Abstract
The study explores the formulation and applications of nanoemulsions to enhance the delivery of hydrophobic drugs, which often suffer from poor water solubility and low bioavailability. Nanoemulsions, with droplet sizes ranging from 20 to 200 nm, provide increased surface area, improved solubility, and controlled drug release. They are stabilized using surfactants, co-surfactants, and polymers. Preparation techniques include high-energy methods like ultrasonication and low-energy methods like spontaneous emulsification. Nanoemulsions have wide-ranging applications in oral, topical, parenteral, and pulmonary drug delivery. Despite their potential, challenges like scalability and stability require further research to optimize their use in pharmaceutical and industrial applications
Keywords
Nanoemulsions, Hydrophobic Drug Delivery, Bioavailability Enhancement, Controlled Drug Release, Pharmaceutical Application, Drug Stability.
Introduction
Nanoemulsions are finely dispersed mixtures of two immiscible liquids, such as oil and water, stabilized by surfactants. Unlike traditional emulsions, nanoemulsions have droplet sizes ranging from 20 to 200 nanometers, making them visually transparent or translucent. These small droplet sizes lead to enhanced stability against sedimentation or creaming, and they often exhibit unique properties compared to macroemulsions, such as increased bioavailability and faster absorption in biological systems.[2] Nanoemulsions are widely used in various industries, including pharmaceuticals, cosmetics, and food. In drug delivery, for example, they offer an effective way to enhance the solubility of poorly water-soluble drugs, improving their therapeutic efficacy. In cosmetics, nanoemulsions enable the delivery of active ingredients into deeper layers of the skin. Their small size and high surface area also make them ideal for controlled release formulations, improving the effectiveness and duration of active compounds. Despite their advantages, the formulation of nanoemulsions can be complex, often requiring high-energy methods such as ultrasonication or microfluidization to achieve stable dispersions. [3]Their stability and effectiveness depend heavily on the selection of surfactants, co-surfactants, and processing conditions. Nanoemulsions offer several advantages over conventional emulsions, particularly in terms of their optical clarity, enhanced stability, and ability to encapsulate both hydrophilic and lipophilic compounds.[2] Their small droplet size results in increased surface area, which facilitates more efficient interactions between the dispersed and continuous phases. In addition to pharmaceuticals, nanoemulsions have shown great promise in the food industry, where they are used to improve the solubility of flavor oils, vitamins, and antioxidants. Their ability to stabilize sensitive bioactive compounds while maintaining desirable sensory properties makes them valuable in developing functional foods and beverages.[1] Nanoemulsions can also be used to reduce the fat content in food products without compromising texture or taste. Despite their growing applications, the challenges of producing large-scale, cost-effective, and stable nanoemulsions remain, requiring ongoing research and optimization of formulation techniques.[3]
Hydrophobic Drugs
Hydrophobic drugs are a class of pharmaceutical compounds characterized by their poor solubility in water, stemming from their nonpolar molecular structure. The term “hydrophobic” derived from the Greek words “hydro” (water) and “phobos” (fear), refers to the tendency of these drugs to repel water. Unlike hydrophilic drugs, which dissolve easily in aqueous environments, hydrophobic drugs are more soluble in nonpolar substances such as lipids or organic solvents. This property significantly impacts their absorption, distribution, metabolism, and excretion in the body, making drug formulation and delivery challenging. Water solubility is a critical determinant in a drug’s bioavailability-the proportion of the drug that enters circulation and reaches the target tissue. Hydrophobic drugs, due to their low water solubility, often exhibit poor bioavailability, especially when administered orally. They struggle to dissolve in the aqueous fluids of the gastrointestinal tract, limiting their absorption into the bloodstream. As a result, ensuring sufficient therapeutic levels of the drug at the target site can be difficult. However, despite these challenges, hydrophobic drugs play a vital role in treating diseases such as cancer, immune disorders, and infections. One of the key advantages of hydrophobic drugs lies in their affinity for lipid environments, which allows them to cross cell membranes more easily. Cell membranes are composed of lipid bilayers, and hydrophobic drugs can passively diffuse through these membranes to reach intracellular targets. This is particularly useful in treating conditions where the drug needs to act within cells, such as in certain types of cancer or central nervous system diseases. Nonetheless, their poor solubility in water-based biological fluids necessitates the development of advanced drug delivery systems. Pharmaceutical scientists have developed various formulation strategies to enhance the solubility and bioavailability of hydrophobic drugs. Techniques such as nanoparticle carriers, liposomal delivery systems, and the use of solubilizing agents have proven effective in improving the dissolution rate, absorption, and overall therapeutic impact of these drugs. For example, nanoparticles can encapsulate hydrophobic drugs, enhancing their solubility and ensuring more efficient delivery to target tissues. Lipid-based formulations and self-emulsifying drug delivery systems (SEDDS) are also used to improve the transport of these drugs in the body.
Challenges In Delivery of Hydrophobic Drugs
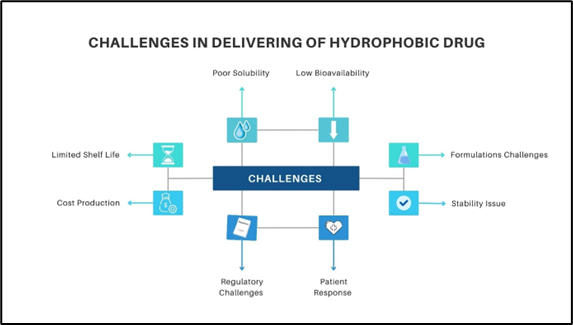
Fig.1. Challenges In Delivering of Hydrophobic Drugs
The delivery of hydrophobic drugs presents several challenges primarily due to their poor solubility in water-based environments like blood, which limits their absorption and bioavailability. As a result, these drugs often struggle to reach therapeutic concentrations in the bloodstream, leading to low bioavailability. Additionally, hydrophobic drugs are prone to rapid clearance through liver metabolism, reducing their effectiveness. To compensate, higher doses may be required, increasing the risk of toxicity and adverse side effects. Inconsistent absorption in the gastrointestinal tract further complicates drug delivery, leading to variable effectiveness. The formulation of stable and effective drug delivery systems for hydrophobic drugs is technically challenging, often necessitating the use of special carriers like liposomes, micelles, or nanoparticles. Moreover, these drugs may encounter difficulty in crossing biological barriers, such as the blood-brain barrier or skin, which limits their therapeutic applications. Finally, hydrophobic drugs may also require specific storage conditions to maintain their stability and prevent degradation. Advanced drug delivery systems are often employed to address these challenges.
Formulation Components for Nanoemulsions
- Oil Phase
The oil phase is critical as it forms the dispersed phase in oil-in-water (O/W) nanoemulsions and solubilizes hydrophobic drugs or active ingredients. The choice of oil influences the drug release rate, bioavailability, and stability of the nanoemulsion.
Types of Oils:
Triglycerides: Medium-chain triglycerides (MCTs), such as caprylic/capric triglycerides, are frequently used due to their digestibility and ability to solubilize lipophilic drugs.
Vegetable Oils: Oils such as soybean oil, olive oil, and sunflower oil are used for their compatibility with biological systems and natural origin.
Essential Oils: These oils, like eucalyptus or peppermint oil, are used for their therapeutic properties.
Esters: Esters such as isopropyl myristate and ethyl oleate are synthetic oils used to enhance the solubilization of active ingredients and ensure better skin penetration in topical applications.[4-5]
- Aqueous Phase
The aqueous phase typically consists of water or a water-based buffer. It forms the external continuous phase in O/W nanoemulsions and acts as the medium in which the oil droplets are dispersed. In water-in-oil (W/O) nanoemulsions, the aqueous phase forms the internal phase.[6]
Buffer Solutions: Phosphate-buffered saline (PBS) or other buffers can be used to maintain the pH stability of the system, which is particularly important for biological applications like drug delivery. Deionized Water: Pure deionized water is used to avoid contamination or reactions from ions present in regular tap water.
- Surfactants
Surfactants are amphiphilic molecules that reduce interfacial tension between oil and water, stabilizing the nanoemulsion. The choice of surfactant impacts droplet size, surface charge, and the overall stability of the system.
Non-Ionic Surfactants: Non-ionic surfactants like Tween 80 (polysorbate 80) or Span 20 (sorbitan monolaurate) are popular due to their low toxicity and ability to stabilize nanoemulsions. They are often used in food and pharmaceutical formulations.
Ionic Surfactants: These include cationic (e.g., cetyltrimethylammonium bromide) and anionic surfactants (e.g., sodium dodecyl sulfate). They are less commonly used in biomedical applications due to their potential for irritation but can offer excellent emulsification properties.
Natural Surfactants: Lecithin, a phospholipid found in soy or egg yolk, is often used for its biocompatibility, especially in pharmaceutical and cosmetic formulations.[7-8]
- Co-Surfactants
Co-surfactants help enhance the flexibility of the surfactant film at the oil-water interface, thus improving the emulsification process and stabilizing the nanoemulsion. They also help to reduce the amount of surfactant needed.[9]
Examples of Co-Surfactants:
Alcohols: Ethanol, propylene glycol, and polyethylene glycol (PEG) are frequently used to adjust the hydrophilic-lipophilic balance (HLB) and improve the stability of nanoemulsions.
Short-Chain Alcohols: These, such as butanol or pentanol, can fluidize the interfacial layer and enhance droplet formation.
- Stabilizers/Polymers
Stabilizers prevent the coalescence of droplets by creating steric hindrance and increasing the viscosity of the continuous phase. They are particularly important for enhancing the long-term physical stability of nanoemulsions.[9]
Polymers: Natural polymers such as xanthan gum, alginates, or synthetic polymers like polyvinyl alcohol (PVA) are added to prevent the aggregation of droplets by increasing viscosity and imparting steric stability.
Colloidal Stabilizers: Solid particles such as silica or clay can also be used to stabilize nanoemulsions through Pickering stabilization, where solid particles adsorb at the oil-water interface.
- Active Ingredient (Drug or Bioactive Compound)
The active ingredient in nanoemulsions is usually a hydrophobic or poorly water-soluble compound that benefits from being solubilized in the oil phase. Nanoemulsions are used to improve the bioavailability of such compounds due to the small droplet size, which enhances absorption.
Types of Active Ingredients:
Pharmaceuticals: Lipophilic drugs like paclitaxel, cyclosporine, or curcumin are solubilized in the oil phase of nanoemulsions to improve their bioavailability and therapeutic efficacy.
Nutraceuticals: Bioactive compounds like omega-3 fatty acids, vitamins (e.g., vitamin D, E), and antioxidants (e.g., resveratrol) are often delivered using nanoemulsions in food or dietary supplements.
Cosmetic Actives: Ingredients like retinoids, ceramides, or essential oils used for skin care are frequently formulated in nanoemulsions for enhanced penetration and stability.
- Other Additives
pH Adjusters: Substances like citric acid or sodium hydroxide may be added to adjust the pH, ensuring the stability of the active ingredients.
Preservatives: Antimicrobial agents like parabens or benzyl alcohol are included to prevent microbial growth in water-rich formulations.
Antioxidants: To prevent oxidative degradation of oils or active ingredients, antioxidants like vitamin E (tocopherol) or ascorbyl palmitate are added.
Preparation Methods For Nanoemulsions
The preparation of nanoemulsions can be classified into high-energy and low-energy methods
- High-Energy Methods
High-energy methods involve the application of mechanical energy to break down larger droplets into nanosized droplets.
-
- High-Pressure Homogenization
Process:
The oil phase is combined with the aqueous phase and surfactants to form a pre-emulsion. This mixture is then subjected to high pressure (typically 1000 to 2000 bar) in a homogenizer. The high shear forces generated during homogenization break down the oil droplets into nano-sized particles. The process can be repeated multiple times to achieve the desired droplet size.[10]
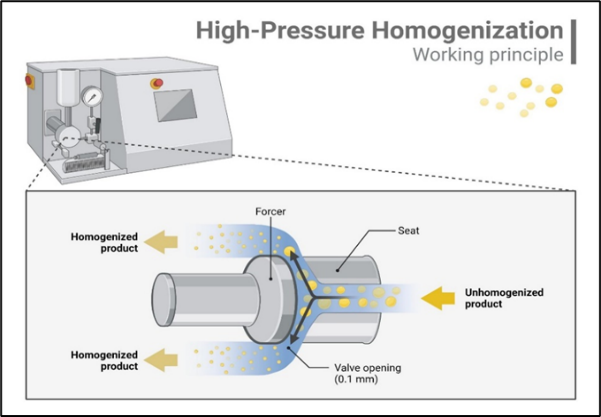
Fig.2. High-Pressure Homogenization
Advantages:
- Produces very stable nanoemulsions with small droplet sizes.
- Can be scaled up for industrial applications.
- Requires specialized equipment and energy-intensive processes.
- Widely used in pharmaceuticals, cosmetics, and food industries.
- Ultrasonication
Process:
This method utilizes ultrasonic waves to create high-frequency sound waves in the liquid phase. The cavitation effect leads to the formation and rapid collapse of bubbles, generating intense shear forces that break up larger droplets into nano-sized droplets. Parameters such as power, frequency, and duration of treatment can be adjusted to optimize droplet size.[11]
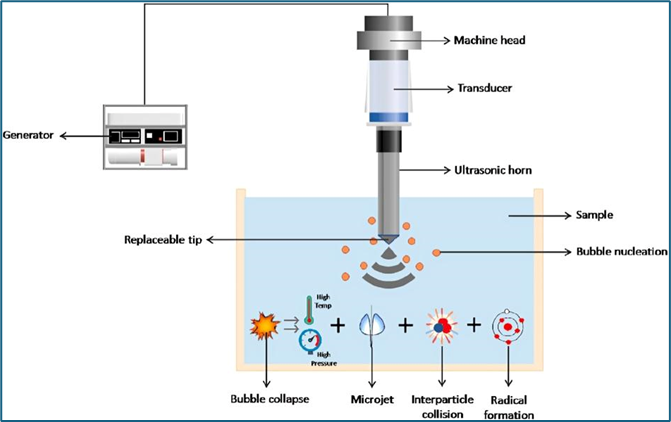
Fig.3. Ultrasonication
Advantages:
- Effective for producing smaller droplet sizes with controlled size distribution.
- Less equipment-intensive compared to high-pressure homogenization.
- Heat generated during ultrasonication can affect temperature-sensitive compounds.
- Commonly used in drug delivery systems, food emulsions, and cosmetic formulations.
- Low-Energy Methods
Low-energy methods rely on spontaneous emulsification or phase transitions and generally require less mechanical energy.
-
- Phase Inversion Temperature (PIT)
Process: This method involves mixing the oil and aqueous phases with surfactants and heating the mixture above a specific temperature known as the phase inversion temperature. At this temperature, the emulsification properties of the surfactants change, allowing for efficient emulsification upon cooling. The cooled mixture forms a stable nanoemulsion.[12]
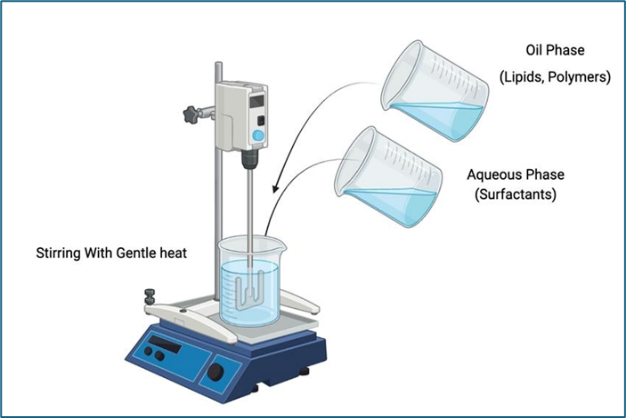
Fig.4. Phase Inversion Temperature
Advantages:
- Requires less energy compared to high-energy methods.
- Produces smaller droplets and can enhance the stability of the emulsion.
- Limited to certain types of surfactants and formulations.
- Useful in pharmaceutical formulations and food products for encapsulating flavors or bioactive compounds.
- Spontaneous Emulsification
Process:
In this technique, the oil phase is gradually added to the aqueous phase containing surfactants at room temperature. The system is then gently mixed, and under specific conditions (like certain surfactant concentrations),emulsification occurs spontaneously. This method often relies on the surfactants’ ability to lower the interfacial tension between oil and water phases.[13]
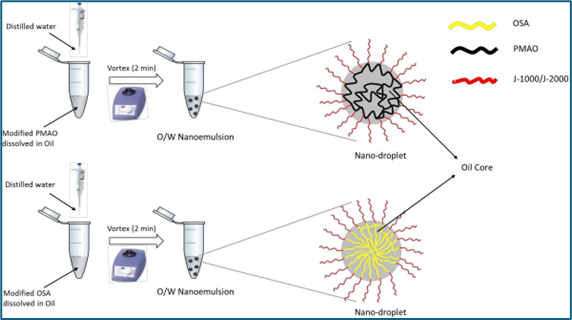
Fig.5. Spontaneous Emulsification
- Simple and easy to execute without the need for high energy input.
- Suitable for thermolabile compounds as it does not involve heating.
- Droplet size may be less uniform compared to high-energy methods.
- Frequently used in food technology and pharmaceutical applications for improving bioavailability.
Characterization Of Nanoemulsions
Characterization of Nanoemulsions is a critical step in understanding their behavior, performance, and suitability for various applications. Here is a more detailed breakdown of the various characterization methods used for nanoemulsions:
1. Droplet Size and Size Distribution
The droplet size is one of the most important properties in nanoemulsions, affecting their stability, appearance, and behavior.
Dynamic Light Scattering (DLS): This technique measures the intensity fluctuations of scattered light from particles in suspension. It provides the hydrodynamic diameter, which is the size of the droplets in their natural environment, and the polydispersity index (PDI), which gives information about the uniformity of the droplet sizes. Smaller droplets (typically between 20-200 nm) lead to nanoemulsions with better physical stability.
Transmission Electron Microscopy (TEM): TEM gives direct visual confirmation of droplet size and morphology at a very high resolution (down to nanometers). Samples must be prepared carefully, often by freezing or drying, to prevent distortion. TEM images can reveal the spherical shape of droplets and can confirm DLS measurements.
Scanning Electron Microscopy (SEM): Like TEM, SEM provides high-resolution images of nanoemulsion droplets, but it primarily shows the surface features. This technique is often used for dried samples and provides complementary information to TEM.
Atomic Force Microscopy (AFM): AFM can also be used to image nanoemulsion droplets, providing topographical information. It can show both size and surface characteristics.
Nanoparticle Tracking Analysis (NTA): NTA directly visualizes and tracks particles in a liquid suspension, measuring size distribution based on Brownian motion. It complements DLS and provides information on the concentration of particles in the system.
2. Zeta Potential
The zeta potential measures the surface charge of the nanoemulsion droplets. It indicates the electrostatic repulsion between droplets, which is crucial for stability.
Measurement Process: A sample of the nanoemulsion is placed in a cell where an electric field is applied. The velocity at which the charged droplets move in response to the field is measured. The zeta potential is calculated from this movement.
Significance: A high positive or negative zeta potential (typically above ±30 mV) indicates strong repulsive forces between droplets, which helps prevent coalescence and enhances the long-term stability of the emulsion. A low zeta potential suggests that the system may be prone to flocculation or coalescence.
3. Viscosity
The viscosity of a nanoemulsion can affect its stability, flow properties, and suitability for applications like drug delivery, cosmetics, or food.
Rheological Measurements: A rheometer is used to measure the viscosity and shear-thinning behavior of nanoemulsions. Rheology can provide insight into the internal structure and interactions between droplets, particularly under different shear conditions (such as mixing, pumping, or application on the skin).
Shear-Thinning Behavior: Many nanoemulsions exhibit non-Newtonian flow, where viscosity decreases with increased shear. This property is advantageous in products like lotions or sprays, where easy flow under pressure but thickening at rest is desired.
4. Optical Properties
Nanoemulsions tend to be translucent or transparent due to the small size of the droplets, which scatter little light.
UV-Visible Spectroscopy: This technique assesses the light scattering properties of nanoemulsions, particularly in the visible range. The transparency or turbidity of a nanoemulsion can indicate the uniformity of droplet size distribution and overall quality.
Turbidity and Clarity: Highly uniform and small-sized nanoemulsions appear more transparent because they scatter less light. Turbidity measurements provide quantitative data on the extent of light scattering.
5. Stability Tests
Nanoemulsion stability is a key factor in their formulation, affecting how they behave during storage and use.
Centrifugation: Accelerated stability testing can be performed by subjecting nanoemulsions to high-speed centrifugation, which simulates the effect of gravity over time. Phase separation, creaming, or sedimentation can be observed, which reveals potential stability issues.
Temperature Cycling: Nanoemulsions are subjected to extreme temperatures in a cyclical pattern to assess their stability under thermal stress. This test simulates real-life temperature fluctuations that products might experience during transportation or storage.
Freeze-Thaw Testing: This test involves freezing and thawing the nanoemulsion repeatedly to assess its resilience to phase changes. Nanoemulsions with poor stability may exhibit coalescence, creaming, or phase separation after freeze-thaw cycles.
Storage Stability: Long-term stability studies are conducted at room temperature and elevated temperatures to observe changes in physical appearance, droplet size, or phase separation over time. This helps determine the shelf life of the nanoemulsion.
6. Surface Tension and Interfacial Properties
The interfacial tension between the oil and water phases is critical in determining the ease of emulsification and the stability of the nanoemulsion.
Surface Tension Measurement: Techniques like pendant drop or Wilhelmy plate methods are used to measure the surface tension of the continuous phase. Lower surface tension indicates that the emulsifier is effective at reducing the energy required to form droplets.
Interfacial Tension: The interfacial tension between the oil and water phases can be measured to understand the stability of the oil droplets in the water phase. Lower interfacial tension generally correlates with better emulsification and long-term stability.
7. pH and Electrical Conductivity
These parameters provide insight into the ionic environment of the nanoemulsion, particularly if it contains ionizable components.
pH Measurement: Monitoring the pH of the nanoemulsion is important for its stability and function, especially in drug delivery or food applications, where the pH can affect the solubility and release of active ingredients.
Conductivity: Electrical conductivity measurements help determine whether the nanoemulsion is oil-in-water (O/W) or water-in-oil (W/O). O/W emulsions tend to have higher conductivity due to the continuous aqueous phase, while W/O emulsions have lower conductivity.
8. Encapsulation Efficiency and Drug Release (if applicable)
For nanoemulsions used in drug delivery, the encapsulation efficiency and release profile of the active ingredient are key performance indicators.
Encapsulation Efficiency: This is measured by separating the free (unencapsulated) drug from the nanoemulsion and calculating the percentage of the drug that is successfully encapsulated within the droplets. Techniques like ultrafiltration or centrifugation followed by analysis using HPLC or UV-Vis spectroscopy can be used.
In Vitro Drug Release: The release of the encapsulated drug from the nanoemulsion can be monitored using techniques like dialysis or diffusion cells. The release profile helps predict the behavior of the drug in vivo, particularly its bioavailability and controlled release properties.
Mechanisms Of Drug Delivery Enhancement
The mechanism of drug delivery enhancement by nanoemulsions, particularly in terms of bioavailability, involves several factors:
1. Improved Solubility and Dissolution Rate
One of the main reasons nanoemulsions enhance bioavailability is their ability to solubilize hydrophobic drugs within the oil phase. This improves the drug's solubility and allows it to be readily dispersed in biological fluids. Since most drugs exhibit poor water solubility, especially hydrophobic compounds, nanoemulsions can enhance their dissolution rate significantly.
Mechanism: Nanoemulsions increase the surface area available for dissolution due to the very small droplet size. This improves drug absorption in the gastrointestinal tract by ensuring that more of the drug is in a solubilized, readily absorbable form.[22]
2. Enhanced Permeability and Absorption
Nanoemulsions improve drug permeability across biological membranes, such as the intestinal epithelium. The small size of the droplets allows them to interact more effectively with the epithelial cells, facilitating the passage of the drug across the intestinal barrier.
Mechanism: The oil droplets in nanoemulsions can facilitate passive diffusion of the drug across lipid-rich cell membranes. The small droplet size and uniform distribution in the gastrointestinal fluids enhance interaction with the intestinal mucosa, leading to improved drug absorption.[18]
3. Protection from Enzymatic Degradation
Many drugs are susceptible to enzymatic degradation in the gastrointestinal tract, which limits their bioavailability. Nanoemulsions can protect drugs from being degraded by enzymes (e.g., proteases, lipases) before they are absorbed.
Mechanism: Encapsulation of drugs within the oil droplets of the nanoemulsion protects them from the harsh gastrointestinal environment. This protection ensures that more of the drug reaches systemic circulation intact, thereby improving bioavailability.[15]
4. Increased Lymphatic Absorption
Nanoemulsions can enhance the absorption of drugs via the lymphatic system, especially for lipophilic drugs. The lymphatic route bypasses the liver's first-pass metabolism, which is a significant pathway for drug degradation when administered orally.
Mechanism: Lipophilic drugs solubilized in the oil phase of nanoemulsions are absorbed into the lymphatic system via chylomicron formation. Since the lymphatic route bypasses the liver, nanoemulsions help avoid first-pass metabolism, allowing a larger fraction of the drug to reach systemic circulation.[20]
5. Prolonged Residence Time in the Gastrointestinal Tract
Nanoemulsions can prolong the residence time of the drug in the gastrointestinal tract, enhancing the opportunity for absorption.
Mechanism: The small size of the nanoemulsion droplets increases their interaction with the mucosal surfaces in the gut, which can improve adhesion and retention. The enhanced retention time allows for more effective absorption, increasing the overall bioavailability of the drug.[17]
6. Reduced Inter-Subject Variability
Nanoemulsions offer more predictable and consistent drug absorption across different patient populations.
Mechanism: Due to the improved solubility and absorption mechanisms provided by nanoemulsions, they can reduce the variability in bioavailability that often occurs with poorly soluble drugs. This results in more uniform drug plasma levels across different patients.[21]
7. Improved Stability of Drugs
Nanoemulsions can enhance the chemical and physical stability of drugs, particularly those prone to degradation (e.g., oxidation or hydrolysis), by protecting them within the oil phase.
Mechanism: Encapsulation in the oil droplets of nanoemulsions can shield the drug from exposure to degrading factors like light, oxygen, and moisture. This increases the drug’s stability, reducing degradation before absorption and enhancing bioavailability.[16]
8. Facilitating Paracellular Transport
In some cases, nanoemulsions can modulate tight junctions between epithelial cells, allowing for paracellular transport of drugs, which is typically a limiting factor for hydrophilic drugs.
Mechanism: Certain components of the nanoemulsion, such as surfactants or emulsifiers, may temporarily open tight junctions, allowing the drug to pass through paracellular routes. This can enhance the absorption of hydrophilic drugs and increase their bioavailability.[21]
9. Bioadhesive Properties
Some nanoemulsions possess bioadhesive properties that help them adhere to mucosal surfaces, such as in the gastrointestinal tract, nasal cavity, or oral cavity, leading to improved local or systemic absorption.
Mechanism: The bioadhesive properties of nanoemulsions, facilitated by certain polymers or surfactants, prolong the contact time between the drug and the absorption site, leading to enhanced drug uptake and increased bioavailability.[15]
10. Control of Drug Release
Nanoemulsions can also be engineered to provide controlled or sustained drug release, which can enhance bioavailability by maintaining therapeutic drug levels over time.
Mechanism: The drug is released gradually from the oil droplets, maintaining a consistent concentration in the bloodstream. This controlled release helps to avoid spikes and troughs in drug levels, ensuring more stable and effective bioavailability.[20]
- Benefits of Nanoemulsions on Bioavailability:
Solubilization of poorly soluble drugs: Increases the drug’s solubility and dissolution rate.
Improved membrane permeability: Enhances absorption through biological membranes.
Protection from degradation: Safeguards drugs from enzymatic or chemical degradation in the GI tract.
Lymphatic absorption: Bypasses first-pass metabolism, particularly for lipophilic drugs.
Prolonged residence time: Increases contact time with absorption sites in the gastrointestinal tract.
Reduced variability: Offers more consistent drug absorption across individuals.
Bioadhesive properties: Increases retention time at mucosal surfaces, enhancing absorption.
Applications In Hydrophobic Drug Delivery
- Nanoemulsions encapsulate hydrophobic drugs in their oil phase, significantly improving their solubility in aqueous environments, which is crucial for drugs with poor water solubility.
- By enhancing solubility and reducing the size of drug particles to nanoscale, nanoemulsions allow for more efficient absorption in the body, leading to higher bioavailability.
- The nanometer-sized droplets in nanoemulsions can be tailored for slow and controlled drug release, allowing for prolonged therapeutic effects and reducing the need for frequent dosing.
- Nanoemulsions provide a protective environment for hydrophobic drugs, preventing degradation from environmental factors such as light, heat, and oxidation, thereby improving the stability and shelf life of the drug.
- Nanoemulsions can be functionalized with ligands or antibodies that allow them to target specific cells or tissues, reducing systemic side effects and increasing the drug concentration at the desired site.
- Nanoemulsions can be administered via oral, intravenous, topical, ocular, or pulmonary routes, offering flexibility in the delivery method depending on the drug and therapeutic requirement.
- Due to their ability to improve drug targeting and control drug release, nanoemulsions minimize the systemic distribution of the drug, thus reducing off-target effects and toxicity.
- Nanoemulsions are relatively easy to prepare using high-energy methods (e.g., ultrasonication, high-pressure homogenization) or low-energy methods, making them scalable for industrial applications.[23]
Role Of Nanoemulsion In Different Route Of Administration
- Oral Administration: Nanoemulsions enhance the solubility and absorption of poorly water-soluble drugs, improving bioavailability and protecting drugs from degradation in the stomach.
- Topical/Transdermal: They increase drug penetration through the skin barrier and provide controlled release, improving the effectiveness of both hydrophilic and lipophilic drugs.
- Parenteral (Intravenous): Nanoemulsions can deliver drugs rapidly to target tissues, reduce systemic toxicity, and provide a rapid onset of action, often through targeted delivery.
- Ophthalmic: In eye applications, nanoemulsions enhance drug retention and bioavailability while being non-irritating and comfortable for the user.
- Pulmonary: Nanoemulsions can be used in inhalers or nebulizers to improve drug deposition in the lungs, allowing deep penetration and faster absorption for respiratory treatments.
- Nasal: They facilitate quick absorption through the nasal mucosa, offering a rapid onset of action and bypassing first-pass metabolism for systemic effects.
- Buccal and Sublingual: Nanoemulsions enhance drug absorption through the mucosal tissues in the mouth, providing fast action and avoiding first-pass metabolism.
- Rectal: They improve drug absorption through the rectal mucosa, useful for both local and systemic effects, especially in patients unable to take oral medications.
- Vaginal: Nanoemulsions provide enhanced delivery for local treatments, such as infections or inflammation, and can offer sustained drug release.
- Otic (Ear): They improve drug penetration in the ear, useful for treating infections or inflammation.
- Intrathecal: Used for targeting the central nervous system, nanoemulsions can deliver drugs directly into the cerebrospinal fluid, bypassing the blood-brain barrier.
- Intra-articular: Nanoemulsions can be injected into joints, providing sustained release and improved retention for treating conditions like arthritis.
Future Directions Of Nanoemulsion In Drug Delivery
- Personalized Medicine
Nanoemulsions can be tailored for personalized drug delivery, offering patient-specific treatments. Advances in nanotechnology will allow for the creation of customizable formulations based on an individual’s genetic profile, disease condition, and drug response.[24]
- Targeted Drug Delivery
Future nanoemulsions will be designed for enhanced targeted delivery to specific tissues or cells, such as tumors or inflamed tissues. By incorporating ligands, antibodies, or targeting molecules into nanoemulsion formulations, drugs can be delivered directly to the site of action, reducing side effects and increasing therapeutic efficacy.[24]
- Combination Therapies
Nanoemulsions can facilitate the co-delivery of multiple drugs or therapeutic agents in a single formulation, enabling combination therapies. This is especially beneficial in treating complex diseases like cancer, where combining chemotherapy, immunotherapy, or other treatments can improve outcomes.[24]
- Responsive and Smart Nanoemulsions
The development of “smart” nanoemulsions that respond to specific stimuli (e.g., pH, temperature, enzymes) is a growing area of interest. These stimuli-responsive nanoemulsions could release drugs in a controlled manner based on changes in the biological environment, improving the precision and effectiveness of treatment.[25]&[26]
- Gene and Nucleic Acid Delivery
Nanoemulsions are being explored as carriers for gene therapy, RNA-based treatments (like siRNA or mRNA), and other nucleic acid therapies. Their ability to protect and deliver genetic material to target cells could revolutionize treatments for genetic disorders, cancer, and viral infections.[26]
- Oral Nanoemulsions for Hydrophobic Drugs
Future advancements will focus on improving the stability and bioavailability of oral nanoemulsions, particularly for poorly water-soluble drugs. This could significantly improve the treatment of diseases where oral delivery is preferred but challenging due to poor solubility and absorption.[27]
- Nanotoxicology and Safety Improvements
While nanoemulsions offer significant advantages, concerns regarding the toxicity of nanomaterials remain. Future research will likely focus on improving the biocompatibility and safety of nanoemulsions, particularly by using natural, biodegradable materials as carriers and surfactants.[28]
- Nanoemulsions for Vaccines and Immunotherapy
Nanoemulsions are being explored as adjuvants and carriers for vaccines and immunotherapies. They have the potential to enhance immune responses and facilitate the delivery of antigens, making vaccines more effective. This is especially relevant in the development of next-generation vaccines and treatments for infectious diseases like COVID-19.[25]
- Improved Manufacturing Techniques
The scalability and reproducibility of nanoemulsion production remain challenging. Future advancements in manufacturing technologies, such as microfluidics and continuous production systems, will allow for more efficient, cost-effective production of nanoemulsions with consistent quality.[25]&[26]
- Longer Shelf Life and Stability
Researchers are working on enhancing the stability and shelf life of nanoemulsions. Advances in formulation techniques, such as the use of solid lipid carriers or freeze-dried nanoemulsions, could extend the lifespan of these formulations, making them more commercially viable.[28]
- Transdermal and Topical Applications
Nanoemulsions hold great promise in transdermal and topical drug delivery. Future formulations may focus on improving the penetration of nanoemulsions through the skin barrier for conditions like pain management, dermatological diseases, and localized treatments.[27]
- Sustained and Controlled Drug Release
Advances in nanoemulsion design will focus on developing formulations that offer sustained and controlled release of drugs over time. This could reduce the frequency of dosing and improve patient compliance, especially for chronic conditions.[29]
CONCLUSION
The preparation of nanoemulsions presents a robust platform for enhancing the delivery of hydrophobic drugs. By addressing the challenges of poor solubility, absorption, and bioavailability, nanoemulsions allow for improved therapeutic efficacy and controlled drug release. Future research should focus on optimizing scalable preparation techniques and exploring the potential for targeted drug delivery using nanoemulsion systems.
REFERENCES
- McClements, D. J. (2011). Edible nanoemulsions: Fabrication, properties, and functional performance. Soft Matter, 7(6), 2297-2316. https://doi.org/10.1039/C0SM00549E
- Mason, T. G., Wilking, J. N., Meleson, K., Chang, C. B., & Graves, S. M. (2006). Nanoemulsions: Formation, structure, and physical properties. Journal of Physics: Condensed Matter, 18(41), R635-R666. https://doi.org/10.1088/0953-8984/18/41/R01
- Gupta, A., Eral, H. B., Hatton, T. A., & Doyle, P. S. (2016). Nanoemulsions: Formation, properties, and applications. Soft Matter, 12(11), 2826-2841. https://doi.org/10.1039/C5SM02958A
- Lawrence, M. J., & Rees, G. D. (2000). Microemulsion-based media as novel drug delivery systems. Advanced Drug Delivery Reviews, 45(1), 89-121. https://doi.org/10.1016/S0169-409X(00)00123-8
- Kaur, L., Cannella, D., Sosa-Hernandez, J. E., & Parisi, O. I. (2019). Nanoemulsions: Preparation methods and applications in agriculture. Sustainable Agriculture Reviews, 41, 257-285. https://doi.org/10.1007/978-3-030-05871-5_7
- Gupta, A., Eral, H. B., Hatton, T. A., & Doyle, P. S. (2016). Nanoemulsions: Formation, properties and applications. Soft Matter, 12(11), 2826-2841. https://doi.org/10.1039/C5SM02681E
- McClements, D. J. (2012). Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter, 8(6), 1719-1729. https://doi.org/10.1039/C2SM06977B
- Anton, N., & Vandamme, T. F. (2011). Nano-emulsions and micro-emulsions: Clarifications of the critical differences. Pharmaceutical Research, 28(5), 978-985. https://doi.org/10.1007/s11095-011-0360-8
- Tadros, T., Izquierdo, P., Esquena, J., & Solans, C. (2004). Formation and stability of nano-emulsions. Advances in Colloid and Interface Science, 108, 303-318. https://doi.org/10.1016/j.cis.2004.01.005
- Kaur, G., & Khurana, S. (2019). Development of nanoemulsion for enhancing the solubility of lipophilic drugs. International Journal of Pharmaceutical Sciences and Research, 10(2), 692-703. https://doi.org/10.13040/IJPSR.0975-8232.10(2).692-03
- Raghavendra, K. P., Bhat, S. R., & Sandeep, K. S. (2019). Formulation and characterization of a nanoemulsion for the enhanced oral bioavailability of curcumin. Journal of Drug Delivery Science and Technology, 52, 433-442. https://doi.org/10.1016/j.ddst.2019.05.005
- Kaur, G., Kaur, M., & Kaur, R. (2020). Formulation and characterization of nanoemulsion for enhanced solubility and bioavailability of simvastatin. Asian Journal of Pharmaceutics, 14(2), 159-167. https://doi.org/10.22377/ajp.v14i2.3076
- Anupama, M., Muthusamy, S., & Kumar, K. M. (2018). Formulation and evaluation of curcumin nanoemulsions for enhancing oral bioavailability. Asian Journal of Pharmaceutics, 12(4), 1-8. https://doi.org/10.22377/ajp.v12i4.2372
- Anton, N., Benoit, J. P., & Saulnier, P. (2008). Design and production of nanoparticles formulated from nanoemulsion templates—a review. Journal of Controlled Release, 128(3), 185-199. https://doi.org/10.1016/j.jconrel.2008.02.007
- Ghosh, S. K., & Satpathy, S. (2020). Nanoemulsions: A versatile drug delivery system. Current Pharmaceutical system,26(38),4912-4923. https://doi.org/10.2174/1389201026666200312161312
- Mason, T. G., & Wilking, J. N. (2011). Nanoemulsions: A new class of materials for the pharmaceutical industry. Nature Reviews Drug Discovery, 10(2), 118-126. https://doi.org/10.1038/nrd.2010.196
- Nagarwal, R. C., & Singh, P. (2012). Nanoemulsion: A review. International Journal of Pharmacy and Pharmaceutical Sciences, 4(2), 1-6.
- Sharma, A., & Pathak, K. (2018). Nanoemulsion: A novel approach for drug delivery. Journal of Drug Delivery Science and Technology, 43, 84-90. https://doi.org/10.1016/j.jddst.2017.10.005
- Shah, A., & Jadhav, K. (2018). Nanoemulsion: A potential drug delivery system. Pharmaceutical Sciences and Research, 8(3), 482-490.
- Singh, P., & Gupta, V. (2020). Recent advances in nanoemulsion-based drug delivery systems. Current Drug Delivery, 17(1), 2-10. https://doi.org/10.2174/1567201817666191118105726
- Soni, S. K., & Soni, P. (2018). Nanoemulsion: A review. International Journal of Pharmacy and Pharmaceutical Sciences, 10(1), 1-9.
- McClements, D. J. (2012). Nanoemulsions: Formation, properties, and applications. Food Structure, 1(1), 19-33. https://doi.org/10.1016/j.foodstruct.2012.10.002
- Kotta, S., Khan, A. W., Pramod, K., Ansari, S. H., Sharma, R. K., & Ali, J. (2015). Exploring nanoemulsions for drug delivery: A comprehensive review. Current Nanoscience, 11(1), 99-120.
- Patel, A. R., & Velikov, K. P. (2014). Nanoemulsions as a pharmaceutical delivery system: From formulation to therapeutic applications. Journal of Agricultural and Food Chemistry, 62(47), 12522-12530. https://doi.org/10.1021/jf5046067
- Solans, C., Izquierdo, P., Nolla, J., Azemar, N., & Garcia-Celma, M. J. (2005). Nano-emulsions. Current Opinion in Colloid & Interface Science, 10(3-4), 102-110. https://doi.org/10.1016/j.cocis.2005.06.004
- Gupta, A., Eral, H. B., Hatton, T. A., & Doyle, P. S. (2016). Nanoemulsions: Formation, properties, and applications. Soft Matter, 12(11), 2826-2841. https://doi.org/10.1039/C5SM02958A
- Shakeel, F., Baboota, S., Ahuja, A., Ali, J., Shafiq, S., & Khan, S. (2008). Nanoemulsions as vehicles for transdermal delivery of aceclofenac. AAPS PharmSciTech, 9(4), 1117-1125. https://doi.org/10.1208/s12249-008-9157-0
- Aboofazeli, R. (2010). Nanometric-scaled emulsions (nanoemulsions). Iranian Journal of Pharmaceutical Research, 9(4), 325-326. https://doi.org/10.22037/ijpr.2010.800
- Salvia-Trujillo, L., Soliva-Fortuny, R., Rojas-Grau, M. A., & Martin-Belloso, O. (2013). Nanoemulsions as a novel edible delivery system of essential oils and active compounds: A review. Journal of Food Engineering, 114(3), 302-308. https://doi.org/10.1016/j.jfoodeng.2012.08.032
- Date, A. A., & Nagarsenker, M. S. (2008). Design and evaluation of microemulsions for improved parenteral delivery of propofol. AAPS PharmSciTech, 9(1), 138-145. https://doi.org/10.1208/s12249-008-9023-4
- Gutti, R. V. R., & Mukkanti, K. (2011). Nanoemulsions for drug delivery through different routes. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 2(3), 699-709.
- Salvia-Trujillo, L., Soliva-Fortuny, R., & Martín-Belloso, O. (2013). Emulsion-based delivery systems for lipophilic bioactive compounds: Nanoemulsion formation, characterization, and their potential use. Comprehensive Reviews in Food Science and Food Safety, 12(5), 510-523. https://doi.org/10.1111/1541-4337.12026.


 Kapil Gawai*
Kapil Gawai*
 Harishkumar Rathod
Harishkumar Rathod
 Swati Deshmukh
Swati Deshmukh





 10.5281/zenodo.14724728
10.5281/zenodo.14724728