Abstract
The preliminary screening of phytochemicals and essential oils in selected herbs and spices aims to identify bioactive compounds that have potential therapeutic and medicinal benefits. Herbs and spices are rich sources of natural antioxidants, flavonoids, alkaloids, tannins, and essential oils, which contribute to their traditional use in treating various ailments. This study focuses on the extraction of phytochemicals and essential oils from selected spices and herbs and qualitative and quantitative screening of these bioactive compounds using standard methods. Selected herbs and spices, including Fennel, Ajwain, Bay leaves, Dill seeds, Coriander seeds, Kalonji, Dill leaves, Scallion were analyzed to detect the presence of key phytochemicals like flavonoids, phenolic acids, alkaloids, saponins, and terpenoids. Essential oils extracted from these plants were also subjected to gas chromatography-mass spectrometry (GC-MS) analysis to profile their volatile components and their important functional groups. Phytochemical screening of the extracts showed the presence of tannins, saponins, flavonoids, alkaloids, terpenoids, etc. The results of GC-MS analysis shows the significant presence of bioactive compounds with potential antioxidant, anti-inflammatory, antimicrobial, and anticancer properties. Further in-depth analysis of these phytochemicals and essential oils could contribute to the development of new, natural therapeutic agents for various diseases. This study highlights the importance of herbs and spices as a sustainable and rich source of bioactive compounds with diverse health benefits.
Keywords
Antioxidant; Antimicrobial; Anti-inflammatory; Bioactive compound; Flavonoids; Phytochemicals; Essential oils.
Introduction
Natural products have been crucial globally in treating and preventing fungal infections and diseases with significant inflammatory components, with numerous studies highlighting the therapeutic potential of these metabolites.[1] Herbs and spices are vital resources, commonly used in everyday life as food additives, flavor enhancers, fragrances, pharmaceuticals, colorants, or directly in medicinal applications. The practice of utilizing plants in these ways has a long-standing history worldwide, and over the centuries, humans have improved techniques for extracting phytochemicals and essential oils from these natural sources. Essential oils consist of complex mixtures of volatile compounds, typically present in low concentrations. They are intricate mixtures of volatile compounds that are usually found in low concentrations. Before these compounds can be analyzed, they must be extracted from their source material. [2] Several methods are available for extraction, including hydro-distillation (HD), steam distillation, Soxhlet extraction, and simultaneous distillation–extraction. However, these molecules are known to be sensitive to heat and prone to chemical alterations, making careful extraction crucial. [3]
Phytochemicals are essential for creating therapeutic agents with natural alternatives. A novel method for identifying medicinally active substances in different plant species is the screening of plant extracts. Flavonoids, tannins, saponins, alkaloids, and terpenoids are examples of phytochemicals with a variety of biological characteristics, such as antioxidant, anti-inflammatory, anti-diarrhea, anti-ulcer, and anticancer effects. [4, 5].
MATERIALS AND METHODS
Materials
The spices such as Anise seeds, Ajwain seeds, Bay leaves, Dill seeds, Coriander seeds, Kalonji seeds and herbs such as Dill leaves, Scallion (Spring onion leaves) were identified and collected from the local market. The herbs and spices were air dried at room temperature and crushed into fine powder for further extractions. Analytical grade chemicals were used for the research work.
Methodology
I. Extraction of phytochemicals
Phytochemicals were extracted using Soxhlet’s distillation method. 100 g of dried powdered samples were extracted using 200 ml of ethanol in Soxhlet apparatus for 48 hours. The crude extracts were concentrated by rotary vacuum flash evaporation, weighed and stored at refrigerated condition [6, 7].
II. Extraction of Essential oils
500 grams of dried samples in powdered form were subjected to hydrodistillation in a Clevenger apparatus for 4-5 hours. The essential oil was collected, dried under anhydrous sodium sulphate and stored at 00C. [7]
III. Phytochemical Screening
Phytochemical screening of secondary metabolites such as alkaloids, flavonoids, terpenoids, saponins, phenolic compounds and tannins, phytosterols, carbohydrates, starch, fixed oils and fats were carried out using the standard procedures as described by Harborne, [8]
Khandelwal, [9] and Daniel M. [10]
IV. GC-MS analysis
The phytochemicals and essential oils were analysed by gas chromatography coupled to mass spectrometry (GC–MS) (Perkin Elmer Clarus 600 Gas chromatograph) using a fused-silica-capillary column with an apolar stationary phase GSBP-5MS (30M x 0.25mm x 0.2 micrometer).
GC–MS were obtained using the following conditions: carrier gas He (99.99%); flow rate 1.0 mL/min; split ratio 10:1; injection volume 1 micro L; injection temperature 280°C; Initial oven temperature was 50°C which progress to 180°C at 5°C/min, from 180 to 260°C at 5°C/min, from 260 to 280°C at 6°C/min and holding at 280°C for 40 min; the ionisation mode used was electronic impact at 70 eV. The detector used was Single Quadrupole mass analyzer. Identification of the components was achieved from their linear retention indices on GsBP-5MS column, determined with reference to an homologous series of C8–C22 n-alkanes, and by a comparison of their mass spectral fragmentation patterns with those stored in the data bank NIST (National Institute of Standards and Technology) Library and FFNSC 3 (Flavors and Fragrances of Natural and Synthetic Compounds) Wiley Library and the literature. [11, 12]
RESULTS AND DISCUSSION
Extraction Yields
The yield of ethanolic extracts and essential oils obtained is represented in Figure 1 and Figure 2 respectively. Different spices and herbs were used to prepare the ethanolic extracts and the yield of phytochemicals in all spices and herbs were compared. Use of ethanol gave good yield for ajwain seeds (42%), followed by coriander seeds (38%), dill leaves (34%), bay leaves (31%), dill seeds (27%), kalonji seeds, (22%), anise seeds (18%) and scallions (14%). The yield of essential oil obtained after hydrodistillation was highest for kalonji seeds (11%) and ajwain seeds (4.3%). Dill seeds (2.6%), bay leaves (2.5%), anise seeds (1.8%) and coriander seeds (1.3%) gave an average yield, whereas very poor yield of essential oils was obtained with scallions (0.9%) and dill leaves (0.6%). The total yield of the essential oils of different spices and herbs coincide with the findings of literature papers. [13] The phytochemical yield of spices and herbs is influenced by a variety of factors, including species, cultivation and harvesting practices, and extraction techniques. Previously carried out research consistently shows that optimal growing conditions, correct harvest timing, dying methods and suitable extraction techniques can enhance the yield of bioactive compounds such as flavonoids, phenolics, terpenoids, and essential oils.[13]
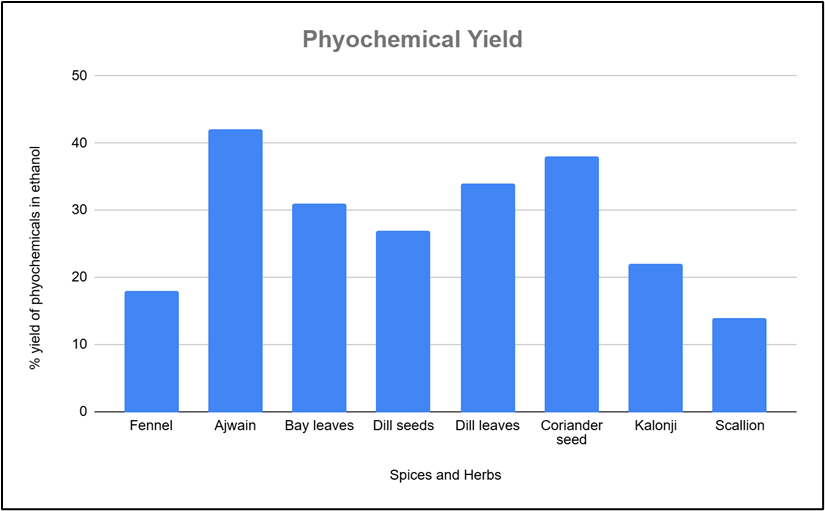
Fig 1: Yield of Phytochemicals in ethanolic extract

Fig 2: Yield of Essential Oils
Phytochemical screening
The qualitative phytochemical screening results for eight ethanolic extracts prepared using commercial spices and herbs is outlined in Table 1
The results of the qualitative screening indicates that all the tested spices and herbs namely, Anise seeds, Ajwain seeds, Bay leaves, Coriander seeds, Dill leaves, Dill seeds, Kalonji seeds, and Scallions are rich in phytochemicals such as alkaloids, flavonoids, terpenoids, phenols and tannins. Kalonji seeds extract showed an abundance of fixed oils and fats. The extracts of coriander seeds and kalonji seeds revealed the absence of phytosterols; whereas absence of terpenoids was reported in the extracts of coriander seeds and dill seeds.
Table 1: Qualitative screening of Phytochemicals in ethanolic extracts
|
|
Anise
seed
|
Ajwain Seed
|
Bay Leaves
|
Coriander seed
|
Dill leaves
|
Dill seeds
|
Kalonji seeds
|
Scallions
|
|
Alkaloids
|
-
|
+
|
-
|
-
|
+
|
+
|
+
|
-
|
|
Carbohydrates
|
+
|
+
|
+
|
+
|
+
|
+
|
+
|
+
|
|
Starch
|
+
|
+
|
+
|
-
|
+
|
+
|
+
|
+
|
|
Fixed oils and fats
|
-
|
-
|
-
|
-
|
-
|
-
|
+
|
-
|
|
Phenolic compounds and Tannins
|
+
|
+
|
+
|
+
|
+
|
+
|
+
|
+
|
|
Phytosterols
|
+
|
+
|
+
|
-
|
+
|
+
|
-
|
+
|
|
Saponins
|
-
|
-
|
+
|
-
|
+
|
+
|
+
|
-
|
|
Flavonoids
|
+
|
+
|
+
|
+
|
+
|
+
|
+
|
+
|
|
Terpenoids
|
+
|
+
|
+
|
+
|
+
|
-
|
+
|
+
|
Key- + positive/ presence of test component, - = Negative/ Absence of test component
Similar results were reported for phytochemical screening of spices and herbs.[14] The present study showed abundance of tannins, organic acids and phenolic acid derivatives in the spices and herbs.. In general, phenolic acids and their derivatives show good anti-oxidation potential, anti-inflammatory and antimicrobial properties.[15] The phytochemical constituents of plants can vary based on soil conditions (pH, acidity, fertility, microbiome and moisture), environmental factors (temperature, humidity) during growth or variation in cultivars. These plants contain a wide variety of complex bioactive chemicals that have anti-inflammatory, anti-cancer, anti-oxidant, and antibacterial qualities, among other important health advantages. [15, 16]
GC- MS analysis of phytochemicals and essential oils
The phytochemicals and essential oils of the eight plants were found to contain a wide variety of chemical constituents. The major compounds in each of them were identified as listed in Table 2. Ajwain exhibited a high presence of thymol. Similarly, Bay leaves showed the presence of anethole. Anise was found to contain linalool and pinene, a potent compound known for its aromatic properties and digestive benefits. Coriander seeds contained cymene, while Dill seeds and leaves exhibited a high content of apiole and carvone, both of which have antimicrobial properties. Scallions demonstrated the presence of diallyl sulfide and kalonji seeds contained apiole, both of which are responsible for the aromatic and medicinal properties of these plants.
Table 2 GC-MS Analysis of ethanolic extracts and essential oils
|
Spices and Herbs
|
Components detected in ethanolic extracts
|
Components detected in essential oils
|
|
Anise seeds
|
(E)-Anethole
Octadecanoic acid
Octadec-(13Z)-enal
|
(E)-Anethole
Estragole
L-Fenchone
|
|
Ajwain seeds
|
Thymol
n-Decanoic acid
14-methyl-Hexadec-8Z-enal
|
gamma-Terpinene
para-Cymene
Thymol
|
|
Bay Leaves
|
3-Pentanol
Trimethyl(2-phenylethoxy)-silane
6-Camphenone
|
para-Cymene
alpha-Phellandrene
3-Carene
|
|
Coriander seeds
|
Linalool
Geranyl formate
n-Decanoic acid
|
Linalool
alpha Pinene
Neryl Propionate
n-Hexadecanoic acid
|
|
Dill leaves
|
1,10-Hexadecanediol
Pentanol
Heptyl alcohol
n-Hexadecanoic acid
|
3-Pentanol
14-methyl-Hexadec(8Z)-enal
n-Decanoic acid
Terpinen-4-ol
|
|
Dill seeds
|
Apiole
Octadec-(13z)-enal
Carvone
|
Carvone
D-Limonene
(E)-dihydro-Carvone
Apiole
|
|
Kalonji seeds
|
2-Pentacosanone
Octadec-(13Z)-enal
Tetradecanoic acid
|
alpha-Phellandrene
Apiole
beta-Phellandrene
|
|
Scallions
|
Hexadec-(11Z)-enal
n-Decanoic acid
alpha-Thujenepara-Cymene
|
alpha-Phellandrene
diallyl disulfide
3-pentanolEugenol
|
The GC-MS analysis revealed that the essential oils of these plants contain a variety of terpenes, phenolic compounds, and sulfur-containing compounds.[17] Thymol, anethole, carvone, and linalool were the most abundant compounds in their respective essential oils, with notable bioactivity profiles related to antimicrobial, antioxidant, and anti-inflammatory effects. [18, 19] The phytochemical screening further confirmed the presence of compounds that have been associated with beneficial health effects. The combination of terpenoids and phenolic compounds, particularly in Ajwain, Bay Leaves, Anise, and Kalonji, suggests these plants could be explored for use in medicinal formulations targeting digestive, inflammatory, and microbial-related disorders. [18, 19]
CONCLUSION
This study concludes that herbs and spices, including Fennel, Ajwain, Bay leaves, Dill seeds, Coriander seeds, Kalonji, Dill leaves, and Scallion, are rich sources of bioactive compounds with substantial therapeutic potential. Qualitative screening showed the presence of key phytochemicals such as flavonoids, phenolic acids, alkaloids, saponins, and terpenoids. Terpenes being an essential component were analysed using GC MS Technique. Preliminary findings indicate that these bioactive compounds have notable antioxidant, anti-inflammatory, antimicrobial, and anticancer properties, supporting the traditional medicinal use of these plants. The antibacterial and antifungal efficacy of the essential oils suggests potential applications in food and pharmaceutical industries. Overall, this research underscores the potential of herbs and spices as a sustainable, natural source for developing new therapeutic agents, highlighting their role in advancing natural medicine and health-promoting products.
REFERENCES
- Lai PK, Roy J. Antimicrobial and Chemopreventive properties of spices and herbs. 2004. Current Medicinal Chemistry 11: 1451-1460
- Singh S, Das SS, Singh G, Schuff C, Lampasona MP, Catalán CA. Composition, In Vitro Antioxidant and Antimicrobial Activities of Essential Oil and Oleoresins Obtained from Black Cumin Seeds (Nigella sativa L.). 2014. BioMed Research International. http://dx.doi.org/10.1155/2014/918209
- Damayanti A, Setyawan E. Essential Oil Extraction of Fennel Seed (Foeniculum vulgare) Using Steam Distillation. 2012. Int. J. Sci. Eng. 3(2):12-14.
- Basak SS, Candan F. Effect of Laurus nobilis L. Essential Oil and its Main Components on ?-glucosidase and Reactive Oxygen Species Scavenging Activity. 2013. Iranian Journal of Pharmaceutical Research. 12 (2): 367-379
- Yin MC, Hsu PC, Chang HH. In Vitro Antioxidant and Antibacterial Activities of Shallot and Scallion. 2003. Journal of Food Science. 68: 281-284
- Delaquis PJ, Stanich K, Girard B, Mazza G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. 2002 International Journal of Food Microbiology. 74:101– 109
- Jyothiprabha V, Venkatachalam V. Preliminary Phytochemical Screening of Different Solvent Extracts of Selected lndian Spices.2016 Int.J. Curr.Microbiol.App.Sci. 5(2): 116-122
- Harborne JB. Phytochemical methods. London: Chapman and Hall, Ltd; 1973.
- Khandelwal, K.R., Practical pharmacognosy, Nirali Prakashan, 5 thed. 1998.
- Daniel, M., Methods in Plant Chemistry and Economic Botany, Kalyani Publishers, New Delhi, 1991.
- Roby MH, Sarhana MA, Selima KH, Khalela KI. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare L.) and chamomile (Matricaria chamomilla L.) 2013. Industrial Crops and Products. 43:587– 595.
- Orhan IE, Senol FS, Ozturk N, Celik SA, Pulur A, Kan Y. Phytochemical contents and enzyme inhibitory and antioxidant properties of Anethum graveolens L. (dill) samples cultivated under organic and conventional agricultural conditions. 2013. Food and Chemical Toxicology. 59:96–103
- Abdela Befa, Desta Fikadu, Beriso Mieso, Mamud Aman, (2021). Effect of drying method and storage days on essential oil yield and quality of Rue (Ruta chalepensis) leaves. J. Nutrition and Food Processing, 4(3); DOI:10.31579/2637-8914/049
- Kumaravel, Shunmugam & Alagusundaram, K.. (2014). Antimicrobial activity and Phytochemical analysis of selected Indian spices. Journal of Pure and Applied Microbiology. 8. 4131-4136.
- Kumar N, Goel N (2019) Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol Rep 24:e00370. https://doi.org/10.1016/j.btre.2019.e00370
- Man G, Xu L, Wang Y, Liao X, Xu Z (2022) Profiling phenolic composition in pomegranate peel from nine selected cultivars using UHPLC-QTOF-MS and UPLC-QQQ-MS. Front Nutr 8:807447. https://doi.org/10.3389/fnut.2021.807447
- Hassanen N, Eissa A, Hafez S. Mosa.E (2015). Antioxidant and antimicrobial activity of celery (Apium graveolens) and coriander (Coriandrum sativum) herb and seed essential oils. Int.J.Curr.Microbiol.App.Sci. 4(3): 284-296
- Kazemi M. (2014)Phenolic profile, antioxidant capacity and anti-inflammatory activity of Anethum graveolens L. essential oil. Natural Product Research, http://dx.doi.org/10.1080/14786419.2014.951934
- Bassolé, I. H., & Juliani, H. R. (2012). Essential oils in combination and their antimicrobial properties. Molecules, 17(4), 3989–4006. https://doi.org/10.3390/molecules17043989


 Roonal Pritam Kataria*
Roonal Pritam Kataria*


 10.5281/zenodo.14332882
10.5281/zenodo.14332882