022). Improved Topical Drug Delivery: Role of Permeation Enhancers and Advanced Approaches. Pharmaceutics, 14(12), 2818. https://doi.org/10.3390/pharmaceutics14122818. PMCID: PMC9785322. PMID: 36559311.
- Liu, L., Zhao, W., Ma, Q., Gao, Y., Wang, W., Zhang, X., Dong, Y., Zhang, T., Liang, Y., Han, S., Cao, J., Wang, X., Sun, W., Ma, H., & Sun, Y. (2023). Functional nano-systems for transdermal drug delivery and skin therapy. Nanoscale Advances, 5(6), 1527-1558. https://doi.org/10.1039/d2na00530a. PMCID: PMC10012846. PMID: 36926556.
- Al-Japairai, K. A. S., Mahmood, S., Almurisi, S. H., Venugopal, J. R., Hilles, A. R., Azmana, M., & Ramana, S. (2020). Current trends in polymer microneedle for transdermal drug delivery. International Journal of Pharmaceutics, 587, 119673. https://doi.org/10.1016/j.ijpharm.2020.119673. PMCID: PMC7392082. PMID: 32739388.
- Qu, F., Geng, R., Liu, Y., & Zhu, J. (2022). Advanced nanocarrier- and microneedle-based transdermal drug delivery strategies for skin diseases treatment. Theranostics, 12(7), 3372-3406. https://doi.org/10.7150/thno.69999. PMCID: PMC9065205. PMID: 35547773.


 Jasnath P.*
Jasnath P.*
 Anagha S. Raj 4
Anagha S. Raj 4





 10.5281/zenodo.12740601
10.5281/zenodo.12740601