Abstract
The administration of pharmacological substances to produce therapeutic effects in people or animals is known as drug delivery. Treatment of disorders thro0ugh the nose and lungs is becoming more and more significant, especially when using peptides and proteins. For these approaches, a number of drug delivery systems, including as liposomes, pro-liposomes, microspheres, gels, prodrugs, cyclodextrins, and nanoparticles comprised of biodegradable polymers, are being investigated. Because they can target specific lung sites or cell populations, release drugs in a controlled manner, form aerosols, withstand aerosolization forces, ensure biocompatibility, and degrade within appropriate time frames, biodegradable polymer-based nanoparticles hold great promise. These characteristics make them appropriate for overcoming obstacles related to pulmonary and nasal medication administration. Drug delivery to particular organs and tissues has emerged as a key area of study in modern pharmacological research. The ineffectiveness of traditional dose forms un getting medications to the right places in the body calls for creative methods and techniques in drug administration. Newer methods include lipid-, protein-, and polymeric-based systems that are intended to provide long-term drug release, improve drug distribution in the body, shield pharmaceuticals from deterioration under abrasive conditions, and reduce premature clearance. Numerous of these cutting-edge drug delivery systems have already entered the market because of their many benefits. An overview of these contemporary carriers and their most recent advancements in medication administration is given in this paper, emphasizing how they have the potential to completely transform patient outcomes and therapeutic efficacy.
Keywords
Drug Delivery, Pro-Liposomes, Microspheres, Gels, Prodrugs, Cyclodextrins, Nanoparticles, Pulmonary Drug Delivery , Sustained Drug Release
Introduction
It takes a lot of money and effort to develop new medication compounds. Personalized drug therapy, dose modification, and therapeutic drug monitoring are just a few of the tactics used to improve the safety and effectiveness of currently available medications. In addition, there is a lot of emphasis on attaining focused and gradual drug delivery, as well as regulated drug delivery rates. These are very attractive strategies that are still being actively explored. Curiously, alongside efforts from the USA and Europe, a substantial quantity of study and publications have been made to this topic by Indian researchers. Our comprehension of pharmacokinetic and pharmacodynamic principles has been improved by studies employing strong opioid analgesics, inhalation aesthetics, sedative/hypnotics, and muscle relaxants. Alternative delivery routes for analgesics and aesthetics, such as the skin, buccal, and nasal mucous membranes, are being investigated. [1] Controlled-release technology (CRT) includes a wide range of cutting-edge apparatuses and systems. Transdermal and transmucosal delivery systems, drug-infused lozenges, encapsulated cells, nasal and buccal aerosol sprays, oral soft gels, iontophoretic devices for transdermal drug administration, and implantable, programmable drug-delivery devices are a few examples. The goal of these developments is to get around the drawbacks of traditional drug administration techniques. [2] These cutting-edge tools and methods are attracting interest for a number of reasons. Since the late 1950s, there have been fewer new chemical entities introduced due to factors such as shorter effective patent lives, decreased research efforts by pharmaceutical businesses, and rising expenses associated with medication development. [3] Since the late 1950s, there have been fewer new chemical entities introduced due to factors such as shorter effective patent lives, decreased research efforts by pharmaceutical businesses, and rising expenses associated with medication development. A new drug's journey from invention to clinical trials and regulatory approval today costs over $120 million and takes about ten years. Novel medication delivery technologies were predicted to account for up to 40% of the US pharma product industry by the year 2000. [4] Advanced technologies called drug delivery systems (DDS) are made to package and store drug molecules in forms that are appropriate for effective administration, including tablets or liquids. Their main objective is to maximize therapeutic effectiveness while avoiding off-target effects by delivering drugs to specified target areas within the body as quickly as possible. Various methods, such as oral buccal and sublingual, nasal and ocular, transdermal and subcutaneous, anal and transvaginal, and intravesical, can be used to administer drugs to the body. The way medications behave in the body and the physiological changes they cause when taken are largely determined by their physicochemical characteristics. [5] By improving systemic circulation and managing the pharmacological effects of drugs, DDS have made a substantial contribution to the treatment of disease and improvement of health in recent decades. Since the 1950s, controlled-release formulations have been developed as a result of developments in pharmacology and pharmacokinetics, which highlight the crucial role that controlled drug release plays in maximizing therapeutic effects. By releasing medications at predetermined rates and durations regardless of physiological conditions, these formulations provide substantial advantages over conventional drugs and allow for spatial control over drug release with constant or changing rates. Furthermore, target site accumulation, efficacy, pharmacological activity, pharmacokinetic characteristics, patient acceptance, compliance, and decreased medication toxicity are all improved by controlled drug delivery systems. [6] Novel drug delivery systems (NDDS), which provide improved convenience, control, and targeting capabilities, have been developed recently. Every NDDS functions according to its distinct features, which include its morphological, chemical, and physical attributes and affect its affinity for various pharmacological molecules. The main mechanisms that control the release of drugs are diffusion, solvent and chemical reactions, and stimuli-responsive controls. For instance, enhanced permeability and retention, or EPR, takes use of the lymphatic and blood arteries in cancer cells' porous nature to help medications diffuse passively into target areas. [7] Despite worries over its selectivity and possible toxicity, electrophoretic radiation therapy (EPR) has been extensively researched and used in the delivery of chemotherapy drugs. By affixing certain ligands or molecules to carriers that bind to target tissue surfaces actively, active targeting strategies overcome the drawbacks of passive targeting. This minimizes uptake by non-target cells and lowers toxicity and side effects. The complete development of actively targeted medications is still hampered by issues like ligand selectivity, immunogenicity, and possible destruction during macrophage endocytosis. [8] Moreover, responsive stimuli targeting helps deliver drugs to particular cells or tissues by utilizing chemical or physical characteristics like pH, temperature, ultrasonic, magnetic, or electric fields. In today's pharmaceutical research, targeted drug delivery to particular organs and tissues has emerged as a critical area of study. A comprehensive scientific strategy is necessary to make considerable progress in boosting site-specific bioavailability and enhancing therapeutic efficacy in the frontier field of innovative drug delivery techniques and mechanisms. It is now possible to overcome obstacles like the permeability of the blood-brain barrier, making it possible to use treatments that were previously unfeasible when using traditional dose forms. By regulating the drug release profile, these cutting-edge drug delivery methods mitigate a number of issues, such as low solubility, sensitivity to environmental variables including photodegradation and pH fluctuations, and the possibility of dose dumping. [9] Moreover, they allow for accurate targeting at the site of action, minimizing exposure to tissues that are not the target, improving therapy efficacy and lowering the risk of toxicity and adverse consequences. This better targeting encourages patient compliance and convenience while also improving therapeutic outcomes. Pharmaceutical applications require biocompatibility, and creating a dosage form that complements a drug's physicochemical characteristics is difficult. The flexible and biodegradable polymers poly(D,L-lactide-co-glycoside) (PLGA) are used in modern methods to get across pathological and physiological barriers that are present in targeting tactics. [10] By employing ligands to deliver greater dosages of medications to particular organs, this approach seeks to improve the pharmacokinetic characteristics of medications while maintaining controlled release and breakdown into non-toxic by products. Since oral administration is still the most practical method of medication delivery, efforts have been made recently to design carriers that can get past biological barriers like the gastrointestinal (GI) tract. These carriers need to shield medications from the hostile gastrointestinal environment, extend the duration of drug residence via bio adhesion, and target certain cells to enhance absorption and maybe lower the frequency of dosage. Many drug delivery technologies are being researched to improve treatment efficacy and overcome issues frequently related to traditional dosage forms. Furthermore, the study of the cellular milieu and its interaction with these new dosage forms has been the focus of recent breakthroughs. [11] These innovations are similar to the old idea of the "magic bullet," first put forth by Paul Ehrlich, which sought to deliver medications exactly to their target areas while reducing systemic adverse effects . [12]
Types of Drug Delivery System [13]

Delivery System [13]
- Red blood cell membrane camouflaged nanoparticles
Scholars have discerned the prospective benefits of nanotechnology in markedly enhancing drug delivery techniques in the long run. Nanoparticles disguised like red blood cell membranes offer a new class of drug delivery vehicles. Red blood cells (RBCs) are perfect candidates for drug delivery vehicles due to their biological characteristics, including their abundance in circulation, biocompatibility (non-immunogenicity), biodegradability, and extended half-life. Engineered red blood cells have demonstrated potential as delivery systems for a range of bioactive materials, such as proteins, big molecules, medicines, and enzymes. [14] Red blood cell membranes are abundant and have favourable biological properties that make them a suitable "camouflage" for nanoparticles, combining the benefits of nanomaterials with RBC membranes. Therapeutic chemicals can be loaded onto red blood cells (RBCs) using a variety of techniques that preserve the cells' physiological and structural integrity. RBC disguised nanoparticles are typically made by sonication, but other methods such extrusion, microfluidic electroporation, and in-situ polymerization are also used; each has benefits and drawbacks in terms of synthesis. [15] Prior to fusion, fresh whole blood is hypotonic treated (dialysis, haemolysis, or dilution) to assist eliminate undesired cells and plasma and produce RBC membrane-derived vesicles. By using this technique, the final nanoparticles are guaranteed to retain the vital traits of RBC membranes. Because of their low immunogenicity and extended systemic circulation (RBCs have a lifespan of around 120 days), RBC membrane-coated nanoparticles offer considerable promise for application in medication delivery systems. Additionally, due to their high drug-loading capabilities, biodegradability, and natural biocompatibility, red blood cell vesicles promote greater accumulation at target areas. Nano-formulations coated with erythrocyte membranes have been effectively used in a number of areas, such as cardiovascular illness, encephalopathy treatment, and anticancer research.[16]
- Hyaluronic acid-based drug nanocarriers drug delivery systems
Hyaluronic acid (HA) is being used in drug delivery systems more and more. HA is a linear macromolecular mucopolysaccharide made up of N-acetylglucosamine and glucuronic acid repeating units. It has a reputation for being highly viscoelastic, biodegradable, and compatible with certain cell surface receptors. Because it is a normal part of eye tissue and is essential to wound healing, these properties make HA a great carrier for ocular medication delivery. Red blood cell membrane-coated nanoparticles (RBCM-NPs) can also be prepared synthetically for anticancer activity. 17] Pure red blood cells are obtained from fresh blood by centrifugation and washing, which eliminates plasma and other undesirable cells. Following hypotonic haemolysis, these cells are employed to coat selected nanoparticles. Intravenous injection of these RBCM-NPs allows them to enter tumour cells through endocytosis, sustain extended systemic circulation, and permeate tumour tissues through the increased permeability and retention (EPR) effect. Please refer to the corresponding article's online version for visual references and full figures. [18]
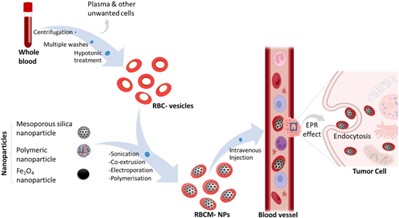
Figure 2Hyaluronic acid-based drug nanocarriers drug delivery systems
Drug targeting is improved and drug thickening, prolonged release, and transdermal absorption are all improved by hyaluronic acid (HA). Drug distribution to cancer cells has significantly improved when using HA-based drug nanocarriers. Furthermore, as biocompatible carriers with the ability to deliver drugs to certain regions with minimal side effects and tissue damage, HA-coated lipid nanoparticles have been produced. HA-based nanocarriers have advantages such better drug distribution, increased therapeutic efficacy, higher cytotoxicity, decreased tumour development, and the possibility of targeted treatment for malignancies with heightened expression of the CD44 receptor. [19] Another application is the development of a CD44-targeting anti-cancer drug delivery system using HA-based nanocarriers in conjunction with doxorubicin (DOX) and cisplatin (CDDP). Significant tumor suppression capabilities of this system against CD44+ breast cancer cells have been shown both in vitro and in vivo. In particular, these dual drug-loaded HA micelles (HA-DOX-CDDP) have demonstrated greater suppression of cellular growth, higher cellular uptake, and improved drug release under acidic conditions in 4T1 (CD44+) breast cancer cells when compared to free pharmaceuticals. Due to their great biocompatibility, biodegradability, and ability to target CD44, HA-DOX-CDDP micelles exhibit good tumour accumulation with few side effects. [20] These characteristics suggest that HA-DOX-CDDP micelles may be useful in the chemotherapeutic treatment of breast cancer.
To specifically target CD44 receptors on cancer cells, hyaluronic acid and its derivatives are used in a variety of drug delivery systems (DDS), such as gel DDS, cationic polymer DDS, and nanoparticle DDS. Research has demonstrated that, following drug delivery, HA and drug conjugates combine at the tumour site, preserving continuous drug release. The reticuloendothelial system's (RES) ability to resist systemic clearance is aided by the negative surface charge of HA-based nanocarriers. By actively targeting CD44 receptors and producing an increased permeability and retention (EPR) effect, HA-based therapeutic nanocarriers are able to preferentially enter cancer cells. [21]
- Hexagonal Boron Nitride nanosheet drug delivery system
An expanding range of materials are being investigated to improve medication delivery systems as science and technology continue to progress. One such substance is boron nitride (BN), a crystalline molecule whose nitrogen (N) and boron (B) atom stoichiometry is balanced. There are several different types of BN, such as wurtzite BN (w-BN), rhombohedral BN (r-BN), cubic BN (c-BN), and hexagonal BN (h-BN). [22] Because of its structural resemblance to graphite, hexagonal Boron Nitride (h-BN), often known as white graphene, stands out among these for its two-dimensional (2D) layered structure with sp2 hybridized B-N links. Van der Waals forces, with a bond length of 1.466 Å and an interlayer spacing of 3.331 Å, hold the compound's layers together while the B–N atoms in h-BN create strong covalent connections that result in interlocking rings. Polar B–N bonds are the outcome of this partial ionic nature. The insulator h-BN has been used in a number of industries, including dentistry, cosmetics, cement, ceramics, and most importantly, medicine, where it is used as a drug carrier similar to graphene or graphene oxide. [23] Drug delivery systems and research have found utility in h-BN. For instance, when exposed to h-BN coated with gold particles, MCF-7 breast cancer cells proliferated less than normal L929 cells, according to studies by Jedrzejczak-Silicka and colleagues. The h-BN was functionalized with gold particles, exfoliated using a modified Hummers' process, and then its effectiveness was assessed using the Neutral Red (NR) uptake assay. [24]

Figure 3 The application of hyaluronic acid-based nanocarriers in cancer treatment. (a) and (b)
Another work altered h-BN nanosheets by adding in-situ deposited palladium (Pd) to give them photothermal characteristics, which improved their ability to carry the anticancer medication doxorubicin. After giving this modified h-BN to mice for two weeks, there was a notable decrease of tumour growth. The study demonstrated that near-infrared (NIR) radiation exposure and elevated glutathione content coincided with the nanohybrids' release of doxorubicin in response to an acidic environment. [25] An additional fruitful investigation demonstrated the efficacy of h-BN coupled with copper (II) phthalocyanine (CuPc) and DNA oligonucleotide as a therapeutic agent for photodynamic treatment (PDT), in situ monitoring, and miR-21 imaging. These results highlight the potential of boron compounds as strong chemotherapeutic agents, especially h-BN. By utilizing these cutting-edge materials, drug delivery is developing and opening up new avenues for more effective and focused treatments. [26]
- Polymer-lipid hybrid nanoparticles drug delivery system
Sustained drug release, better targeting of disease cells, and greater stability during storage, nanocarriers are becoming more and more attractive as drug delivery methods. Polymeric and liposome nanoparticles are the most commonly employed of all the nanoparticles used in medication delivery. Liposomes are lipid-based nanoparticles with good biocompatibility, however they have drawbacks such medication leakage and instability in storage. [27] Conversely, polymeric nanoparticles, which are built on polymers, tend to be less biocompatible but offer great stability, drug loading capacity, and encapsulation. Researchers created polymer-lipid hybrid nanoparticles (PLHNPs), which combine the benefits of polymeric and liposomal nanoparticles, to overcome these drawbacks and provide an efficient nanomaterial. The criteria for biocompatibility, high encapsulation, sustained drug release, low drug leakage, tiny particle size, and good storage stability are all met by PLHNPs. This hybrid approach is presently employed for a number of therapeutic and diagnostic purposes
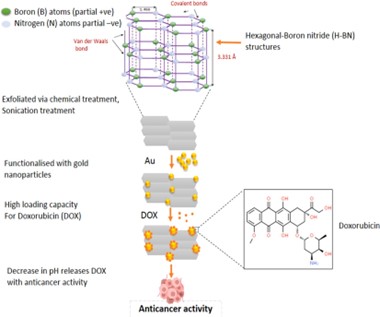
Figure 4 Schematic explanation of the h-BN nanosheet drug delivery system: h-BN nanosheets are exfoliated through chemical treatment and then functionalized with gold (Au) particles.
hybrid approach is presently employed for a number of therapeutic and diagnostic purposes as a result. [28]
Doxorubicin (DOX), a chemotherapeutic medication, has a high loading capacity as a result of its functionalization. When the pH drops, the medication is released within the tumour [29] cells. PLHNPs are made up of three different parts:
- A polymeric core that efficiently encases medications that are hydrophilic or hydrophobic, resulting in a prolonged release.
- A lipid shell with strong stability and biocompatibility.
- A layer of lipid-polyethylene glycol (PEG) on the exterior that lengthens circulation duration, improves steric stability, and blocks immunological recognition. [30]
Because of these characteristics, PLHNPs can be used in a wide range of settings, such as ultrasound, photothermal therapy, photodynamic therapy, siRNA and DNA gene transfer, and chemotherapy administration. Their extensive utility is further demonstrated by the fact that they are employed in imaging, immunological stimulation, vaccine distribution, and alternate magnetic field (AMF) applications. [31]
- Self-micro emulsifying drug-delivery system
Lipid-based medication formulations have attracted a lot of attention lately, especially those that use self-micro emulsifying drug-delivery systems (SMEDDS). A major obstacle in the development of oral dose formulations is insufficient bioavailability. Bioavailability depends on minimal hydrophilicity since medications must be in solution to pass through the gastrointestinal tract (GIT) and be absorbed. The water solubility of many chemical substances that have significant pharmacological effects is a problem. Moreover, around 50% of novel therapeutic compounds and approximately 30% of pharmaceuticals that are currently on the market are hydrophobic, or have low water solubility. [32] Lipid-based carrier systems have become more and more common as a means of improving the bioavailability of drugs that are poorly soluble in water. This formulation's main goal is to keep hydrophobic ingredients in solution during the digestive process. Different forms of lipid-based carriers exist, such as microemulsions, suspensions, dry emulsions, and self-emulsifying drug-delivery systems (SEDDS). Hydrophobic medicines have been demonstrated to be included into SEDDS, which have been further developed into self-nanoemulsifying drug-delivery systems (SNEDDS) and SMEDDS.Emulsions are produced through the dispersion of a macroscopic particle-containing liquid phase into a surfactant-containing liquid phase. [33] These semi-transparent, thermodynamically unstable emulsions have characteristics of viscous liquids. Emulsions come in three varieties: multiple emulsions, oil-in-water, and water-in-oil. Unlike SMEDDS, conventional micro-or nano-emulsions self-emulsify following oral consumption. [34]
Emulsifying agents in microemulsions are categorized as co-surfactants (CoSs) and surfactants (S). Co-surfactants are primarily soluble in the oil phase, whereas surfactants are primarily soluble in water. Co-surfactants are essential for bringing the two liquid phases' interfacial tension down to the ideal level needed for the creation of microemulsions. The formation of nano emulsions, which have droplet sizes smaller than 100 nm, requires the use of chemical or mechanical energy. Nano emulsions show long-term stability over several months, despite being kinetically stable because of their extremely low disintegration rate. In contrast to microemulsions, which are more sensitive to temperature and dilution variations, nano emulsion globules are stable in a variety of environments. [35]

Figure 5 The formation of a Polymeric-Lipid Hybrid Nanoparticle
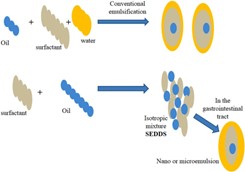
Figure 6Mechanism of Self-emulsification in aqueous environment. SEDDS comprise a mixture drugs, surfactants, oil, stabilizers, and cosolvents.
- In-situ gel drug delivery system
Any drug delivery system's main goal is to efficiently alter the medication's pharmacokinetic characteristics and tissue distribution. Considerable work has gone into creating dependable and controlled drug delivery systems during the last 60 years. One of the most cutting-edge methods among them is in-situ gel medication administration. This system makes use of its special capacity to change from a sol (liquid) to a gel state, allowing for a more gradual and regulated release of drug, improving patient comfort and compliance. [33] Generally, when formulations enter the body in solution form, they transform into gel form under certain physiological conditions. Numerous triggers, such as variations in pH, temperature, or solvent exchange, can cause this transition. The oral, nasal, injectable, vaginal, rectal, ophthalmic, intraperitoneal, and parenteral routes have all been investigated for in-situ gel drug delivery systems. Many polymeric drug delivery methods have been created, and these polymers experience a sol-gel transition in response to physiological cues. In-situ gel medication delivery devices are made of both synthetic and natural polymers. [34]
There are four basic ways in which in-situ gel biomaterials might form:
- Variations in pH and Temperature: The gelation process can be triggered by variations in pH or temperature.
- Physical Property Changes: The production of gel can be caused by variables such swelling and solvent exchange.
- Biochemical Modifications: The sol-gel transition can be brought about by chemical and enzymatic reactions. [35]
- Photo-Polymerization: Gel formation is a possible outcome of light-induced polymerization.
- Because of these principles, flexible and efficient in-situ gel drug delivery devices that can adjust to different physiological conditions for optimal drug release and therapeutic efficacy have been developed. [36]
Additional Controlled Drug Delivery Systems [ACDDS]
Preparations for extended, gradual, and sustained release have been developed by the pharmaceutical industry and pharmacy departments. Their release pattern and bioequivalence have been examined in vitro and in vivo, respectively. [37]
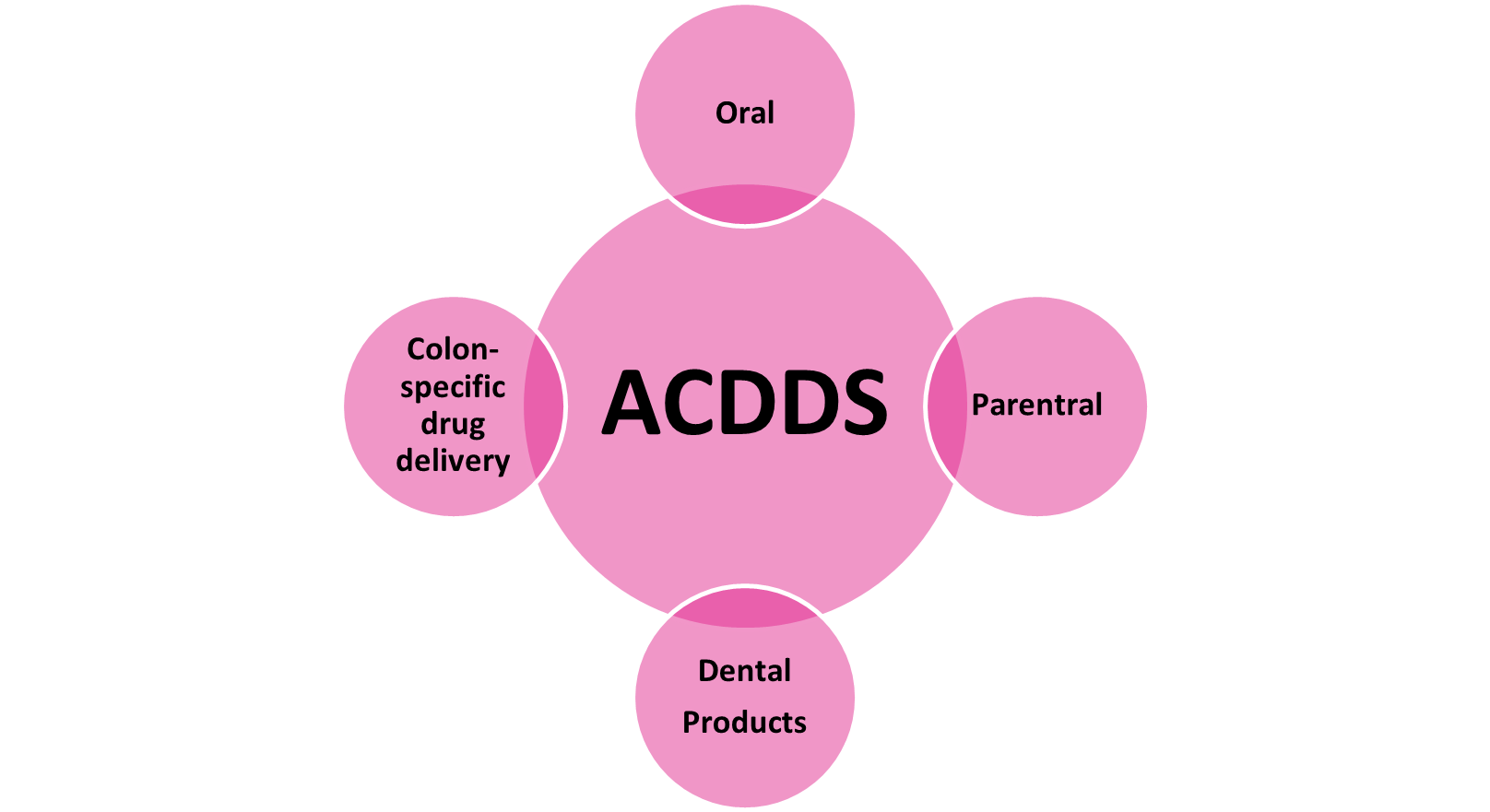
Figure 7 Additional Controlled Drug Delivery Systems
- Oral
The development of specialized devices for targeted administration to the gut employing therapeutic agent-incorporated microspheres was spurred by the ongoing challenges associated with the oral delivery of protein and peptide medicines. [38] The in vitro resistance of gelatine capsules covered with different amounts of sodium alginate and cross-linked with calcium chloride to the stomach and intestinal environments was evaluated. After demonstrating encouraging outcomes in vitro, capsules coated with 20% w/v polymer were assessed for their in vivo gastrointestinal behaviour in human subjects. According to radiographic studies, uncoated gelatine capsules fell apart in the stomach 15 minutes after being consumed, while capsules coated with alginate stayed whole in the stomach for as long as three hours before moving to the ileocecal area of the intestine, where they finally fell apart. Using contemporary palletization techniques, Varanasi and Nageswara created pellets containing prochlorperazine maleate in diameters of 1 mm and 1.65 mm. [39] Using a USP dissolving equipment, these pellets were coated with ethyl cellulose and assessed for in vitro release. They noticed that as ethyl cellulose concentration increased, prochlorperazine maleate release reduced. Rangaiah et al. used hydroxypropyl methylcellulose (HPMC), Eudragit RL, and RS to create theophylline sustained release tablets. Studies on bioavailability in volunteers showed that the formulations of Eudragit and HPMC sustained plasma concentrations. [40] A modified Wurster coating equipment was used to create sustained release nifedipine capsules in a different investigation. These capsules included a solid dispersion form for the quickly available loading dose and microparticles coated with a film of polyvinyl acetate (molecular weight 45,000) for the sustained release portion. These capsules released the therapeutic dose for the first time in 45 minutes and continued to do so for 11 to 12 hours. [41]

Figure 8 Oral Drug Delivery system
- Parenteral
For drug delivery systems, Kushwaha studied a combination of natural macromolecule gum Arabic and synthetic polymer polyvinyl alcohol. She noted that the drug load, drug solubility in the matrix, and the makeup of plasticizers, homopolymers, and cross-linkers all affect the release kinetics and duration. This method enables customized control over the kinetics of medication release. Chitosan microspheres with diameters ranging from 45 to 300 ?m were used to administer progesterone under control. [42] Research carried out in vitro and in vivo demonstrated that over 40 days, heavily cross-linked microspheres released only 35 percent of the integrated steroids, whereas those with less cross-linking released 70 percent. Cross-linked chitosan microspheres appear promising for long-term steroid delivery, as evidenced by bioavailability studies conducted on rabbits using intramuscular injection of the microsphere formulation. These studies showed that plasma concentrations of 1-2 ?g/ml were maintained for up to 5 months without significant initial burst effects. [43] To facilitate the delivery of vaccines through a single point of contact, cross-linked dextran beads were created. With the advent of bioresorbable surgical sutures twenty years ago, biodegradable polymeric devices have been the subject of extensive research. Poly(lactide) (PLA), poly(glycoside) (PGA), and their copolymer poly(lactide-co-glycoside) (PLGA) are examples of thermoplastic aliphatic polyesters that have attracted a lot of attention because of their mechanical strength, biodegradability, and biocompatibility. [44] These polymers are FDA-approved for drug delivery applications and can be used to create a variety of devices that carry proteins, peptides, vaccines, and small molecules. Poly(DL-lactide-co-glycoside) (PLG) microparticles were studied by Dhiman and Khullar as potential delivery systems for the 71-KDa cell wall-associated protein of Mycobacterium tuberculosis H37Ra. Their investigations in mice showed that 71-KDa PLG vaccination, as opposed to emulsified 71-KDa in Freund's incomplete adjuvant (FIA) and BCG vaccination, produced stronger T-cell activation and cytokine release. [45] Additionally, 71-KDa PLG demonstrated improved bacterial load clearance from livers and lungs in comparison to 71-KDa FIA, offering superior protection against Mycobacterium tuberculosis H37Rv challenge. In a different investigation, rats with adjuvant-induced arthritis received subcutaneous injections of diclofenac administered using poly(lactide-co-glycoside) microspheres. [46] The results of pharmacokinetic and pharmacodynamic analyses showed that although in situ gel-forming systems produced higher initial drug concentrations and prolonged anti-inflammatory effects for roughly 10 days, microspheres were able to sustain therapeutic drug levels in plasma for about 16 days after injection. [47]
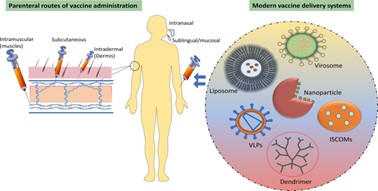
Figure 9 : Parenteral Route of Vaccine Drug delivery System
- Dental
Tetracycline and metronidazole were delivered via ethyl cellulose strips by Soumyajit et al. with the intention of decreasing subgingival bacteria in periodontal pockets. Following supragingival scaling, the patients were separated into five groups according to how long the medicine was applied for. Applications of metronidazole, tetracycline, and placebo were assigned to specific sites. Gram staining and culture techniques were used to gather baseline microbiological samples following site marking and isolation. Subgingival samples were obtained again after therapy. [48] After removing the ethyl cellulose strips, the remaining drug content was examined. The outcomes showed that subgingival bacteria were efficiently inhibited over a number of days by both metronidazole and tetracycline. At lower concentrations, metronidazole completely reduced subgingival bacteria, but tetracycline showed quicker release kinetics. In a different study, a topical gel for dentistry was compared with a saliva-activated bio-adhesive drug delivery system for lidocaine hydrochloride. The drug delivery device showed deeper anesthesia than the commercially available topical gel, adhering to the gingiva in just one minute and reaching peak anesthesia in fifteen minutes. [49]
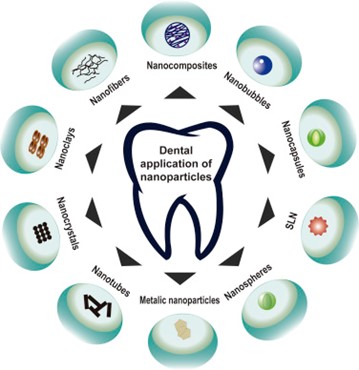
Figure 10 Dental Application of Nanoparticles
- Colon-specific drug delivery
The growing interest in peptide and protein medications emphasizes the necessity of creating dosage forms with site-specific release capabilities. When it comes to medications that are not well absorbed from the upper gastrointestinal tract, oral delivery that targets the colon presents a number of advantages versus parenteral administration. This method is very helpful for treating conditions localized within the colon, such as Crohn's disease, ulcerative colitis, and colorectal cancer. [50] A colon's prolonged release is also beneficial for ailments like arthritis, angina, and nocturnal asthma. For colon-specific medication delivery, oligonucleotides, proteins, peptides, and vaccinations are all excellent options. For the treatment of inflammatory bowel disease (IBD), several medications, including sulfasalazine, unsalaried, and olsalazine, have been developed into colon-specific delivery systems. More and more research is being done on using the unique microbiota and enzymatic environment of the colon to release medications in in this region. One of the challenges is getting through the stomach and small intestine and into the colon, which requires adjusting to the varied pH levels and longer transit periods. Drugs can now be coated with pH-sensitive and bacterially degradable polymers, embedded in matrices broken down by colonic bacteria, and designed as prodrugs thanks to recent advancements in pharmaceutical technology. [51] By using pH variations in the gastrointestinal tract to initiate drug release, these technologies improve the targeted delivery of medications to the colon. Effective colon-specific targeting has been achieved through the use of polysaccharide and azopolymer coatings, which are resistant in the upper digestive tract but degradable by colonic bacteria. The advancement and assessment of colon-specific drug delivery systems for therapeutic application has been hastened by the creation of ideal preclinical models and clinical evaluation techniques. [52]
Comprehensive in vitro and in vivo experiments have been conducted to evaluate these systems, which exhibit in vivo site specificity. There are several therapeutic benefits of administering medications directly to the colon, especially when those medications are sensitive to pancreatic enzymes or stomach acid. It is advantageous for the effective delivery of vermicides and diagnostic agents at lower dosage needs, as well as for the treatment of localized colonic disorders such as ulcerative colitis, colorectal cancer, and Crohn's disease. [53]
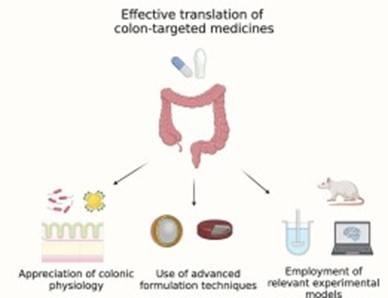
Figure 11colon target drug delivery system
Novel Drug Transporting Systems
The process of microencapsulation is essential for the creation of microspheres that contain hydrophilic and hydrophobic medications within biocompatible polymers, which aids in the discovery of novel therapies. These carriers' major objective is to minimize systemic absorption while achieving controlled drug release, sustaining therapeutic levels for a predetermined amount of time. Numerous industries, including food, cosmetics, medicines, and gene delivery, use these systems. Microparticles, which include both microcapsules and microspheres, are made of polymers or lipids (like liposomes) and are usually between 1 and 250 µm in size, although ideally, they are smaller than 125 µm. [54] Occasionally, they can even reach up to 1000 µm in size. Because of the longer release durations and improved absorption made possible by the adhesive qualities of microparticles, this technology is essential to medication administration. Moreover, strong connections between in vitro and in vivo experiments have been noted, suggesting their efficacy in biological systems. Because biodegradable microparticles are readily eliminated by physiological processes, their build-up in cells and tissues does not pose a risk of cytotoxicity. Active ingredients may be contained within the particle or adsorbed onto the polymer's surface. The use of pH-sensitive or thermosensitive microparticles allows for tailored controlled release, which is especially advantageous for intravenous delivery. [55] A variety of materials, including peptides (like insulin and calcitonin), cosmetics, antiviral medications, antihypertensives, and anticancer medicines, have been encapsulated in microparticles. Microparticles can be made in a number of ways, such as by synthesizing premade polymers or polymerizing synthetic monomers. Submicron-sized particles, however, have a number of important advantages over bigger microparticles. For example, a study comparing PLGA micro- and nanoparticles showed that the latter were more readily absorbed by Caco-2 cells (41% vs. 15%). Additionally, nanoparticles may be a more effective way to target particular tissues, such as malignant or inflammatory regions. [56]
- Micro Sponges
Made of artificial polymers, micro sponges are inert, physiologically porous particles that can hold an active agent up to their own weight. They provide the medication with defense against external elements and enable controlled release. Products like Carnac®, which contains fluorouracil to treat actinic keratosis, and Retin-A Micro®, which treats acne vulgaris, are marketed with the use of micro sponge technology. [57]
- Nanotechnology
The notion of nanomedicine and commercially viable products have emerged as a result of the rapid growth of nanotechnology in drug delivery. Utilizing materials at the nanoscale scale to develop novel therapeutic strategies is known as nanomedicine. Materials at this scale have distinct physicochemical characteristics because of their small size, high surface area, and surface structure. These characteristics allow for the intracellular absorption of nanoparticulate systems to particular cellular targets, thereby circumventing the drawbacks of conventional formulations. As a result, nanotechnology has been incorporated into a number of industries, such as imaging, diagnostics, and medicine and gene delivery. [58]
- Immunoconjugates
Recombinant antibodies covalently bonded to a drug through a linker are known as antibody-drug conjugates (ADCs) or immunoconjugates. By utilizing the specificity of monoclonal antibodies (mAbs), this approach seeks to deliver powerful medications to specific areas while reducing harm to non-targeted organs. ADCs can be customized to target a variety of diseases by choosing the right molecular domains. Nevertheless, preliminary research has shown certain difficulties, such as brief half-lives, immunogenicity, and ineffective interactions. Many approaches, including PEGylation, conjugation with proteins like albumin, and the use of chimeric, humanized, and fully human mAbs, have been developed to overcome these problems. Consequently, Mylotarg (gemtuzumab ozogamicin), the first immunoconjugate to be authorized, was launched to treat acute myeloid leukemia. [59] Phase 3 clinical trials are presently underway for a number of different immunoconjugates, such as Brentuximab vedotin for Hodgkin lymphoma and Mapatumumab for advanced renal illness. Furthermore, novel approaches for the utilization of antibodies coupled to liposomes and nanoparticles—known as immunoliposomes and immunonanoparticles, respectively—have been developed. Multiple medications can be contained in these systems, providing protection from the outside world and allowing for regulated release. Additionally, by focusing on transferrin, insulin, or glutathione receptors, they can target difficult regions like the blood-brain barrier (BBB) and cause receptor activation and subsequent internalization. [60]
- Viruses
Because viruses are naturally able to infect particular cells and deliver genetic information to the nucleus, they are intriguing carriers for gene and medication therapy. Recombinant viruses have the potential to improve drug delivery by increasing transfection efficiency and avoiding lysosomal degradation. The primary obstacle, nevertheless, is developing viral vectors that can infect mammalian cells even in the absence of replication machinery. Lentiviruses, retroviruses, and adenoviruses are often utilized viruses in this setting. The use of viruses presents serious safety problems despite its potential, including the possibility of insertional mutagenesis and proto-oncogene activation, undesired viral replication, and severe immune responses. [61] Retroviruses are better suited for ex vivo administration since they can only infect dividing cells and have a limited capacity for loading genetic material. On the other hand, adenoviruses remain extrachromosomal while lentiviruses and adenoviruses can transfer genes into non-dividing cells, lowering the possibility of damaging the host genome. These viral systems hold special significance in the context of cytotoxic gene therapy. Nonviral vectors, such liposomes (virosomes) and nanoparticles, are becoming more and more popular since they are easier to synthesize and elicit a lesser immune response. For these nonviral techniques to be applied effectively, issues including poor gene expression and ineffective gene transfer must be resolved. [62]
General Mechanisms Consideration
- Tissue-targeting design, controlled release, and surface functionalization
Many techniques have been investigated to direct medications to certain locations, either actively, by employing ligands to reach desired targets, or passively, as a result of physiological processes. Interaction with cell receptors is made possible by surface modification of drug carriers with bioactive molecules that can be adsorbed, coated, conjugated, or connected to them. This interaction shows a preferential affinity for particular tissues or cells, which improves medication absorption. Additionally, modified coatings that include chitosan and albumin can stop enzymatic breakdown in the gastrointestinal tract and plasma. The application of monoclonal antibodies (or fragments) with non-antibody ligands, such as carbohydrates that are particular to cell surfaces and called lectins, has been studied. Small compounds or peptide agonists/antagonists for receptors that are overexpressed in particular organs (such as folate, transferrin, and galactosamine) have been developed more recently. However, non-antibody ligands may express themselves non-selectively, targeting ligands might improve distribution to unwanted regions, and immunoconjugates present problems with immunogenicity and retention in the reticuloendothelial system (RES). [63] Coatings that change the lipophilicity/hydrophilicity profile, inhibit immune cell absorption, and enhance cell identification are examples of carrier surface modification. For instance, after an intravenous injection, opsonization and subsequent phagocytosis by RES cells frequently result in the nanoparticles being removed from the plasma in a matter of minutes. Applying surface ligands such as PEG, a hydrophilic polymer that increases resistance to plasma protein binding and inhibits serum-induced aggregation, can lessen opsonization. By doing this, immunological reactions are prevented by decreasing opsonization and identification by phagocytes. PEG also limits the ability of enzymes to reach dendrimer scaffolds, which lessens breakdown. [64] PEG-coated liposomes and nanoparticles lengthen residence lengths in vivo by up to 200 times in humans and considerably lengthen circulation times. However, surface density, chain length, and avoiding liver absorption all affect how effective PEG is. Notwithstanding their benefits, PEG carriers may cause an immunological reaction following repeated injections, referred to as the accelerated blood clearance (ABC) phenomenon, which increases build-up in the spleen and liver. As a result, novel approaches are being investigated, such as substituting polyomino acid polyhydroxy ethyl-L-asparagine (PHEA) for PEG, which has demonstrated favourable extended circulation durations and a decreased ABC phenomenon. [65] Furthermore, employing a hydrazine-cholesteryl hemi succinate linkage to PEG has demonstrated pH-responsiveness at pH 5.5 and stability at physiological pH, making it cleavable by esterase. Along with promoting extended circulation and stealth shielding, poly(ethylene oxide) (PEO) and its derivatives have good biocompatibility and low toxicity. In addition to having some stealth qualities, polymers like PLA and PLGA can aid in gastrointestinal absorption. Another important component of targeting is the surface charge (zeta potential) of carriers, which influences interactions with surfaces, cell membranes, and plasma proteins, ultimately affecting clearance and distribution patterns. Because of their overall anionic charge, cationic surfaces—like those made from chitosan coating—show high interaction with surfaces and cell membranes. Nevertheless, the minor negative surface of PLGA nanoparticles tends to limit their interaction and intracellular uptake of negatively charged plasmids. [66] Drugs can be delivered from carriers into cells in two primary ways: either the drug is released from the carrier and absorbed by the cell, or the carrier is absorbed into the cell and releases its contents gradually. There are several ways that cells can absorb nanoparticles, but the most important one is endocytosis. For particles bigger than 200 nm, caveolae-mediated endocytosis is frequently the main mechanism; however, other mechanisms that contribute to nanoparticle uptake include lipid raft-associated receptors, actin and clathrinid, microtubules, and processes that are dependent on cholesterol. The uptake of nanoparticles is significantly influenced by their surface charge. [67] Anionic dendrimers, for example, are generally endocytosed by clathrinid-dependent mechanisms that encourage tight junction opening, but are not dependent on caveolin-mediated endocytosis. Previous research on PAMAM dendrimers revealed that electrostatic interactions between their negatively charged proteoglycan surfaces on mammalian cell surfaces and their cationic primary amine surface groups drive their internalization by inducing micropinocytosis and clathrinid-mediated endocytosis. In order to guarantee efficient drug administration, nanoparticles need to be engineered to withstand degradation in the acidic lysosome environment—a typical cell degradation process. Dendrimers are discovered in endosomes once they have saturated the lysosomal pathway. [68] Nanotechnology advancements have made it possible to build carriers that may specifically target the nucleus of cells or other disease-causing organelles, enabling a concentrated and direct release of the medication at the desired location. Using outside stimuli to momentarily boost cell junction permeability—such as ultrasound—is another strategy to improve cellular drug uptake. For example, liposomes could profit from this method. In order to boost drug accumulation at particular areas, passive targeting makes use of physiological characteristics such as the increased permeability and retention (EPR) effect, which is frequently employed in cancer therapy. Nevertheless, controlling the release profile of novel drug carriers is crucial since sudden releases of highly potent medications, particularly those used to treat chronic illnesses, can have unanticipated harmful effects. [69] Bi-association of liposomes enclosed in polymeric particles and the use of chitosan and alginate, which have been demonstrated to inhibit burst releases, are two methods for achieving controlled release. Controlled release can also be affected by surfactants, industrial techniques, and microparticles incorporating nanoparticles. Heterogeneous drug distribution, temperature during solvent removal, the physicochemical nature of the polymer matrix (e.g., using non-water-soluble polymers to prevent water uptake), porosity, recovery techniques, and the concentration of the incorporated drug are additional factors that impact controlled release. [70]
- Combining Treatment with Drugs Simultaneously Encapsulated
Multiple medications may be delivered at once by these drug delivery systems. PLGA nanoparticles, for example, can be loaded with vincristine sulphate and verapamil hydrochloride to suppress the P-glycoprotein (P-gP) efflux system and deliver chemotherapeutic drugs. This method can improve the therapeutic index and overcome tumour resistance. Comparable tactics have been used to administer cyclosporine A and doxorubicin. According to recent research, PLGA-PEG interacts with P-gP, which could increase these systems' effectiveness. The properties of the medications to be encapsulated must be carefully considered when designing these systems. Hydrophobic medications are apt to be encapsulated in hydrophobic polymers, whereas hydrophilic drugs are more likely to be encapsulated in hydrophilic polymers. [71] In order to solve this, new polymers have been created, such as PCL-PEG or (PLA-PEG-PLA)n, which may successfully encapsulate hydrophilic and hydrophobic medications, such as retinoic acid and calf thymus DNA, respectively. Another tactic to improve treatment efficacy, especially in cancer therapy, is to design systems with distinct release rates for every medication. For instance, to efficiently counteract cancer medication resistance mechanisms, paclitaxel and C6-ceramide have been encapsulated in a regulated blend polymer of PLGA-PbAE. [72]
- Distribution of Carriers
The reticuloendothelial system (RES) can be internalized and removed from the systemic circulation, it presents serious hurdles to drug carrier systems, especially in the liver and spleen. After intravenous (IV) delivery of PLGA nanoparticles, the liver absorbs most of them (about 40%), followed by the kidneys (26%) heart (12%) brain (13%) and other organs (less than 25%). Very little of the nanoparticles end up in the plasma. PLGA-PbAE nanoparticles have shown comparable dispersion patterns. Because intraperitoneal (IP) injection causes a variable distribution due to lymphatic clearance, the route of administration is also very important in determining the distribution pattern. More hydrophilic particles are quickly removed from the system, which is influenced by the lipophilicity of the carriers. [73] Furthermore, the pH of the media can modify the surface charge of the nanoparticles, changing their absorption by cells and overall dispersion throughout the body. The route of administration has been found to significantly affect the distribution of 10 nm gold nanoparticles functionalized with different groups to achieve varied zeta potentials (neutral, negative, positive, and zwitterionic). After intravenous administration, neutral and zwitterionic particles showed improved circulation, but positively charged particles showed a tenfold decrease in peak plasma concentration and were removed in 15 minutes. The main cause of these differences in bioavailability is opsonization, a process that also occurs with dendrimers and entails local macrophages recognizing the nanoparticle. [74]
PROBLEMS WITH THE WAY THAT DRUGS ARE DELIVERED NOWADAYS
New developments in drug delivery technologies have made it possible to direct medications made from different plant sources to certain body parts that require therapy. But even with these achievements, there are still a number of restrictions and difficulties. A significant obstacle impeding the advancement of medication delivery devices is the scarcity and diversity of existing literature. Research must be advanced by a thorough literature, especially in the field of nanomedicine. Translating nanotechnology from research to therapeutic applications is hampered by the variable characterization of experimental details and the heterogeneity of published data. Although tailored administration and increased efficacy are two of the many potential advantages of nanoparticles, questions about their safety and potential interactions with non-specific proteins and unwanted organs still need to be addressed. Their broad use in medical therapies is complicated by the absence of a thorough knowledge of these elements. Large particles are used as carriers in a lot of current delivery systems, which can have disadvantages like low solubility, poor absorption, instability in vivo, and unfavourable side effects after administration. By boosting target-specific delivery, reducing side effects in the human biological system, and increasing bioavailability, the switch to smaller particles presents a viable answer to these problems. [75]
FUTURE DIRECTIONS AND CONCLUSION:
Indeed, drug delivery and nanomedicine have emerged as two very fascinating areas of study in recent years, drawing a lot of interest from both academic and clinical research. Notwithstanding the obstacles that have impeded the extensive clinical implementation of multimodal delivery methods, the present progress exhibits considerable potential. Collaboration between multiple disciplines, such as academic theory, laboratory experiments, medical understanding, and pharmaceutical experience, is necessary to achieve effective drug transport from bench to bedside. Scholars such as Vargas et al. support the use of cell treatments to overcome the problems with current drug delivery methods' bio-acceptability. According to their proposal, cell treatments have the ability to overcome innate biological barriers and trigger natural responses within the body, thereby offering a sustainable source of complex biologics. Adepu proposes novel approaches to address the current problems in drug delivery, including the use of molecular imprinting polymers, microfluidics, and inorganic mesoporous nanoparticles. According to Khalid et al., priming agents can optimize tissue interactions without having a negative impact on patients by changing the biological milieu at the administration site and increasing the efficacy of drug delivery. Furthermore, investigating cell-based medication delivery methods within biomaterial frameworks—which combine human system-native Nano biomaterials with cells—represents an innovative and promising strategy. This innovative approach has the potential to produce highly efficient and targeted medication delivery patterns. Even with these developments, thorough research and clinical trials are still necessary to improve the effectiveness and get over the difficulties that come with using contemporary drug delivery systems. In order to fully utilize medication delivery and nanomedicine in clinical practice, several issues must be resolved. [76]
REFERENCES
- Panchagnula R. Transdermal delivery of drugs. Indian J Pharmacol. 1997;29:140–56. [Google Scholar]
- Rao PR, Diwan PV. Formulation and in vitro evaluation of polymeric films of diltiazem hydrochloride and indomethacin for transdermal administration. Drug Dev Indian Pharm. 1998;24:327–36. [PubMed] [Google Scholar]
- Rao PR, Diwan PV. Permeability studies of cellulose acetate free films for transdermal use: Influence of plasticizers. Pharm Acta Helv. 1997;72:47–51. [PubMed] [Google Scholar]
- Thacharodi D, Rap KP. Development and in vitro evaluation of chitosan-based trandermal drug delivery system for the controlled delivery of propranolol hydrochloride. Biomaterials. 1995;16:145–8. [PubMed] [Google Scholar]
- Krishna R, Pandit JK. Carboxymethylcellulose-sodium based transdermal drug delivery system for propranolol. J Pharm Pharmacol. 1996;48:367–70. [PubMed] [Google Scholar]
- Bhat M, Shenoy DS, Udupa N, Srinivas CR. Optimization of delivery of betamethasone - dipropionate from skin preparation. Indian Drugs. 1995;32:211–4. [Google Scholar]
- B.M. Rayaprolu, J.J. Strawser, G. Anyarambhatla, Excipients in parenteral formulations: selection considerations and effective utilization with small molecules and biologics, Drug Dev. Ind. Pharm. 44 (2018) 1565–1571, https://doi.org/10.1080/03639045.2018.1483392.
- A.M. Vargason, A.C. Anselmo, S. Mitragotri, The evolution of commercial drug delivery technologies, Nat. Biomed. Eng. 5 (2021) 951–967, https://doi.org/ 10.1038/s41551-021-00698-w.
- M.S. Alqahtani, M. Kazi, M.A. Alsenaidy, M.Z. Ahmad, Advances in oral drug delivery, Front. Pharmacol. 12 (2021), 618411, https://doi.org/10.3389/ fphar.2021.618411.
- D. Sahoo, R. Bandaru, S.K. Samal, R. Naik, P. Kumar, P. Kesharwani, R. Dandela, in: P. Kesharwani, S. Taurin, K.B. T.-T., A. of N.N. GreishGreish (Eds.), Chapter 9 - Oral Drug Delivery of Nanomedicine, Academic Press, 2021, pp. 181–207, https://doi.org/10.1016/B978-0-12-820466-5.00009-0.
- J.O. Morales, P.R. Vuddanda, S. Velaga, Controlled drug delivery via the buccal and sublingual routes, in: Fundam. Drug Deliv., 2021, pp. 433–448, https:// doi.org/10.1002/9781119769644.ch17.
- N.R. Hussein, H.K. Omer, A.M.A. Elhissi, W. Ahmed, in: W. Ahmed, D.A. Phoenix, M.J. Jackson, C.P.B.T.-A. in M., S.E. Charalambous (Eds.), Chapter 15 - Advances in Nasal Drug Delivery Systems, Academic Press, 2020, pp. 279–311, https://doi.org/10.1016/B978-0-12-819712-7.00015-2.
- A. Chauhan, L. Fitzhenry, A.P. Serro, Recent Advances in Ophthalmic Drug Delivery (2022) 1–5.
- Thirunavukkarasu, R. Nithya, J. Jeyanthi, Transdermal drug delivery systems for the effective management of type 2 diabetes mellitus: a review, Diabetes Res. Clin. Pract. (2022), 109996, https://doi.org/10.1016/j.diabres.2022.109996.
- P. Sharma, K. Gajula, N.N. Dingari, R. Gupta, S. Gopal, B. Rai, R.G. Iacocca, Subcutaneous drug delivery: a review of the state-of-the-art modelling and experimental techniques, J. Biomech. Eng. (2022), https://doi.org/10.1115/1.4055758.
- M. Misbah Ul Haq, M. Razzak, M.A. Uddin, N. Ahmed, D. Shahidulla, Rectal drug delivery system: an overview, Clin. Pharmacol. Biopharm. 10 (2021).
- S. Mahant, A.K. Sharma, H. Gandhi, R. Wadhwa, K. Dua, D.N. Kapoor, Emerging trends and potential prospects in vaginal drug delivery, Curr. Drug Deliv. (2022), https://doi.org/10.2174/1567201819666220413131243.
- M. Cho, M. Joo, K. Kim, Y. Wook, S. Lee, Y. Mi, I. Ho, Biochemical and Biophysical Research Communications the immunotherapeutic effects of recombinant Bacillus rin resistant to antimicrobial peptides on Calmette-Gu e bladder cancer cells, Biochem. Biophys. Res. Commun. (2018), https://doi.org/10.1016/j. bbrc.2018.12.097.
- L. Palugan, M. Cerea, M. Cirilli, S. Moutaharrik, A. Maroni, L. Zema, A. Melocchi, M. Uboldi, I. Filippin, A. Foppoli, A. Gazzaniga, International Journal of Pharmaceutics : X Intravesical drug delivery approaches for improved therapy of urinary bladder diseases, Int. J. Pharm. X. 3 (2021), 100100, https://doi.org/ 10.1016/j.ijpx.2021.100100.
- R. Verma, S. Garg, Current Status of Drug Delivery Technologies and Future Directions, 2001.
- R.A. Keraliya, C. Patel, P. Patel, V. Keraliya, T.G. Soni, R.C. Patel, M.M. Patel, Osmotic drug delivery system as a part of modified release dosage form, 2012, ISRN Pharm (2012), 528079, https://doi.org/10.5402/2012/528079.
- B.D. Mattos, O.J. Rojas, W.L.E. Magalh, Biogenic silica nanoparticles loaded with neem bark extract as green, slow-release biocide 142 (2017) 4206–4213, https://doi.org/10.1016/j.jclepro.2016.11.183.
- Ding, Z. Li, A review of drug release mechanisms from nanocarrier systems, Mater. Sci. Eng. C. 76 (2017) 1440–1453, https://doi.org/10.1016/j. msec.2017.03.130.
- K. Chen, X. Chen, Design and Development of Molecular Imaging Probes, 2010, pp. 1227–1236.
- A.M. Faheem, D.H. Abdelkader, Novel Drug Delivery Systems, 1, Elsevier LTD., 2020, https://doi.org/10.1016/B978-0-08-102548-2.00001-9.
- Pathak, F.U. Vaidya, S.M. Pandey, Chapter 3 - Mechanism for Development of Nanobased Drug Delivery System, Elsevier Inc., 2019, https://doi.org/ 10.1016/B978-0-12-814029-1.00003-X.
- F. Danhier, O. Feron, V. Pr´eat, To exploit the tumor microenvironment : passive and active tumor targeting of nanocarriers for anti-cancer drug delivery, J. Contr. Release 148 (2015) 135–146, https://doi.org/10.1016/j.jconrel.2010.08.027.
- V. Agrahari, Novel Drug Delivery Systems, Devices, and Fabrication Methods, 2018, pp. 303–306.
- J.K. Patra, G. Das, L.F. Fraceto, E. Vangelie, R. Campos, P. Rodriguez, L. Susana, A. Torres, L. Armando, D. Torres, R. Grillo, Nano based drug delivery systems : recent developments and future prospects, J. Nanobiotechnol. (2018) 1–33, https://doi.org/10.1186/s12951-018-0392-8.
- X. Li, C. Malardier-jugroot, Folic Acid-Conjugated Amphiphilic Alternating Copolymer as a New Active Tumor Targeting Drug Delivery Platform, 2016, pp. 4101–4110.
- V.P. Torchilin, Nanoparticulate Systems for Drug Delivery, Nat. Publ. Gr., 2014, https://doi.org/10.1038/nrd4333
- A. Vincy, S. Mazumder, Amrita, I. Banerjee, K.C. Hwang, R. Vankayala, Recent progress in red blood cells-derived particles as novel bioinspired drug delivery systems: challenges and strategies for clinical translation, Front. Chem. 10 (2022). https://www.frontiersin.org/articles/10.3389/fchem.2022.905256.
- P.B. Patil, S.K. Datir, R.B. Saudagar, A review on topical gels as drug delivery system, J. Drug Deliv. Therapeut. 9 (2019), https://doi.org/10.22270/jddt.v9i3- s.2930.
- T. Hanley, R. Vankayala, C.-H. Lee, J.C. Tang, J.M. Burns, B. Anvari, Phototheranostics using erythrocyte-based particles, Biomolecules 11 (2021), https://doi. org/10.3390/biom11050729.
- L.Z. Wang C, X. Sun, L. Cheng, S. Yin, G. Yang, Y. Li, Multifunctional theranostic red blood cells for magnetic-field-enhanced in vivo combination therapy of cancer, Adv. Mater. 26 (2014) 4794–4802, https://doi.org/10.1002/adma.201400158.
- L.H. Jiawei Chen, Nanoscale delivery system for nutraceuticals: preparation, application, characterization, safety, and future trends, Food Eng. Rev. 12 (2020) 14–31, https://doi.org/10.1007/s12393-019-09208.
- A. Zinger, J.P. Cooke, F. Taraballi, Biomimetic nano drug delivery carriers for treating cardiovascular diseases, Nanomed. Nanotechnol. Biol. Med. 33 (2021), 102360, https://doi.org/10.1016/j.nano.2021.102360.
- A. Gao, X. Chu, W. Gong, J. Zheng, X. Xie, Y. Wang, M. Yang, Z. Li, C. Gao, Y. Yang, Neuron tau-targeting biomimetic nanoparticles for curcumin delivery to delay progression of Alzheimer’s disease, J. Nanobiotechnol. 18 (2020) 71, https://doi.org/10.1186/s12951-020-00626-1.
- T. Mamo, E.A. Moseman, N. Kolishetti, C. Salvador-Morales, J. Shi, D.R. Kuritzkes, R. Langer, U. Von Andrian, O.C. Farokhzad, Emerging nanotechnology approaches for HIV/AIDS treatment and prevention, Nanomedicine 5 (2010) 269–285, https://doi.org/10.2217/nnm.10.1.
- Joseph Anthony Moss, HIV/AIDS review, Radiol. Technol. 84 (2013) 247–267.
- L. Alaniz, P.V. Cabrera, G. Blanco G, G. Ernst, G. Rimoldi, E. Alvarez, S.E. Hajos, Interaction of CD44 with different forms of hyaluronic acid. Its role in adhesion and migration of tumor cells, Cell Commun. Adhes. 9 (2002) 117–130, https://doi.org/10.1080/15419060214522.
- L. Kang, Z. Gao, W. Huang, M. Jin, Q. Wang, Nanocarrier-mediated co-delivery of chemotherapeutic drugs and gene agents for cancer treatment, Acta Pharm. Sin. B. 5 (2015) 169–175, https://doi.org/10.1016/j.apsb.2015.03.001.
- S.A.A. Rizvi, A.M. Saleh, Applications of nanoparticle systems in drug delivery technology, Saudi Pharmaceut. J. 26 (2018) 64–70, https://doi.org/10.1016/j. jsps.2017.10.012.
- T. Yu, Y. Li, X. Gu, Q. Li, Development of a hyaluronic acid-based nanocarrier incorporating doxorubicin and cisplatin as a pH-sensitive and CD44-targeted anti-breast cancer drug delivery system, Front. Pharmacol. 11 (2020), https://doi.org/10.3389/fphar.2020.532457.
- Y. Jia, S. Chen, C. Wang, T. Sun, L. Yang, Hyaluronic acid-based nano drug delivery systems for breast cancer treatment: recent advances, Front. Bioeng. Biotechnol. 10 (2022). https://www.frontiersin.org/articles/10.3389/fbioe.2022.990145.
- G. Huang, H. Huang, Application of hyaluronic acid as carriers in drug delivery, Drug Deliv. 25 (2018) 766–772, https://doi.org/10.1080/
- Allemann, J. Leroux and R. Gurny, “Polymeric Nanoand Microparticles for the Oral Delivery of Peptides and Peptidomimetics,” Advanced Drug Delivery Reviews, Vol. 34, No. 2-3, 1998, pp. 171-189. doi:10.1016/S0169-409X(98)00039-8
- P. Couvreur and F. Puisieux, “Nano- and Microparticles for the Delivery of Polypeptides and Proteins,” Advanced Drug Delivery Reviews, Vol. 10, No. 1993, pp. 141-162.
- S. Freiberg and X. X. Zhu, “Polymer Microspheres for Controlled Drug Release,” International Journal of Pharmaceutics, Vol. 282, No. 1-2, 2004, pp. 1-18. doi:10.1016/j.ijpharm.2004.04.013
- J. Panyam and V. Labhasetwar, “Biodegradable Nanoparticles for Drug and Gene Delivery to Cells and Tissue,” Advanced Drug Delivery Reviews, Vol. 55, No. 3, 2003, pp. 329-347. doi:10.1016/S0169-409X(02)00228-4
- C. Pinto Reis, R. J. Neufeld, A. N. J. Ribeiro and F. Veiga,“Nanoencapsulation I. Methods for Preparation of DrugLoaded Polymeric Nanoparticles,” Nanomedicine: Nanotechnology, Biology, and Medicine, Vol. 2, No. 1, 2006, pp. 8-21.doi:10.1016/j.nano.2005.12.003
- M. P. Desai, V. Labhasetwar, E. Walter, R. J. Levy and G. L. Amidon, “The Mechanism of Uptake of Biodegradable Microparticles in Caco-2 Cells Is Size Dependent,” Pharmaceutical Research, Vol. 14, No. 11, 1997, pp. 1568- 1573. doi:10.1023/A:1012126301290
- Y. Avnir, K. Turjeman, D. Tulchinsky, A. Sigal and P. Kizelsztein, “Fabrication Principles and Their Contribution to the Superior in Vivo Therapeutic Efficacy of Nano-Liposomes Remote Loaded with Glucocorticoids,” PLoS One, Vol. 6, No. 10, 2011, p. e25721.
- M. Taglietti, C. N. Hawkins and J. Rao, “Novel Topical Drug Delivery Systems and Their Potential Use in Acne Vulgaris,” Skin Therapy Letter, Vol. 13, No. 5, 2008, pp. 6-8.
- W. Geldenhuys, T. Mbimba, T. Bui, K. Harrison and V. Sutariya, “Brain-Targeted Delivery of Paclitaxel Using Glutathione-Coated Nanoparticles for Brain Cancers,” Journal of Drug Targeting, Vol. 19, No. 9, 2011, pp. 837- 845. doi:10.3109/1061186X.2011.589435
- O. Taratula, O. B. Garbuzenko, P. Kirkpatrick, I. Pandya and R. Savla, “Surface-Engineered Targeted PPI Dendrimer for Efficient Intracellular and Intratumoral siRNA Delivery,” Journal of Controlled Release, Vol. 140, No. 3, 2009, pp. 284-293. doi:10.1016/j.jconrel.2009.06.019
- V. V. Mody, R. Siwale, A. Singh and H. R. Mody, “Introduction to Metallic Nanoparticles,” Journal of Pharmacy and Bioallied Sciences, Vol. 2, No. 4, 2010, pp. 282-289. doi:10.4103/0975-7406.72127
- Brambilla, B. Le Droumaguet, J. Nicolas, S. H. Hashemi and L. P. Wu,“Nanotechnologies for Alzheimer’s Disease: Diagnosis, Therapy, and Safety Issues,” Nanomedicine, Vol. 7, No. 5, 2011, pp. 521-540. doi:10.1016/j.nano.2011.03.008
- A. Beck, J. F. Haeuw, T. Wurch, L. Goetsch and C. Bailly, “The Next Generation of Antibody-Drug Conjugates Comes of Age,” Discovery Medicine, Vol. 10, No. 53, 2010, pp. 329-339.
- M. Wu and P. D. Senter, “Arming Antibodies: Prospects and Challenges for Immunoconjugates,” Nature Biotechnology, Vol. 23, No. 9, 2005, pp. 1137-1146. doi:10.1038/nbt1141
- L. Nelson, “Antibody Fragments: Hope and Hype,” MAbs, Vol. 2, No. 1, 2010, pp. 77-83. doi:10.4161/mabs.2.1.10786
- J. M. Reichert, “Antibody-Based Therapeutics to Watch in 2011,” MAbs, Vol. 3, No. 1, 2011, pp. 76-99. doi:10.4161/mabs.3.1.13895
- J. C. Olivier, R. Huertas, H. J. Lee, F. Calon and W. M. Pardridge, “Synthesis of Pegylated Immunonanoparticles,” Pharmaceutical Research, Vol. 19, No. 8, 2002, pp. 1137-1143. doi:10.1023/A:1019842024814
- J. C. Olivier, “Drug Transport to Brain with Targeted Nanoparticles,” NeuroRx, Vol. 2, No. 1, 2005, pp. 108- 119. doi:10.1602/neurorx.2.1.108
- H. M. Blau and M. L. Springer, “Gene Therapy—A Novel Form of Drug Delivery,” The New England Journal of Medicine, Vol. 333, No. 18, 1995, pp. 1204-1207. doi:10.1056/NEJM199511023331808
- Y. Z. Chen, X. L. Yao, Y. Tabata, S. Nakagawa and J. Q. Gao, “Gene Carriers and Transfection Systems Used in the Recombination of Dendritic Cells for Effective Cancer Immunotherapy,” Clinical and Developmental Immunology, Vol. 2010, 2010, Article ID 565643, 12 Pages. doi:10.1155/2010/56564
- R. Li, L. Xie, Z. Zhu, Q. Liu and Y. Hu, “Reversion of pH-Induced Physiological Drug Resistance: A Novel Function of Copolymeric Nanoparticles,” PLoS One, Vol. 6, No. 9, 2011, p. e24172. doi:10.1371/journal.pone.0024172
- J. Thompson, D. Hansford, S. Higgins, C. Rostron and G. A. Hutcheon, “Evaluation of Ibuprofen-Loaded Microspheres Prepared from Novel Copolyesters,” International Journal of Pharmaceutics, Vol. 329, No. 1-2, 2007, pp. 53-61. doi:10.1016/j.ijpharm.2006.08.019
- S. Jhunjhunwala, G. Raimondi, A. W. Thomson and S. R. Little, “Delivery of Rapamycin to Dendritic Cells Using Degradable Microparticles,” Journal of Controlled Release, Vol. 133, No. 3, 2009, pp. 191-197. doi:10.1016/j.jconrel.2008.10.011
- S. Lee, S. C. Yang, C. Y. Kao, R. H. Pierce and N. Murthy, “Solid Polymeric Microparticles Enhance the Delivery of siRNA to Macrophages in Vivo,” Nucleic Acids Research, Vol. 37, No. 22, 2009, p. e145. doi:10.1093/nar/gkp758
- Allemann, J. Leroux and R. Gurny, “Polymeric Nanoand Microparticles for the Oral Delivery of Peptides and Peptidomimetics,” Advanced Drug Delivery Reviews, Vol. 34, No. 2-3, 1998, pp. 171-189. doi:10.1016/S0169-409X(98)00039-8
- P. Couvreur and F. Puisieux, “Nano- and Microparticles for the Delivery of Polypeptides and Proteins,” Advanced Drug Delivery Reviews, Vol. 10, No. 1993, pp. 141-162.
- S. Freiberg and X. X. Zhu, “Polymer Microspheres for Controlled Drug Release,” International Journal of Pharmaceutics, Vol. 282, No. 1-2, 2004, pp. 1-18. doi:10.1016/j.ijpharm.2004.04.013
- J. Panyam and V. Labhasetwar, “Biodegradable Nanoparticles for Drug and Gene Delivery to Cells and Tissue,” Advanced Drug Delivery Reviews, Vol. 55, No. 3, 2003, pp. 329-347. doi:10.1016/S0169-409X(02)00228-4
- Pinto Reis, R. J. Neufeld, A. N. J. Ribeiro and F. Veiga, “Nanoencapsulation I. Methods for Preparation of DrugLoaded Polymeric Nanoparticles,”Nanomedicine:Nanotechnology, Biology, and Medicine, Vol. 2, No. 1, 2006, pp. 8-21. doi:10.1016/j.nano.2005.12.003
- M. P. Desai, V. Labhasetwar, E. Walter, R. J. Levy and G.L. Amidon, “The Mechanism of Uptake of Biodegradable Microparticles in Caco-2 Cells Is Size Dependent,” Pharmaceutical Research, Vol. 14, No. 11, 1997, pp. 1568- 1573. doi:10.1023/A:1012126301290
- Y. Avnir, K. Turjeman, D. Tulchinsky, A. Sigal and P. Kizelsztein, “Fabrication Principles and Their Contribution to the Superior in Vivo Therapeutic Efficacy of Nano-Liposomes Remote Loaded with Glucocorticoids,” PLoS One, Vol. 6, No. 10, 2011, p. e25721.
- Y.S. Youn, Y.H. Bae, Perspectives on the past, present, and future of cancer nanomedicine, Adv. Drug Deliv. Rev. 130 (2018) 3–11, https://doi.org/10.1016/j. addr.2018.05.008.
- Pasut, Grand challenges in nano-based drug delivery, Front. Med. Technol. 1 (2019) 10–13, https://doi.org/10.3389/fmedt.2019.00001.
- S. Sharma, R. Parveen, B.P. Chatterji, Toxicology of nanoparticles in drug delivery, Curr. Pathobiol. Rep. 9 (2021) 133–144, https://doi.org/10.1007/s40139- 021-00227-z.
- A. Khalid, S. Persano, H. Shen, Y. Zhao, E. Blanco, J. Wolfram, W.C. Medicine-qatar, W.C. Medicine, W.C. Medicine, W.C. Medicine, HHS Public Access 14 (2018) 865–877, https://doi.org/10.1080/17425247.2017.1243527.Strategies.
- S.M. Zargar, D.K. Hafshejani, A. Eskandarinia, M. Rafienia, A.Z. Kharazi, A review of controlled drug delivery systems based on cells and cell membranes, J. Med. Signals Sens. 9 (2019) 181–189, https://doi.org/10.4103/jmss.JMSS_53_18.


 Nandkumar M. chaudhari*
Nandkumar M. chaudhari*
 Amrita M. Singh
Amrita M. Singh











 10.5281/zenodo.13337185
10.5281/zenodo.13337185