Abstract
In the pharmaceutical field, the development and assessment of non-effervescent tablets are essential for ensuring the controlled release of active ingredients such as Pregabalin. Non-effervescent tablets are specifically designed to release medication gradually and consistently over an extended period, which is particularly advantageous for drugs like Pregabalin utilizing HPMC K100M and Carbopol 971P as release-retarding polymers. The objective of this study was to create and assess controlled release matrix tablets containing Pregabalin. The tablets were produced through wet granulation and assessed for different parameters like angle of repose, bulk density, true density, compressibility index, Hausner ratio and evaluation of various physical and chemical properties like- swelling characteristics, in vitro buoyancy, adhesion retention, and in vitro drug release, drug content. The optimized formulation exhibited the desired drug release profile and was determined to be comparable to the product Lyrica CR. Stability tests performed on the optimized formulation demonstrated that it remained physically and chemically stable under both accelerated and real-time storage conditions.
Keywords
Pregabalin, Controlled release, non-effervescent tablets, HPMC K100M(Hydroxypropyl Methylcellulose), Carbopol 971P
Introduction
Oral drug administration is the primary method for administering drugs for systemic effects, with 90% of all drugs used to produce systemic effects being administered by this route [1]. Sustained release dosage forms are designed to enhance the pharmaceutical activity of the drug, reduce dosage frequency and side effects, and improve patient convenience. Gastro retentive drug delivery systems are designed to be retained in the stomach for extended periods, allowing for sustained and prolonged drug input to the upper part of the gastrointestinal tract [2]. These systems are particularly useful for drugs acting locally in the stomach, having an absorption window, unstable in the intestinal or colonic environments, or having low solubility at high pH values. Techniques for forming successful gastro retentive drug delivery systems include floating dosage forms, low density systems, raft systems, bio adhesive or muco-adhesive systems, high density systems, super-porous hydrogel, and magnetic systems. These systems offer advantages such as sustained blood levels, attenuation of adverse effects, and improved patient compliance. Sustained release (SR) drug release systems are a crucial aspect of oral medication delivery due to their ease of production and cost. These systems can be classified into dissolution-controlled release systems, osmotic pump systems, and erosion-controlled release systems. Dissolution-controlled systems control the rate of drug dissolution in the intestinal juice, while diffusion-controlled systems involve reservoir devices and matrix devices. Osmotic pump systems use a constant inflow of water across a semipermeable membrane into a reservoir containing an osmotic agent, allowing constant release unaltered by the gastrointestinal tract environment. Active pharmaceutical ingredients (APIs) are chemical-based compounds produced mainly in the USA, Europe, China, and India. Modern medicines consist of two main components: chemically and biologically active components [3]. Excipients in dosage forms play a significant role in the final product, including manufacturing, stability, dose uniformity, effective delivery, and organoleptic properties [4]. Pharmaceutical excipients are typically included in dosage forms in larger quantities than the API, making up to 90% of the total mass/volume of medicinal products [5]. Pregabalin is a calcium channel blocker that inhibits calcium influx into nerve cells, reducing neuronal hyper-excitability and contributing to its analgesic effects [6]. It is used for treating seizure disorders, fibromyalgia, and neuropathic pain. The pharmacological activity of pregabalin is known for its stereo isomeric structure, (S)-enantiomer, which has gained importance in the pharmaceutical industry [7]. Neuropathy pain (NP) is characterized by sensory pathway dysfunction and can be of high intensity and long duration, impacting mood, personality, and social relationships. Pregabalin is effective for both central and peripheral NP and achieves rapid pain reduction. Recently, the US FDA approved once-daily sustained release tablets for post-therpetic neuralgia. However, the product must be taken after an evening meal and a high calorie diet to ensure the same extent of absorption. The current study aims to develop oral controlled release matrix tablets of Pregabalin, using hydrophilic matrices like carbopol 971P, HPMC K100M and Crospovidone.
MATERIALS AND METHODS
Materials
Formulation and Preparation of Pregabalin Non-Effervescent Tablets
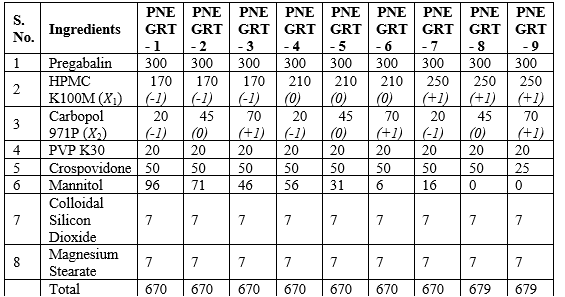
Tablets containing 300 mg of Pregabalin were prepared by wet granulation technique. For each formulation, Pregabalin, Hydroxy Propyl Methyl Cellulose (HPMC) K100M, Carbopol 971P, Povidone, Crospovidone and Mannitol were separately passed through #40 mesh sieve. Pregabalin, Hydroxy Propyl Methyl Cellulose (HPMC) K100M, Carbopol 971P and Povidone (presieved through #40 mesh) were geometrically mixed and then the blend was passed through #40 mesh followed by manually blending homogeneously. Moisture content of powder blend was measured using moisture analyzer. The blend was then granulated manually with Isopropyl Alcohol. The wet mass was passed through sieve #12 and dried in tray dryer at 50oC until moisture content of dried granules was similar to that of powder blend. The dried granules were passed through #18 mesh and then blended with Crospovidone and Mannitol (presieved through #40 mesh). The blend was further lubricated by mixing with Magnesium Stearate and Colloidal Silicon Dioxide (presieved through #80 mesh). Lubricated blend was compressed in Rotary Tablet Compression Machine using round, 10 mm diameter, concave D-tooling punch.
Table 2: Composition of Pregabalin Non-effervescent Gastro-retentive Tablets (PNEGRT)
Pre-Formulation Studies
First the dosage formulation was studied to generate information useful in formulating acceptable, safe, stable. Different parameters like angle of repose, bulk density, true density, compressibility index, Hausner ratio were evaluated. Angle of repose is evaluated from funnel method. Bulk density is evaluated from bulk density apparatus.
In vitro Dissolution Studies
The in-vitro dissolution studies were performed using the USP-II (Paddle) dissolution apparatus (Lab India) at 50 rpm. Dissolution media used was 6.0 pH Methanol buffer maintained at 37±0.5oC. A 5 ml was withdrawn at specific time intervals and same volume of fresh medium was replaced. The withdrawn samples were diluted with pH 6.0, filtered and analyzed on chromatogram using pH 6.0 methanol buffer as a blank. Percentage cumulative drug release was calculated.
FINDINGS
Compatibility study
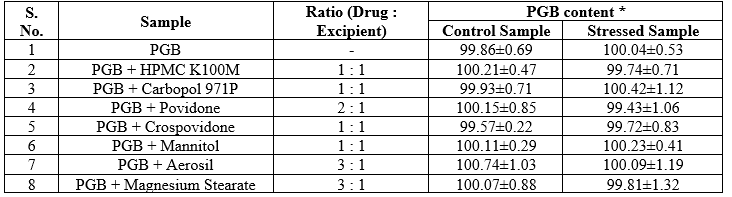
In isothermal stress testing, it was observed that there was no physical change (color and appearance) as well as drug content of stressed and control samples. As seen from Table 2, the change in drug content of IST samples was less than 1?ter 3 weeks of storage under stressed conditions.
Table 2: Results of isothermal stress testing study of Pregabalin after 3 weeks of storage at stressed conditions
Pre-formulation evaluation
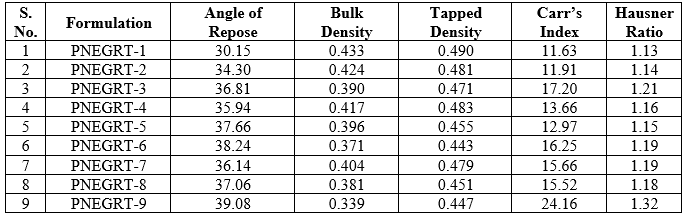
The powder blend of each formulation of Pregabalin tablet prior to compression were evaluated for flow and compressibility using different parameters. The angle of repose of various powders ranged from 30.15o – 39.08o and exhibited moderate flow. The values obtained for Carr’s Index and Hausner Ratio of various formulations ranged from 11.63 – 24.16 % and 1.13 – 1.32 respectively. It was observed that powder blend containing low amount of HPMC K100M and Carbopol 971P polymers had better flow properties and compressibility characteristics. With increase in amount of matrix forming polymers, especially Carbopol 971P, the flowability of powder was reduced. Similar observation was reported by Birsan et al. and Omeh et al [8]. Reduced flowability with increased Carbopol 971P could be due to its very fine particle size and static charge, thus rendering poor flowability [9]. The result of pre-formulation parameters is shown in table 3.
Table 3: Result of pre-granulation powder and dried granules during tablet preparation of various criteria:
Post compression evaluation
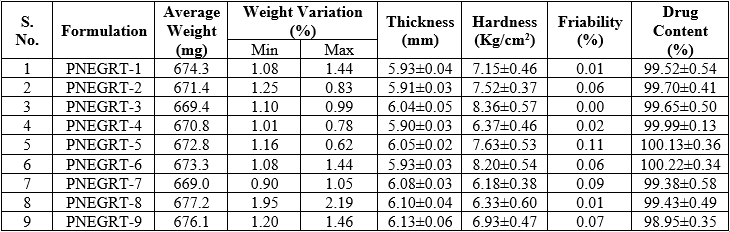
Controlled-release non-effervescent gastro-retentive tablets of PGB were developed using release-retarding gel-forming polymer(s) like HPMC K100M and Carbopol 971P. Mannitol was used as a water-soluble filler. Crospovidone was used as swelling agent which is insoluble in water and can enhance water uptake in hydrophilic matrix system. As seen in Table 4, all the tablet formulations showed acceptable physicochemical properties and complied with the pharmacopoeial specifications for weight variation, friability and drug content. The weight of the tablets ranged from 669.0 – 677.2 mg. The tablets were compressed at a thickness of about 6 mm and the thickness was found in the range of 5.90 ± 0.03 – 6.13 ± 0.06 mm. Hardness of tablet formulations was in the range of 6.18 ± 0.38 – 8.36 ± 0.57 Kg/cm2. Gambhire et al. studied the influence of tablet hardness on the floating lag time and the release of diltiazem hydrochloride from floating matrix tablet and concluded that tablet hardness had no (or little) effect on the drug release profile but was a determining factor in buoyancy of the tablets [10]. Friability of all formulations was less than 1%, indicating good mechanical resistance. Drug content was found to be uniform for all batches; percentage of drug content ranged from 98.95 ± 0.35% – 100.22 ± 0.34%.
Table 4: Result of post-compression evaluation of tablets
Swelling ability
The swelling indices obtained from the aqueous medium uptake studies in of all the formulations is shown in Table 5 and Figure 1. Swelling index of formulations after 24 hours in 0.06N HCl (HydrochloricAcid) with stirring at 50 rpm (Rotation Per Minute) ranged from 3.52 ± 0.13 to 4.98 ± 0.06. The hydration ability of the formula is important as it has impact on tablet buoyancy, adhesion ability of swellable polymers, HPMC K100M and Carbopol 971P, in contact with the test fluid, and drug release kinetics. Crospovidone, a super disintegrating agent, which causes immediate disintegration of tablets due to its swelling nature when in contact with aqueous medium, is used as swelling agent in the formulations. Because of swelling character as well as wicking nature of Crospovidone, it is utilized in preparation of floating, swellable, extended-release tablets [11]. Percentage of Crospovidone was kept constant in formulae PNEGRT-1 to 8 and lower in formula PNEGRT-9.
Table 5: Result of Swelling Ability of Pregabalin Gastro-retentive Tablets
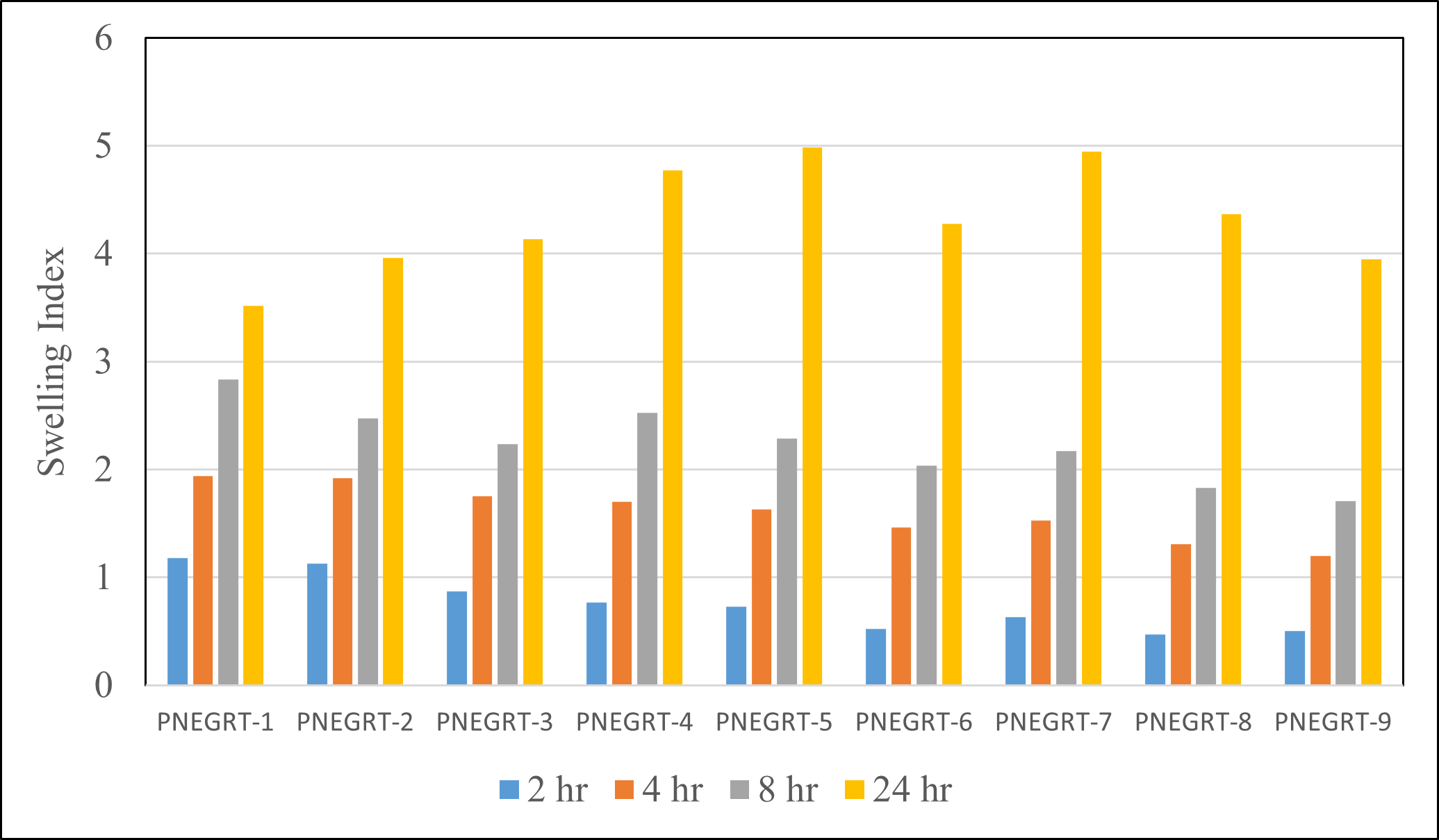
Figure 1: Swelling behaviour of Pregabalin Non-effervescent Gastro-retentive Tablets
In vitro drug release study
In this study, two different polymers including Carbopol 971P and HPMC K100M were chosen to combine with Crospovidone to explore their controlled release capability. The in-vitro release of Pregabalin from prepared matrix tablet formulations was mainly affected by the concentration of matrix forming hydrophilic polymers and depends on swelling behaviour of the formulations. All formulations PNEGRT-1 to 9 showed sustained release of drug. The dissolution rate of Pregabalin was highest in PNEGRT-1 and maximum retardation of release was observed in PNEGRT-9. As shown in Figure 2, the release rate of pregabalin tended to increase with the decrease of HPMC K100M and Carbopol 971P content. During dissolution, the drug diffusion from the core to dissolution media is inhibited by a viscously hydrated layer. The larger amount of release retarding polymers in the formulation implies a thicker diffusion barrier and slower dissolution rate, which concur well with previous results for effervescent and non-effervescent floating dosage forms obtained by Garg and Gupta [12]. The upper surface of the floating tablet is not available for dissolution, and it must be wet from the lower surface to enable drugs to diffuse out. Hence, the tablets inherently release drug more slowly when they are floating than when they are immersed in the medium using sinkers. This tendency was also reported by Hwang et al [13]. The drug release from PNEGRT followed the biphasic model, with an initial higher release attributed to the drug molecules on the peripheral surface of the tablet which initially come in contact with the dissolution medium followed by slower release due to thick diffusion barrier created by swelling of matrix-forming release-retarding polymers.
Table 6: Results of in vitro drug release from Pregabalin Non-effervescent Gastro-Retentive Tablets

Drug release kinetic studies
The model dependent approaches evaluated for the drug release kinetics were zero order, first order, Higuchi, Hixson-Crowell and Korsmeyer-Peppas. The correlation coefficients of Zero order, First order and Higuchi kinetics of all formulations were determined and depicted. The best fit model was determined on the basis of correlation coefficients (R2). The release from PNEGRT formulations was found to follow Higuchi diffusion model with R2 value close to 1. From the plot of log Mt/M? vs log t (Korsmeyer–Peppas model), the value of release exponent (n), which indicates the mechanism of drug release, was determined and depicted in Table 7. The n value of entire formulations of PNEGRT was within the limit of 0.545 – 0.631 which indicates that the drug release followed anomalous diffusion (0.5 < n>
Table 7: In vitro drug release model-dependent kinetics

32 randomized full factorial design: Response surface methodology
A 32 randomized full factorial design was employed for investigating the effect of two independent process variables (factors), i.e., amount of HPMC K100M and amount of Carbopol 971P on the dependent variables like time required to release 25% of drug (t25), 50% of drug (t50) and 80% of drug (t80) and optimization of non-effervescent gastro-retentive tablets of Pregabalin. The purpose of creating the Response Surface Methodology (RSM) was to establish a method for the accurate modulation of these drug release characteristics. 3-level full factorial design method was chosen for response surface methodology as it allowed the exploration of quadratic response surfaces and a polynomial model was constructed which helped in optimizing the process for the fabrication of PNEGRT formulation.
Model fit study
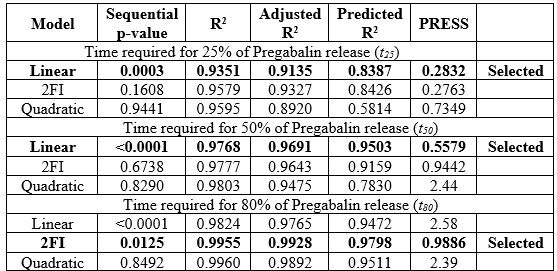
From the model fit analysis, it was found that linear model fit well for t25 and t50 responses, and 2FI (two-factor interaction) model fit well for t80 response based on all the model terms mentioned in Table 8. The sequential p-value for linear model is significant for t25 and t50 and insignificant for 2FI and quadratic model, whereas both linear and 2FI model were significant for t80. Model with R2 close to 1 and predicted R2 being in reasonable agreement with the adjusted R2 as well as minimum PRESS (Predicted Residual Error Sum of Squares) was determined to be the best fit.
Table 8: Model fit summary to evaluate models
Effects of Formulation variables on t25 (Y1), t50 (Y2) and t80 (Y3)
Stat-Ease 360 software generated contour plots and three-dimensional response surface plots are presented in Figure 2 for Y1 (t25), Y2 (t50) and Y3 (t80). 3D surface plots show that a downward trend of the wire mesh was at lower level (?1) and the upward trend was at higher level (+1) of both HPMC K100M and Carbopol 971P concentration. It is predicted that the dependent variables (t25, t50 and t80) are directly proportional to both the independent variables.

Figure 3: Contour plots (A) and Three-dimensional response surface plots (B) showing the effect of polymers on t25 (1), t50 (2) and t80 (3).
Optimization of PNERGT by Response Surface Methodology
The criteria for dependent variables t25, t50 and t80 were set to 1.6 hr, 5 hr and 12.1 hr as per the in vitro dissolution profile graph of reference product shown in Figure 4. The recommended amount of the independent variables was calculated by the Stat-Ease 360 software which has the highest desirability near to 1.0. The optimum values of selected variables obtained using the software were 196.4 mg of HPMC K100M and 62.8 mg of Carbopol 971P with the desirability of 0.978 as shown in Desirability plot for numerical optimization and Overlay plot for graphical optimization (Figure 3 A and B). The final composition of optimized formulation (PNEGRT-O) is shown in Table 9.

Figure 4: Optimization of Pregabalin non-effervescent gastro-retentive tablets (A) Desirability plot; (B) Overlay plot
Table 9: Composition of optimized Pregabalin Non-effervescent Gastro-retentive Tablet (PNEGRT-O)

Stability Study
The optimized formulation (PNEGRT-O) was evaluated for 2 months of storage at accelerated stability condition (40 ?C ± 2 ?C and 75% ± 5% RH) and real time stability condition (30 ?C ± 2 ?C and 75% ± 5% RH). The tablets were evaluated for physical appearance, hardness, friability, floating characteristics, drug content and in vitro drug release. Stability studies showed the tablets complies with the physical properties and no any significant change was observed in drug content and dissolution profile indicating that the formulation is physically and chemically stable.
Summarizing the findings
Formulations of non-effervescent tablets for controlled realease of pregabalin using polymers were possible. Drug content of all nine formulations were uniform ranged from 98.85±0.35% to 100.22± 0.34%. Crospovidone was utilized in preparation of floating, swellable, extended released- tablets, besides crospovidone, HPMC K100M & carbopol 971P also have swelling ability. Floating lag time increased in carbopol 971P polymer due to increase in hardness of tablet. Tablets adhered to agar plates and retention period by different formulations ranged from 12.59 ± 1.46 min to 55.02 ± 4.47. The dissolution rate of PNEGRT-1 was highest and maximum retardation of release was observed in PNEGRT-9, the release rate of pregabalin tended to increase with the decrease of HPMC K100M & carbopol content. Optimized formulation PNEGRT-0 was same as criteria of physiochemical properties. The optimized formulation (PNEGRT-O) was evaluated for 2 months of storage at accelerated stability condition (40 ?C ± 2 ?C and 75% ± 5% RH) and real time stability condition (30 ?C ± 2 ?C and 75% ± 5% RH). The tablets were evaluated for physical appearance, hardness, friability, floating characteristics, drug content and in vitro drug release. Stability studies showed the tablets complies with the physical properties and no any significant change was observed in drug content and dissolution profile indicating that the formulation is physically and chemically stable. Hence, formulations were successful and may lead to enhance patient compliance by reducing doses frequency.
CONCLUSION
In conclusion, the preparation and evaluation of non-effervescent tablets for controlled release of Pregabalin require precision and expertise. By understanding the nuances of formulation and employing stringent quality control measures, pharmacists can create effective medications that meet the needs of patients. Mastering this process is key to unlocking the full potential of controlled-release drug delivery systems.
REFERENCES:
- S Hitesh N. Jain, Parth P. Patel, Millin R. Globel, and Umesh M. Upadhyay. Formulation and Evaluation of Pregabalin Sustained Release Tablets, Indo Am J. P. Sci, 2016; 3(11)
- Vinit Sharma, Shalini Sharma, Sukhbir Lal Khokra, Ram Kumar Sahu, Rajendra Jangde, Jagdish Singh. Formulation, development and evaluation of Pregabalin sustained release matrix tablets Scholars Research Library Der Pharmacia Lettre 2011: 3 (5) 326-331
- V. Kumar et al., “Active pharmaceutical ingredient (API) chemicals: a critical review of current biotechnological approaches,” Bioengineered, vol. 13, no. 2, pp. 4309–4327, 2022.
- K. H. Kim et al., “Development of a novel controlled-release tablet of pregabaliFormulation variation and pharmacokinetics in dogs and humans,” Drug Des. Devel. Ther., pp. 445–456, 2020.
- J. Van der Merwe, J. Steenekamp, D. Steyn, and J. Hamman, “The role of functional excipients in solid oral dosage forms to overcome poor drug dissolution and bioavailability,” Pharmaceutics, vol. 12, no. 5, p. 393, 2020.
- E. Ben?Menachem, “Pregabalin pharmacology and its relevance to clinical practice,” Epilepsia, vol. 45, pp. 13–18, 2004.
- Y. U. Dongre and O. C. Swami, “Sustained-release pregabalin with methylcobalamin in neuropathic pain: an Indian real-life experience,” Int. J. Gen. Med., pp. 413–417, 2013.
- R. C. Omeh et al., “DESIGN AND EVALUATION OF ALBENDAZOLE FLOATING TABLETS FOR ENHANCED GASTRO-RETENTIVE AND SUSTAINED RELEASE EFFECTS,” 2022.
- M. Chavanpatil, P. Jain, S. Chaudhari, R. Shear, and P. Vavia, “Development of sustained release gastroretentive drug delivery system for ofloxacin: in vitro and in vivo evaluation,” Int. J. Pharm., vol. 304, no. 1–2, pp. 178–184, 2005.
- U. Bertram and R. Bodmeier, “In situ gelling, bioadhesive nasal inserts for extended drug delivery: in vitro characterization of a new nasal dosage form,” Eur. J. Pharm. Sci., vol. 27, no. 1, pp. 62–71, 2006.
- R. Garg and G. D. Gupta, “Preparation and evaluation of gastroretentive floating tablets of silymarin,” Chem. Pharm. Bull. (Tokyo), vol. 57, no. 6, pp. 545–549, 2009.
- M. N. Gambhire, K. W. Ambade, S. D. Kurmi, V. J. Kadam, and K. R. Jadhav, “Development and in vitro evaluation of an oral floating matrix tablet formulation of diltiazem hydrochloride,” Aaps Pharmscitech, vol. 8, pp. E166–E174, 2007.
- K.-M. Hwang, C.-H. Cho, N .-T. Tung, J.-Y. Kim, Y.-S. Rhee, and E.-S. Park, “Release kinetics of highly porous floating tablets containing cilostazol,” Eur. J. Pharm. Biopharm., vol. 115, pp. 39–51, 2017.


 Chandani Rajak*
Chandani Rajak*










 10.5281/zenodo.13770648
10.5281/zenodo.13770648