Abstract
Chromatography is a vital biophysical method that allows for the separation, identification, and filtering of components in order to conduct quantitative research. High efficiency, quicker separation speed, high flow rate, increased detection sensitivity, and cost savings are all advantages of novel chromatography methods. The goal of using chromatography, which is a technique for quantitative investigation separated from its partition, is to accomplish a satisfactory separation in a reasonable amount of time. The objective of chromatography is to analyze a sample qualitatively and quantitatively and its primary function is to clean and separate at least one component of the sample. It examines new and emerging chromatography techniques, as well as main standards. Phospholipids can be determined qualitatively and quantitatively using a variety of analytical procedures. Current new chromatographic methods are presented in this review.
Keywords
Chromatography, Purification, Novel, Analytical, Identification, Application.
Introduction
separating components of a mixture into distinct entities. It is derived from Greek word, Chromo-Colour, and Graphic-Writing. Chromatography was first utilized in Russia in 1900 by Russian botanist Mikhail Tsvet [1]. The kind of connection between stationary phase, mobile phase and substances contained in the mixture is the fundamental part is effective and division of particles from one phase to another. Chromatography strategies based on partition are very effective. The purpose of applying chromatography is a quantitative analysis apart from its separation. Stationary phase in chromatography is solid phase and liquid phase; liquid phase is coated on surface of solid phase [2]. Mobile phase flowing over the stationary phase is gas or liquid phase. Liquid chromatography is used when the mobile phase is liquid, while gas chromatography is used when the mobile phase is gas. Apart from its separation, it is utilized as a method of quantitative analysis to accomplish sufficient separation with0
in a molecular weight of protein. RNA, DNA particles, and viruses are purified using agar gel chromatography [3].
Types of Chromatography
- Gas chromatography
- Ion exchange chromatography
- High-performance liquid chromatography (HPLC)
- Paper chromatography
- Affinity chromatography
- Thin-layer chromatography (TLC)
- Column chromatography
Gas chromatography
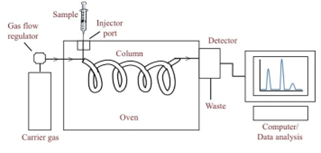
Gas chromatography is a separation technique in which the molecules are separated on the basis of their retention time depending on the affinity of the molecules to the stationary phase. The sample is either liquid or gas that is vaporized in the injection point.
Ion exchange chromatography
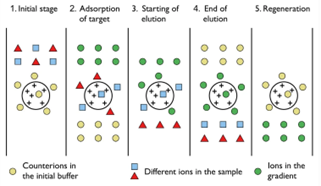
Ion exchange chromatography is the separation technique for charged molecules by their interaction with the oppositely charged stationary phase in the form of ion-exchange resin.
High-performance liquid chromatography (HPLC)
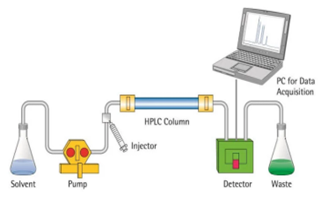
High-performance liquid chromatography is a modified form of column chromatography where the components of a mixture are separated on the basis of their affinity with the stationary phase.
Paper chromatography
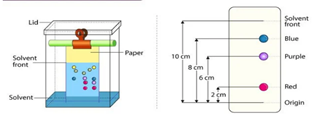
Paper chromatography is a separation technique where the separation is performed on a specialized paper.
Affinity chromatography
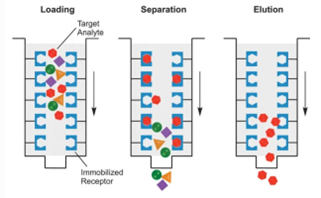
Affinity chromatography is a separation technique where the components of a mixture are separated based on their affinity towards the stationary phase of the system.
Thin-layer chromatography (TLC)
Thin-layer chromatography is a separation technique where the stationary phase is applied as a thin layer on a solid support plate with a liquid mobile phase.
Column chromatography
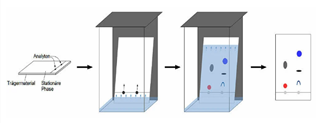
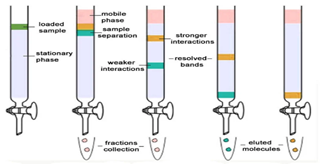
Column chromatography is the separation technique where the components in a mixture are separated on the basis of their differential adsorption with the stationary phase, resulting in them moving at different speeds when passed through a column. It is a solid-liquid chromatography technique in which the stationary phase is a solid & mobile phase is a liquid or gas.
Application
In inorganic chemistry:
- The chromatographic separation of anions can be effectively carried out by using ion pair chromatography
- or the separation of cations ,sulphonated inert polymer resins have been used.
In organic chemistry:
Separationof lipids:Lipids range from hydrocarbons and wax esters to highly polar sugar of phosphoric acid conataining glycol and phospholipids. The polar head group interact with a polar stationary phase
Pharmaceutical Applications
- To control drug stability.
- Tablet dissolution study of pharmaceutical dosages form.
- Pharmaceutical quality control.
Environmental Applications
- Detection of phenolic compounds in drinking water.
- Bio-monitoring of pollutants.
Applications in Forensics
- Quantification of drugs in biological samples.
- Identification of steroids in blood, urine etc.
- Forensic analysis of textile dyes.
- Determination of cocaine and other drugs of abuse in blood, urine etc.
Food and Flavour
- Measurement of Quality of soft drinks and water.
- Sugar analysis in fruit juices.
- Analysis of polycyclic compounds in vegetables.
- Preservative analysis.
Applications in Clinical Tests
- Urine analysis, antibiotics analysis in blood.
- Analysis of bilirubin, biliverdin in hepatic disorders.
- Detection of endogenous Neuropeptides in extracellular fluid of brain etc.
- Advantages
- Chromatography is the simplest method for the separation of components.
- Chromatography can be controlled by a single person.
- Components from a complex mixture can separate using chromatography method.
- The small amount of sample (Gram, PPM, and ng/ml) can be detected by this chromatography.
- It is a rapid and precise method of separation.
- Very few sample volume/quantity is required for analysis.
- It works on a broad range of samples.
- In some chromatography techniques, it is possible to separate different components of a complex mixture.
- Continuous operation possible on a large scale
- The separation of components can be achieved in different methods.
- Different kinds of chromatography tools are available for the separation of the compounds. Some examples are liquid chromatography, Gas chromatography, High-performance liquid chromatography, Column Chromatography, Solid Chromatography, Liquid-liquid Chromatography, Solid-liquid Chromatography,Thin Layer Chromatography.
Disadvantages
- The chromatography equipment can only be operated by a trained person.
- Chromatography instruments are expensive.
- An error occurs due to the overloading of the samples.
- Chromatography equipment must be handled with care because of these parts are expensive and sensitive.
- Some of the chromatography techniques require more solvent to separate the analytes.
- Periodic maintenance and parts need to be changed
- Some of the chromatography methods require high power consumption.
- High operational pressure may be required to achieve efficient separation.
Recent Developments
LC-MS
LC-MS is a technique routinely used in sample analysis that combines liquid chromatography (LC) with mass spectrometry (MS). Extremely sensitive modern MS has helped LC-MS replace several immunoassays. LC-MS has helped improve the efficiency of drug discovery due to its excellent sensitivity and specificity. The technique can be combined with stable isotope dilution for precise and reproducible assays.
GC-MS
Gas chromatography–mass spectrometry (GC-MS) is a hybrid analytical technique that couples the separation capabilities of GC with the detection properties of MS to provide a higher efficiency of sample analyses. While GC can separate volatile components in a sample, MS helps fragment the components and identify them on the basis of their mass. GC-MS provides enhanced sample identification, higher sensitivity, an increased range of analyzable samples, and faster results, which enable a whole new range of applications for GC-MS in several areas.
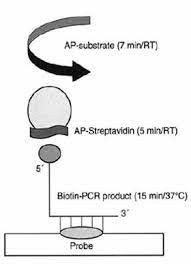
Paper Chromatography Hybridization Assay (Pacha)
It is a DNA hybridization method based on chromatographic relocation of DNA on a nitrocellulose strip that passes through an immobilized test area. PACHA is easier to utilize and has more strength. When PCR enhanced target DNA is introduced to one end of a nitrocellulose strip, narrow powers immobilized in the response zone allow DNA to migrate to the strip far edge [4]. It is located in the middle of a section of a small human papilloma infection that is in the process of hybridization. Hybridization proficiency is limited in this method by the hybridization arrangement stream speed and the volume of improved DNA migrating over immobilized test. This novel technique assures successful hybridization in a variety of tests and appears to be superior to the already available strong stage hybridization method (Fig.2) [5, 6].
Hydrophobic- Interaction Chromatography (HIC)
HIC works on the same principles as ion-exchange and size exclusion chromatography. When sample molecules with hydrophobic and hydrophilic districts are applied to an HIC section in a high salt buffer, the test solutes are less likely to survive. Hydrophobic regions that get revealed are absorbed by the media as salvation fades. Less salt is predicted to enhance binding as the particle becomes more hydrophobic. [7, 8].
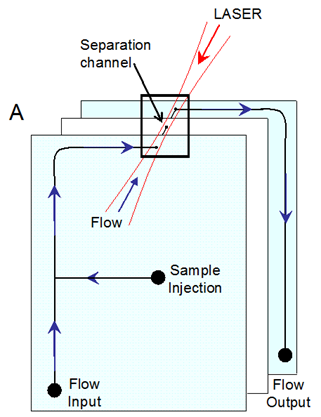
4.5 Optical force chromatography
The balance between optical and liquid drag power acting on particles is required for optical force chromatography [9]. In small size study spanning several logical fields, the use of laser light as a tool for controlling tiny molecule suspension for natural, thermodynamic, and micro fluidic purposes has been realized. For molecular partition, optical chromatography involves freely centering a laser into a liquid stream flowing in the opposite direction of the laser propagation path (Fig.4) [10, 11].
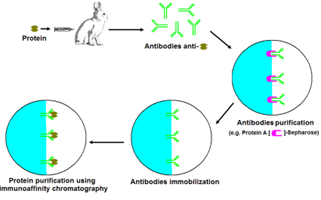
High performance and immuno–affinity chromatography (HPAC) AND (IAC)
High- performance affinity chromatography (HPAC) In an HPAC system, it is a method in which an organically related ligand is used as the stationary phase. HPAC has been used in recent years to investigate the relationship between drugs, chemicals, and other substances and serum proteins. This method is used for detaching or quantifying specialists in complicated samples [12]. Immuno-affinity chromatography (IAC) Immuno-affinity chromatography combines the use of liquid chromatography with the specific restriction of antibodies or other specialized agents. It is used for filtering and centralization of data prior to further analysis using another method. Antibody or antibody-related reagent is the stationary phase. Filtration, immune depletion, direct sample analysis, immunoassay, and joint examination procedures are all approaches that utilize it (Fig.5) [13].
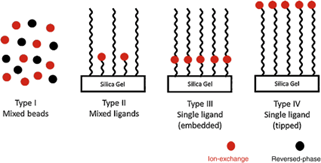
Mixed - Mode Chromatography (MMC)
Multimodal chromatography is another name for mixed mode chromatography. Mixed mode media combine complementing chromatography methods inside a same medium, reducing the number of segment processes necessary throughout the purifying process. In a single ligand, mixed mode components contribute affinity, such as binding and selectivity (Fig.6) [14, 15].
Dye ligand chromatography
It is a purification method with a high degree of selectivity and purification rates. The capacity of multiple compounds to purine nucleotides for cibacton blue is required for improvement of this procedure [16]. The planar ring structure with negatively charged bunches resembles NAD design. The adsorbed proteins are isolated from the column under suitable PH conditions, elution with high ionic strength arrangements and using particles that trade adsorbent properties (Fig.7) [17, 18].
CONCLUSION
A new chromatographic technique has been devised that is simple, precise, quick, accurate, and repeatable. As in the case of herbal pigments, chromatographic methods were utilized to separate compounds depending on their colour. The new chromatographic approach enhances productivity, resolution speed, time consumption, and sensitivity. In the future, three-dimensional chromatographic separation will be possible, which will be useful in a variety of industries. Three-dimensional separation methods may provide a route to large peak capacities. Multidimensional separation is attractive and is commonly employed to separate complicated sample.
REFERENCE
- Nishendu P. Nadpara*, Rakshit V. Thumar, Vidhi N. Kalola, Parula B. Patel, QUALITY BY DESIGN (QBD) : A COMPLETE REVIEW, Int. J. Pharm. Sci. Rev. Res., 17(2), 2012; n? 04, 20-28
- Woodcock J, The concept of pharmaceutical quality. American Pharmaceutical Review, 7(6), 2004, 10–15
- Q9: Quality Risk Management. ICH Harmonized TripartiteGuidelines. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticalsfor Human Use, 2006.
- Q10:Pharmaceutical Quality System, ICH TripartiteGuidelines. International Conference on Harmonization ofTechnical Requirements for Registration of Pharmaceuticals for Human Use, 2007.
- Lionberger RA, Lee LS, Lee L, Raw A ,Yu LX, Quality bydesign: Concepts for ANDAs, The AAPS Journal, 10, 2008,268–276
- AV Ganorkar, KR Gupta. Analytical Quality by Design: A Mini Review. Biomed J Sci & Tech Res 1(6)- 2017. BJSTR.
- Q8 (R1): Pharmaceutical Development, Revision 1, ICH Harmonized Tripartite Guidelines, International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, 2007.
- Asmita Mahapatra,Subramani Nainar Meyyanathan;Analyatical Quality By Design-A Review,JSS college of pharmacy ooty,Ind Res J Pharm & Sci,2020:7(2)
- Delasko, J., Cocchetto, D. M., & Burke, L. B. (2005). Target product profile: beginning drug development with the end in mind. Update.
- Food and Drug Administration CDER. (2007). Draft Guidance for Industry and Review Staff: Target Product Profile- A Strategic Development Tool (March 2007).
- Food and Drug Administration Office of Generic Drugs. (2006). Model Quality Overall Summary
- N. V. V. S. S. Raman, Useni ReddyMallu, and Hanimi Reddy Bapatu, Analytical Quality by Design Approach to Test Method Development and Validation in Drug Substance Manufacturing, Journal of chemistry, 2015
- Q9: Quality Risk Management. ICH Harmonized Tripartite Guidelines. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, 2006.
- Roy S (2012) “Quality by Design-Holistic concept of concept of building quality in pharmaceuticals”. Int. J Pham Biomed Res 3:100-108.
- Ranga S, Jaimini M, Sharma SK, Chauhan BS, Kumar A. A review on design of experiment. International Journal of Research and Reviews in Pharmacy and Applied science. 2013; 3(6): 867-82.
- 16.USP Validation and verification expert panel lifecycle managmen of analyatical procedure Method and development,procedure performance Qualification and procedure performance verification ,http://www.usp.org/sites/default/files/usp_pdf/EN/USPNF/revisions
- Burnham R. How to Select Design of Experiments Software. www.sixsigma.com. Accessed 10 March 2016.
- Yu LX, Amidon G, Khan MA, Hoag SW, Polli J, Raju GK, et al. Understanding pharmaceutical Quality by design. AAPS Journal. 2014; 16:771-83.
- . 19 Sangshetti JN, Zaheer Z, Mahaparale PR and Chitlange SS. Quality by design (QbD) in pharmaceuticals. Unique Publication, Aurangabad. 1st edition; 2015: 20
- 20 Gawade A, Chemate S and Kuchekar A. Pharmaceutical Quality by Design: A New Approach in Product Development. Research and Reviews: Journal of Pharmacy and Pharmaceutical Sciences. 2013; 2:5-11.
- 21.Kadam VR, Patil MP, Pawar VV, Kshirsagar S. A review on: Quality by design (QbD). Asian J Res Pharm Sci 2017;7:197-204.
- Aksu B, Ye?en G. Global regulatory perspectives on quality by design in pharma manufacturing. Pharm Qual Des 2019;2019:19-41.


 Kavita Suresh Hamare*
Kavita Suresh Hamare*
 R. B. Darade
R. B. Darade











 10.5281/zenodo.11261617
10.5281/zenodo.11261617