Abstract
The disease, diabetes, is a complex one with many etiologies and pathophysiologies. The present approach with a single target has not produced the best clinical effects for the treatment of the disease and its complications. Herbal medicine has been utilized for the treatment of various diseases like diabetes for centuries. Many diabetic patients are known to be taking herbal medications along with their conventional Western treatment for better control of the disease and prevention of complications. To use herbal medicines with antidiabetic properties as adjuncts to their mainstream treatments, which may pose either a beneft and/or a potential risk to efective management of their disease. In this review we will evaluate the clinical and experimental literature on herb–drug interactions in the treatment of diabetes. Pharmacokinetic and pharmaco- dynamic interactions between drugs and herbs are discussed, and some commonly used herbs which can interact With anti diabetic drugs summarized. Herb–drug interactions may be a double-edged sword, both predisposing to risks (adverse drug events) and offering benefts (through enhancement). More generally, there is a lack of data on herb–drug interactions. Altogether, more rigorous scientific research is needed to inform clinical practice as well as ensure the wellbeing of diabetes patients. Multiple drugs or polypharmacy received by patients with diabetes mellitus (DM) during therapy can trigger drug-related problems, one of which is drug interactions. The occurrence of drug interactions causes uncontrolled blood sugar levels, which can affect morbidity, mortality, and the quality of life experienced by a patient. This study aims to look at the Description of potential drug interactions in prescribing type 2 DM patients at a Pharmacy in Medan City for the period January-April 2022. This study is a descriptive study and data were taken retrospectively on 126 prescription sheets for type 2 DM patients who met the inclusion criteria. Identification of potential drug interactions using online literature such as Medscape Drug Interaction Checker, Drugs.com, and Drug Interaction Fact 2009 e-book. Data analysis was done univariately to outline the percent of drug interactions. Results: From 126 prescription sheets for type 2 DM patients, there were 108 patients (85.71%) who had the incidence potential drug interactions with a total of 238 potential drug interactions.
Keywords
disease, diabetes, Antidiabetic Medication, prevention of complications.
Introduction
Diabetes mellitus (DM) is a group of metabolic diseases characterized by an increase in blood glucose levels (hyperglycemia) resulting from a lack of insulin secretion, insulin action, or both [1]. The number of people with type 2 DM has increased significantly every year, this is evidenced by the incidence of type 2 DM in the world. Based on data published by the International Diabetes Federation (IDF) in 2019, type 2 DM sufferers in the world reached 463 million people and it is estimated that this will increase in 2030 to 578 million people. In Indonesia, based on Basic Health Research data, DM sufferers increased from 6.9% in 2013 to 10.9% in 2018 and according to the International Diabetes Federation (IDF), Indonesia is the seventh highest ranking in the world. The world that has DM sufferers with a total of 10.7 million people in 2019 [2,3]. Blood glucose levels that are not controlled properly cause complications that interfere with health and cause death. Therefore, various treatments often occur for each symptom that appears, causing the administration of more than one drug and tends to encourage irrational treatment patterns by using more than one kind of drug that is not necessary, resulting in overprescribing or polypharmacy [4,5]. Treatment with several drugs at once (poly pharmacy) can facilitate drug interactions [6]. Polypharmacy is defined as the concurrent use of large amounts of drugs in 1 prescription by a patient but not according to the condition of the patient or the clinical effect indicated [7]. The risk of drug interactions and drug-induced problems increases with the use of multiple drugs [8]. In some clinical conditions, interactions between drugs can be beneficial to the patient, for example, antidotes are injected in cases of overdose), poor interactions (potentially harmful interactions that must be identified early), and unfavorable interactions (interactions that have a little clinical impact). and have a low risk) [9]. The results of research conducted by Ariani and Prihandawati (2021) at a pharmacy in Banjarmasin showed that the number of drug combinations that had potential interactions based on the mechanism of action was 149 (39.52%), including pharmacodynamic interactions with as many as 74 events (48.05%), pharmacokinetic interactions were 33 events (21.43%), and unknown were 47 events (30.52%). Based on the severity level, the serious category was 1 event (0.65%), moderate was 121 (78.57%), and minor was 32 events (20.78%) [10]. the potential for drug interactions in type 2 DM patients at pharmacies still shows a high category. Therefore, this study aims to determine the incidence of potential drug interactions that may occur, the mechanism of interaction, drugs that have the potential to interact, and the severity of their interactions in prescribing type 2 DM patients at a Pharmacy in Medan City.
Literature Review & Background
Type 2 diabetes is progressive in nature and so to control cardiovascular risk, most patients need combinations of oral antidiabetic drugs (OADs) plus or minus insulin. Thus, drug–drug interactions may substantially contribute to harmful effects of intensive glucose lowering therapy. Methods: A Pub Med literature search was performed to select the most recent and relevant publications examining OAD metabolism and the effects of concomitant use of OADs. Results/conclusion: Considering the individual sensitivity to OADs, pharmacogenetic factors could be of critical importance The therapeutic range and efficacy as well as adverse effects of OADs may be significantly affected by genetic polymorphisms of cytochrome P450 drug metabolising enzymes, organic cation transporters or organic anion transporting polypeptides. Although current data suggest that modest pharmacokinetics interferences among some OAD combinations exist, they do not seem to have substantial clinical consequences. As long-term adherence to multi-drug treatment is poor in diabetic patients, the future will show a strong move towards earlier treatment with combination therapies. As metformin is cardiovascular protective and is not metabolized through the hepatic cytochrome P450 system, it is a key compound for any OAD combination. There is an overwhelming amount of small-sized in vitro studies and investigations mostly including healthy volunteers dealing with short-term effects and surrogate parameters of concomitant OAD use. Further evidence from large- scale studies including typical subjects with type 2 diabetes, in particular multimorbid and geriatric patients with polypharmacy, is needed. Post marketing surveillance using large patients' registries could be helpful to improve the early detection of clinically relevant drug–drug interactions. Patients with type 2 diabetes mellitus (T2DM) often do not suffer solely from symptoms of increased blood glucose levels. In the majority of cases, several comorbidities are present with the need of additional pharmacological treatment. Concomitant diseases such as hypertension and high blood lipids can lead to both microvascular and macrovascular complications [Cornier et al. 2008]. Moreover, central nervous disorders such as depression are increased in patients with T2DM compared with the general population [Anderson et al. 2001]. Multifactorial pharmacotherapy significantly reduces the risk of cardiovascular (CV) mortality [Gaede et al. 2008], but an increasing number of medications taken by the patients leads to a higher risk of adverse drug effects and interactions [Freeman and Gross, 2012; Amin and Suksomboon, 2014; Rehman et al. 2015; Valencia and Florez, 2014; Peron et al. 2015]. Applying a multifactorial pharmacotherapy approach, it is important to consider cytochrome P-450 (CYP) enzyme interactions [De Wildt et al. 1999; Dresser et al. 2000], altered absorption properties [Fleisher et al. 1999] and transporter activities [Lin and Yamazaki, 2003.
Aim & Objective
Intention Statement:
- Investigate the impact of drug interactions on antidiabetic medication efficacy: The research aims to analyze how drug-drug interactions influence the effectiveness of antidiabetic medications, specifically focusing on their ability to maintain proper blood glucose levels in diabetic patients.
- Examine pharmacokinetics and pharmacodynamics: A key goal is to understand how these interactions affect the absorption, distribution, metabolism, and excretion (pharmacokinetics) and how they alter the drug’s mechanism of action (pharmacodynamics). This is crucial for determining the real-world efficacy of antidiabetic medications in various therapeutic contexts.
- Increase awareness among healthcare professionals: By identifying specific drug interactions that impair or enhance the action of antidiabetic drugs, this research seeks to educate healthcare providers. The aim is to improve clinicians' ability to detect potential issues early and to adjust treatment plans accordingly.
- Promote patient safety and improve prescribing practices: Another objective is to reduce the risk of adverse effects from drug interactions by guiding safer prescribing and medication management practices. This will ensure that patients receive the full therapeutic benefits of their antidiabetic medications without compromising efficacy due to other drugs in their regimen.
- Enhance patient outcomes: Ultimately, this research intends to improve clinical outcomes in diabetic patients by ensuring that their antidiabetic medications work as intended, minimizing complications related to drug interactions, and ensuring better long- term glycemic control.
Achievements Statement:
Identification of key drug classes affecting antidiabetic medication efficacy: The study successfully identified common drug classes (e.g., corticosteroids, beta-blockers, diuretics) that either reduce or enhance the efficacy of antidiabetic medications. These findings have been critical in helping clinicians predict potential interactions during the treatment planning process.
- Development of clinical guidelines for managing drug interactions: As a result of this research, evidence-based guidelines have been developed for healthcare providers. These guidelines assist in recognizing high-risk interactions, adjusting antidiabetic drug dosages, and selecting alternative treatments where necessary, which improves medication safety and efficacy.
- Implementation of patient education programs: A key achievement has been the integration of research findings into patient education programs. These programs empower patients to better understand their medications, recognize the importance of reporting all drugs they are taking, and manage their medication schedules to avoid interactions.
- Reduction in drug interaction-related complications: The application of this research in clinical practice has led to a noticeable reduction in the number of complications arising from drug interactions, such as hypoglycemia or hyperglycemia. This has contributed to a lower risk of hospitalizations and adverse events in diabetic patients.
Improvement in glycemic control and health outcomes: With better management of drug interactions, patients have experienced improved glycemic control, which is critical for preventing diabetes-related complications like cardiovascular disease, kidney damage, and neuropathy. The overall quality of life and long-term health outcomes for patients on antidiabetic medications have significantly improved as a result.
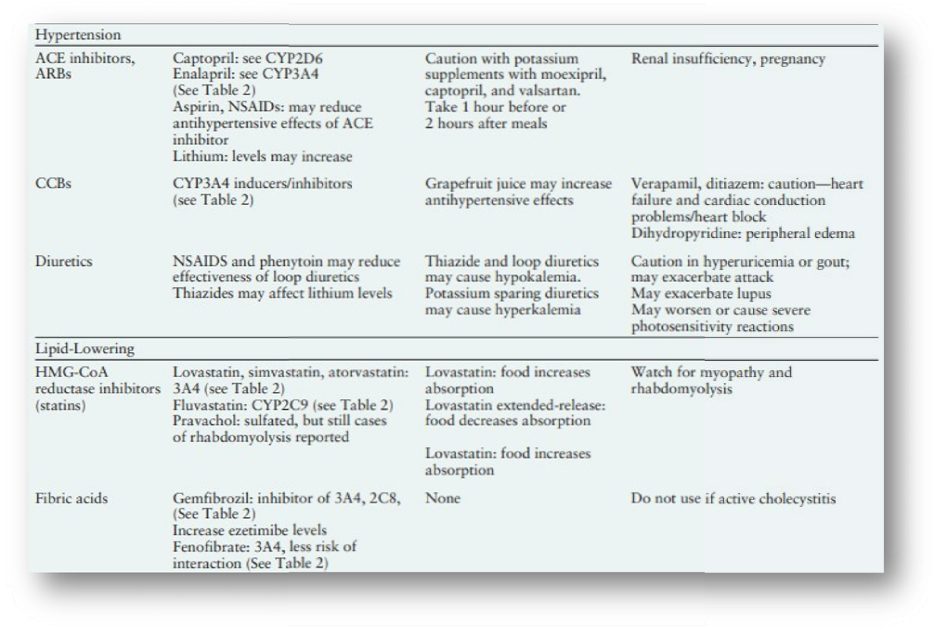
Drug Interactions of Medications Commonly Used in Diabetes
DRUG-DRUG INTERACTIONS
Drug interactions are often categorized as pharmacodynamic or pharmacokinetic in nature. A pharmacodynamic drug interaction is related to the drug’s effect on the body. An example is the combination of alcohol with medications that cause sedation. A pharmacokinetic drug interaction is related to the body’s effect on the drug. An example is an increase in the systemic concentration of a renally eliminated drug because of renal insufficiency. A pharmacokinetic drug interaction can be caused by an alteration in absorption, distribution, metabolism, or elimination of a drug.8
Pharmacodynamic Interactions
Pharmacodynamic drug interactions can be either beneficial or detrimental to patients. A beneficial example is the additive blood pressure–lowering effect when an ACE inhibitor is added to a calcium channel blocker (CCB). Likewise, synergistic blood pressure lowering may be seen if a diuretic is added to an ACE inhibitor. The pharmacodynamic drug interaction can also be detrimental. When alcohol and a medication that causes sedation are combined, additive unwanted sedation may occur. Antagonistic effects may also be encountered, as with the combination of an acetylcholinesterase inhibitor for myasthenia gravis or Alzheimer’s disease with amitriptyline for painful diabetic peripheral neuropathy. The acetylcholinesterase inhibitor increases acetylcholine levels, whereas amitriptyline has antagonistic anticholinergic effects.8
Pharmacokinetic Interactions
Absorption interactions.
Drug absorption is the movement of the drug from its site of administration into the bloodstream (Figure 1). Absorption interactions are changes in a drug’s effects caused by food, drink, or medications taken concurrently. Classically, we think of the oral administration of a medication and absorption from the gastrointestinal system, but it applies to all routes of administration, including injection, inhalation, topical, buccal, sublingual, and others. Drug-food interactions can affect the total amount of drug absorbed (bioavailability), but most often they only slow absorption. For example, the hypoglycemic effect of glipizide may be delayed slightly. If taken with a meal versus 30–60 minutes before a meal, although hemoglobin A1c (A1C) values are unaffected.9,10 Alteration of gastrointestinal motility, as is the case with exenatide (Table 1), or pH may also affect absorption. In addition, components of food may interact. For example, vitamin K intake from green leafy vegetables interacts with warfarin. Similarly, several medications may complex or chelate with administered medications, significantly reducing their absorption.6 For example, levothyroxine absorption is reduced when administered with ferrous sulfate or antacids and should be moved either 1 hour earlier or at least 2 hours after administration of these drugs.7,11 It is best not to administer other medications with antacids because they can reduce the absorption of many medications.
Distribution interactions.
Distribution is the movement of the absorbed drug through the bloodstream and its transport throughout extracellular or intracellular compartments to the site of action (Figure 2). Many medications extensively bind to plasma proteins such as albumin in the bloodstream. When a drug is bound to these plasma proteins, it is not actively distributed to the site of action, and only the “free” drug is available to cause an effect. One drug can displace another from the binding sites on the plasma proteins if its binding is stronger. This increases the amount of “free” drug available to cause an effect. In the past, many protein- displacing interactions were documented in vitro, with in vivo consequences assumed. The majority of protein-displacing interactions have since been documented to be test-tube phenomena and are not clinically important.12 Most of the suspected distribution interactions have now been reclassified as metabolism interactions. Distribution interactions can be significant for drugs that have extremely rapid distribution, narrow safety margins, and possibly nonlinear kinetics.12 No significant distribution interactions are pertinent for oral medications commonly used for diabetes.12
Metabolism interactions.
Drug metabolism is the modification or degradation of drugs. Metabolism can make drugs more or less toxic, active or inactive, or more easily eliminated from the body.13 The primary organ involved in metabolism is the liver, although metabolism has been documented in the kidneys, lungs, gastrointestinal system, blood, and other tissues. The most extensively studied family of isoenzymes found in the liver and gastrointestinal tract is the cytochrome P450 (CYP) system. The name “cytochrome P450” comes from the experimental techniques used to Identify the isoenzymes and is not clinically relevant.14 CYP2D6, for example, includes “2,” the genetic family; “D,” the genetic subfamily; and “6,” the specific gene member. The nomenclature used to classify different subsets of the CYP system has no functional implications but clinically allows us to classify metabolism interactions. Drugs can inhibit (decrease) metabolism, induce (increase) metabolism, or have no effect on each CYP450 isoenzyme subset. Thus, inhibition of metabolism will likely increase the affected drug’s systemic concentrations, whereas induction of metabolism often reduces systemic concentrations. Not all isoenzymes are inducible, and only CYP2C9 and CYP3A4 induction is clinically relevant to people with diabetes. A drug may also be a substrate for (metabolized by) one or more of these enzyme subsets, and clinically, if an inhibitor or inducer affects that isoenzyme, it could affect the efficacy of the drug. Drugs can have a complex profile, being a substrate for or an inhibitor or inducer of multiple subsets. For example, quinidine is a potent inhibitor of CYP2D6, but it is primarily metabolized by CYP3A4. More than 50% of all drugs are metabolized at least in part by CYP3A4 or CYP2D6, and several important diabetes drugs are metabolized by these pathways.15 Phase 2 metabolism (glucuronidation, acylation, sulfation, and so forth) includes attachment of a water-soluble molecule to aid elimination and detoxification of a drug one important drug-drug interaction involving gemfibrozil and several hydroxymethylglutaryl (HMG) CoA reductase inhibitors (statins). High-risk groups for drug interactions include neonates, infants, the elderly, and those with significant organ disease (i.e., renal or hepatic disease) warranting increased screening vigilance. Neonates, infants, and the elderly will often metabolize drugs slower than healthy adults, and lifestyle choices such as smoking (induces metabolism) and alcohol use (may induce or inhibit metabolism) can alter metabolism. Metabolism patterns can also be altered by genetically determined variations. For example, ~ 5–10% of Caucasians, but only 0–1% of Asians, have little CYP2D6 enzyme activity, making them “CYP2D6 poor metabolizers,” the consequences of this are dependent on the drug and alternative pathways available for metabolism.16.


Types of drug interactions
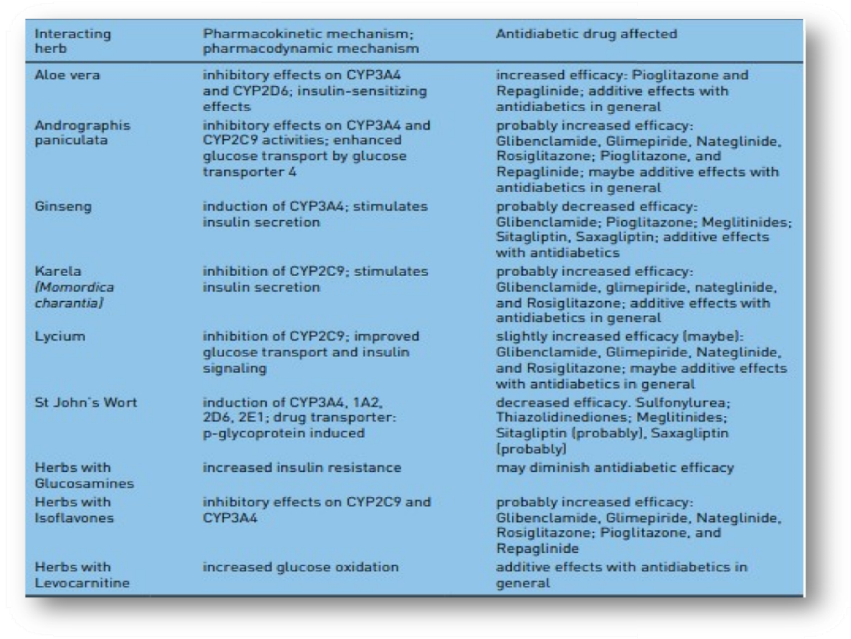
Types of drug interactions A drug interaction is defined either as increase or decrease of a medical diagnostic or therapeutic effect of a specific drug caused by another substance, which may be another drug, plant or a dietary supplement. Mechanisms of drug interactions can be divided into two categories: (1) pharmacokinetic interactions, which influence absorption, distribution, metabolism or excretion of a drug (ADME rule) and thus lead to increased or reduced plasma levels of a drug; and (2) pharmacodynamic interactions, which alter pharmacologic efficacy of a drug while drug plasma levels remain unaltered [Rang and Dale, 2012]. Different targets for drug interactions are shown in Figure 1. Pharmacokinetic interactions In the case of pharmacokinetic drug–drug interactions, at least one drug affects the metabolic pathway of the other concomitantly taken drug. The interaction results in either increased or reduced plasma levels of one or both interacting medications compared with plasma levels when the drugs are taken separately. A frequent mechanism of pharmacokinetic interactions is inhibition or induction of degrading liver enzymes [Dresser et al. 2000]. Even if, in principle, every drug metabolizing enzyme can be the cause for a drug–drug interaction, most interactions are based on oxidative metabolism by the CYP enzyme system [De Wildt et al. 1999], or on an interaction with the drug transporter P-glycoprotein [Lin and Yamazaki, 2003. Furthermore, altered plasma protein binding (only the free fraction of a drug in plasma is pharmacologically active, displacement from plasma protein binding can increase the active proportion of a drug), absorption and excretion (e.g. by influencing tubular reabsorption) can be mechanisms of pharmacokinetic drug interactions [Fleisher et al. 1999]. For instance, altered gastric pH or the formation of insoluble complexes inside the gastrointestinal tract can result in altered absorption rates. In this regard, food intake and nutritional supplements can play a relevant role by causing significant differences in the plasma concentration of several drugs. A clinical example, relevant for treatment of diabetic patients, is the reduced and slightly delayed metformin absorption rate when drug intake takes place simultaneously with food ingestion [Scheen, 1996; Fleisher et al. 1999]. Moreover, there are gender-specific pharmacokinetic and pharmacodynamic differences to mention [Franconi et al. 2007], even if these differences seem to be of rather minor clinical importance and do not appear to play a clinically relevant role in the treatment of diabetes mellitus [Meibohm et al. 2002]. Herbal drugs represent a complex problem when taken concomitantly with a pharmacological treatment. In the majority of cases insufficient information about the intake of herbal drugs is available for the respective physician, because herbal drug preparations are available over-the-counter. However, these preparations often consist of a complex mixture of bioactive substances, which can interact with pharmacological medications in a different and unpredictable manner. An example for an often-used herbal preparation which presents a high interaction potential with several commonly used drugs is St John’s wort due to induction of various hepatic CYP enzymes (CYP3A4, 1A2, 2D6, 2E1) and P-glycoprotein [Dürr et al. 2000; Gurley et al. 2002; Mills et al. 2004]. Thus, St John’s wort affects the disposition of Sulfonylureas and probably Thiazolidinediones, Meglitinides, Sitagliptin, and Saxagliptin.[Xu et al. 2008, Rehman et al. 2014]. Similarly, aloe vera, ginseng, Andrographis paniculata, karela, lycium, and herbs with isoflavones or levocarnitine as ingredients might affect antidiabetic drug metabolism [Rehman et al. 2015].
Table 1. Relevant herb-drug interactions with commonly prescribed antidiabetic drugs [Holstein et al., 2012, Rehman et al., 2014].
Efficacy and Cardiovascular Safety of Antidiabetic Medications
Although rigorous management of DM from the disease onset was shown to reduce the microvascular complications remarkably, the incidence of macrovascular disease from type
1 diabetes mellitus (T1DM) showed a significant reduction only after many years of treatment.3 On the contrary, among patients with type 2 diabetes mellitus (T2DM), tight glycaemic control was found to be associated with a higher incidence of macrovascular complications such as strokes and myocardial infarctions as evidenced by major randomized controlled clinical trials like the Action to Control Cardiovascular Disease in Diabetes (ACCORD) trial4 and the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial.5 Though there is a clear link between the severity of prolonged hyperglycemic state in uncontrolled T2DM and the incidence of CVD, the lack of benefit or even potential harm from rigorous DM control in such patients may be due to the presence of established vasculopathy that gets aggravated by such treatments.6 Therefore, it is imperative to tailor individualized treatment targets and medication regimes to each DM case with due considerations of their age, gender, ethnic and cultural backgrounds, and the other disease comorbidities to ensure that we are not harming our patients. In 2007, the New England Journal of Medicine published a meta-analysis by Nissen et al. from the Cleveland Clinic that demonstrated an increased risk of myocardial infarction and other cardiovascular events with Rosiglitazone, an antidiabetic drug widely used in patients with T2DM.7 Although there was controversy on the methodological accuracy of this study in 2007 itself, the huge public apprehension from media and the concerns raised by the scientific fraternity resulted in the enforcement of sanctions on the drug molecule by the United States Food and Drug Administration (US FDA) and the European Medicine Agency (EMA) in 2010.8,9 Though the FDA restriction was subsequently uplifted in 2013, the drug molecule has never been used commonly since the controversy shook the global drug market. With the eye-opening results from the ACCORD and ADVANCE Trials and Rosiglitazone controversy, the FDA has made it mandatory to ensure cardiovascular safety studies for all the antidiabetic medications before final approval and recommended continued post-marketing surveillance for further safety monitoring. Therefore, now the clinicians across the world are clear that they are not only ensuring the most effective pharmacotherapy for DM, but also the cardiovascular safety in every patient, DM being identified as a CVD equivalent historically from 1998 by the American Heart Association. Insulin, being the best drug molecule for glycaemic management since its discovery in the early 19th century and the lifesaving treatment option in patients with T1DM, a critical appraisal of the efficacy and safety of the molecule is crucial in any analysis of the efficacy of antidiabetic drug classes. In their review titled “Efficacy and cardiovascular safety of Insulins,” Fernandez and Radhakrishnan provided a detailed review of the efficacy, safety issues, and pharmacological properties of different insulins currently available in the global market.10 The glycaemic response of insulins are clearly dose-dependent in contrast to all other antidiabetic medications, and therefore, the efficacy analysis should address the issue of targeted glycated hemoglobin (HbA1c) reduction without the risk of hypoglycemia. Based on multiple large randomized controlled trials (RCTs) and meta-analyses, the authors have portrayed a detailed analysis of the efficacy of individual short acting and long-acting insulin molecules in their review. They have also provided us summaries of switching between different insulin molecules, cost-effectiveness of some insulins, the differences in the use of some formulations of insulins such as premixed insulins, biphasic insulins, and biosimilar insulins, a combination of insulins with glucagon-like peptide-1 receptor analogues (GLP-1RA), and also concentrated insulin (U500), in different situations of DM management. In addition, the use of insulin for the treatment of severe hypertriglyceridemia, emergency management of hyperkalemia, and the potential use in wound management are briefly discussed. Presently, there is only a limited number of CVOTs on insulins since most insulins do not qualify the FDA mandate. The DEVOTE Trial (comparing cardiovascular safety of insulin Degludec vs. insulin glargine in subjects with type 2 diabetes at high risk of cardiovascular events) showed noninferiority of degludec use compared to glargine among patients with T2DM at high risk for cardiovascular events.11 Similarly, the ORIGIN (outcome reduction with initial glargine intervention; the CVOT for glargine) trial comparing insulin glargine with standard care did not exhibit any adverse cardiovascular safety issues.12 However, several observational/ cohort/ retrospective studies,13-18 revealed possible associations between insulin use and allcause mortality, major adverse cardiovascular events (MACE), hospitalisation from heart failure, and cancer although with multiple confounding factors such as the amount of insulin exposed, glycaemic control, weight gain, and hypoglycaemic events. Fernandez and Radhakrishnan have concluded their review by providing some additional outlooks into the areas of uncertainty and emerging new therapeutic agents/devises to fight diabetes with insulins, the wonder drug molecule that revolutionised 20th -century diabetes care with its relentless battle now into the 21st century defeating DM related harm to the sufferers.10 Presently, there is only a limited number of CVOTs on insulins since most insulins do not qualify the FDA mandate. The DEVOTE Trial (comparing cardiovascular safety of insulin Degludec vs. insulin glargine in subjects with type 2 diabetes at high risk of cardiovascular events) showed noninferiority of degludec use compared to glargine among patients with T2DM at high risk for cardiovascular events.11 Similarly, the ORIGIN (outcome reduction with initial glargine intervention; the CVOT for glargine) trial comparing insulin glargine with standard care did not exhibit any adverse cardiovascular safety issues. However, several observational/ cohort/ retrospective studies,13-18 revealed possible associations between insulin use and all-cause mortality, major adverse cardiovascular events (MACE), hospitalization from heart failure, and cancer although with multiple confounding factors such as the amount of insulin exposed, glycemic control, weight gain, and hypoglycemic events. Fernandez and Radhakrishnan have concluded their review by providing some additional outlooks into the areas of uncertainty and emerging new therapeutic agents/devises to fight diabetes with insulins, the wonder drug molecule that revolutionized 20th -century diabetes care with its relentless battle now into the 21st century defeating DM related harm to the sufferers.10 After the rosiglitazone controversy in 2007 and the introduction of newer antidiabetic molecules such as dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 receptor agonists (GLP-1RAs) in the early 21st century, the use of the thiazolidinedione (TZD) group of antidiabetic agents has markedly declined in the developed countries. This is not only because of the cardiovascular safety concerns about TZDs, but also due to the mechanisms of actions of DPP-4 inhibitors and GLP-1RAs, which grossly alter the metabolic and hormonal milieu of the individuals with T2DM and obesity. However, TZDs are still being used in developing countries owing to their economical pricing and reasonable efficacy for HbA1c reduction. In their article, Raveendran et al. have elaborated their mechanisms of action, efficacy in the management of various metabolic disorders, especially T2DM, and the cardiovascular safety of TZDs.24 Based on the evidence from multiple RCTs and metanalyses, the authors have demonstrated that monotherapy with pioglitazone and rosiglitazone results in HbA1c reduction of 1- 1.5%, although associated with a weight gain potential of up to 3 kg. Lobeglitazone, the third molecule in the TZD class mainly available in Southeast Asia, has shown to cause a mean HbA1c reduction of 0.6% and weight gain of
1.52 kg. Raveendran et al. have also provided a detailed account of the benefits of using TZDs in NAFLD, PCOS, prediabetes, and lipodystrophies. As the cardiovascular safety issue was the main concern about TZD use a decade ago, Raveendran et al. analysed the currently available evidence on this hot topic in their extensive review. Although associated with a modest risk of heart failure in high-risk individuals, the authors have demonstrated that pioglitazone use is associated with improvement of cardiovascular outcomes such as MACE, including myocardial infarction and stroke. Even though there were no major evidence on the cardiovascular safety of rosiglitazone in several studies and the subsequent analysis of the controversial study data by Nissen et al.7 , the uncertainty persists even now as described by Wallach et al.25 54% higher risk of heart failure is another major safety concern about this drug molecule. There are no CVOTs with lobeglitazone though animal models showed anti-atherosclerotic potential. Augmented risk of hypoglycemia with insulin and insulin secretagogues, peripheral and macular oedema, reduction of bone mineral density, and fractures along with a signal towards an increased incidence of urinary bladder cancer with pioglitazone were the potential adverse effects of TZDs. Sulfonylureas (SUs) are one of the earliest classes of oral antidiabetic agents available in the market over the past few decades with modest efficacy in the glycemic management of patients with T2DM, especially in the early stages of the disease. These drugs augment insulin production from the pancreatic ? cells, which results in the control of hyperglycemia. Thus, SUs may worsen diabesity (DM resulting from obesity) – the basic pathogenic mechanism; T2DM being a hyperinsulinemia state, and insulin being an anabolic hormone.26 However, considering the affordably low prices for most people, wide availability, and the reasonable safety profile make this medication class an attractive choice in the developing countries, if not in the economically affluent nations. In their article in this special issue, Fernandez et al. have provided an extensive review of the efficacy and safety issues of SUs, which enables readers to use these age-old medications scientifically for T2DM management.27 Based on the currently available evidence from multiple RCTs and meta-analyses, the authors have proved that T2DM treated with SUs results in mean HbA1c reduction ranging from 0.97 – 1.5% with a modest risk of hypoglycemia, ranging from 3.6 – 13.9%, and with weight gain ranging from 2.3 – 2.8 kg, depending on the drug molecule in the subclass used.27-30 Authors have also provided other uses of SUs apart from T2DM, such as treatment of gestational diabetes mellitus, permanent neonatal diabetes, and maturity-onset diabetes of the young (MODY). Based on the evidence derived from various meta-analyses, Fernandez et al. have argued that SU use for the management of T2DM may be associated with a marginal increase in all- cause mortality and cardiovascular mortality, MACE, and stroke risk.27, 31-33 However, most of these study data analyzed were derived from observational studies with inherent methodological flaws that might skew the accuracy of the results. The recently published Cardiovascular Outcome Study of Linagliptin Versus Glimepiride in Type 2 Diabetes (CAROLINA) trial was a high-quality RCT that did not suggest a significant increase in CV risk with glimepiride.34 The ongoing GRADE (Glycaemia Reduction Approaches in Diabetes: A Comparative Effectiveness) study is expected to shed more light on the gray area of the cardiovascular safety concerns surrounding SUs.35 Meglitinides are the next class of oral antidiabetic agents used in the management of T2DM. The two molecules in this drug class, viz., repaglinide and nateglinide, act like SUs by stimulating the pancreatic ? cells for insulin secretion. However, the duration of action is quite short in comparison to SUs, and these two molecules are used mainly to control postprandial hyperglycemia in patients with T2DM and those with erratic eating patterns who are prone to develop hypoglycemia with other long-acting antidiabetics. With extensive analysis of the currently available literature, Philip and Fernandez in this issue of the Journal have provided an up-to-date evidence-based review article on meglitinides.36 The authors have described that the use of these molecules is associated with HbA1c reduction ranging from 0.2 – 1.5?pending on the baseline glycaemic control and the patient characteristics. Combination therapy with other antidiabetic medications except SUs is also associated with modest reductions in HbA1c. Hypoglycemia and weight gain are the main problems with meglitinides, although less than that with SUs. Although there are no long-term well-designed CVOTs on the cardiovascular safety of these drugs, the currently available evidence does not suggest any signals towards major harm. However, being SU- like in their actions, hypoglycemia may be a cardiovascular risk, especially in vulnerable populations such as the elderly and those with advanced renal disease.
Epidemiology
Pancreatic, colorectal, breast, endometrial, ovarian, hepatocarcinoma, and prostate cancer are only a few of the cancers that have been linked to diabetes and are significantly tied with obesity and insulin resistance [14, 61, 62]. On the other hand, several epidemiological studies have shown that certain cancers and T2DM are closely related and diabetes raises a person’s risk of developing cancer of pancreatic, liver, colon, breast, and endometrial cancer [14, 61]. The third most often diagnosed malignancy, colorectal cancer (CRC), accounts for more than 6% of all cancer cases worldwide [63]. There are several theories regarding the association between colorectal cancer and diabetes. In a large cohort study conducted in Canada between 2007 and 2015 on 44,178 participants with CRC, diabetes had a greater impact on non-cancer than cancer mortality risk for patients with CRC [64]. A large study published in 2016 in the British Journal of Cancer reported that diabetes mellitus is significantly associated with larger pancreatic tumors and also may elevate the overall risk of death of pancreatic cancer patients (HR of 1.19) [42]. Another epidemiological study found that sugar consumption is strongly correlated with an increase in both incidence and mortality of breast and colon cancer, independent of obesity [65]. Preclinical studies suggest that high-sucrose or high-fructose diets activate several pathways, including inflammation, glucose, and lipid metabolic pathways [66]. Interestingly, some cancers, such as those of the brain, buccal cavity, esophagus, lung, breast, urinary bladder, and larynx, demonstrated a null or decreased occurrence risk in diabetic patients in some studies [67]. It is noteworthy that several American and European studies have shown that individuals with type 2 diabetes have a lower risk of developing prostate cancer [68, 69]. Furthermore, patients with more than 10 years of T2DM duration showed a stronger protective effect [70]. Men with diabetes had lower testosterone levels [70] than men without the disease, and research has shown that testosterone is linked to a higher risk of prostate cancer [71]. Also, large studies found no correlation between T2DM and the risk of dying from cancers of the lung, bladder, stomach, cervix, esophagus, or leukemia [72, 73]. According to a five-country study on cancers in T1DM patients, there is a correlation between T1DM and the risk of multiple common cancers. Comparing non-sex-specific cancers to the general population, the estimated homologous recombination (HR) and 95% confidence intervals (CIs) for overall cancer were 1.15 (1.11, 1.19) for men and 1.17 (1.13,1.22) for women [74].
Treatment And Prevention
Recent data indicate that metformin [94], besides its benefit for diabetic patients may have also a benefit in cancer patients. Metformin promotes the liver kinase B1 (LKB1)/AMPK signaling pathways and inhibits the mTOR pathway, it decreases insulin levels, protein translation, and circulating levels of insulin and IGF-1 in peripheral blood and may ameliorate dyslipidemia [95, 96]. Currently, the use of metformin in cancer prevention is still under scrutiny [95]. Large epidemiologic data suggest that metformin decreases the incidence of prostate, pancreas, liver, colon, thyroid, endometrial and esophageal cancers [97]. It may also improve the progression free survival of patients with ovarian cancer [39], the prognostic of patients with breast cancer [97] and the overall survival of patients with metastatic non-small cell lung cancer [98] and nasopharyngeal cancer [99]. Intriguingly it has been recently shown both in vitro and in vivo that Metformin may enhance the efficacy of check point inhibitors in lung cancer tumors harboring STK11 mutations [100]. The authors of a systematic review and meta-analysis reported significantly reduction in both overall cancer incidence and mortality in patients taking metformin [48]. Metformin’s potential to upregulate AMP kinase (AMPK), which inhibits mTOR and impairs angiogenesis as well as cell growth and proliferation—both essential for the progression of cancer—may explain how cancer growth is restricted but more mechanisms may be present, and sometimes it’s effect is counter-intuitive [101, 102]. While it was initially thought that AMPK might be a connecting link between diabetes and cancer, emerging studies indicate that the impact of metformin on cancer suppression, despite its activation of AMPK in cancer cells, is not definitive. This ambiguity is highlighted by the fact that metformin, through inhibition of complex 1, can increase glycolysis (Warburg effect), potentially promoting tumor growth in mice via elevating lactate and VEGF levels, although in vitro it leads to growth arrest because of enhanced extracellular acidification as a result of increased glycolysis [103]. Additionally, the role of AMPK in cancer is itself context dependent and appears contradictory [104], with some studies indicating its significant involvement in worsening cancer cell survival and promoting tumorigenesis [102, 105.
Future Developments
Leucine, isoleucine, and valine, collectively known as branched chain amino acids BCAA, are essential amino acids, both for the host and the tumor cells. It has been shown that elevated circulating BCAA levels are related to a number of conditions marked by insulin resistance (IR) and inflammatory response, including obesity and diabetes, both of which are known risk factors for cancer development [101]. Several large studies conducted in US and Japan have demonstrated that elevated circulating BCAA concentrations are early predictors for pancreatic ductal adenocarcinoma (PDAC) [108–110]. Recently, it has been also shown that metformin and sulphonyl urea treatment results in lower BCAA levels [111]. Importantly, BCAA levels decreased as HbA1c levels improved, indicating that improper glucose metabolism may contribute to elevated BCAA levels. As a result, serum BCAA levels could be also a new indicator for assessing metabolic disorders and glycemic management [112]. The relationship between diabetes and cancer, where certain aspects remain under- addressed due to the current limitations in research. For instance, the potential dual role of anti-diabetic drugs as anti-cancer agents, as indicated by some articles and epidemiological studies, needs further exploration. The dual sword role of metformin, on one hand, known to activate AMPK – which in turn inhibits mTOR and suppresses proliferation—but, on the other hand, has also the potential to increase cancer progression, illustrates the intricacy of this relationship.
DISCUSSION
Cancer and T2DM are major public health concerns, and their association has gained significant attention in recent years. Although a direct causal relationship between the two conditions has not been proven, emerging evidence suggests shared risk factors and reciprocal indirect influence. Understanding these connections is crucial for developing preventive strategies and optimizing treatment approaches for both conditions. Hyperglycemia, hyperinsulinemia, chronic inflammation, and obesity contribute to both T2DM and cancer, influencing tumor growth, progression, and metastasis. BCAA levels may represent novel biomarkers that may contribute to both better diabetes control and early pancreatic cancer detection. Further research is needed to elucidate the complex relationship and explore the potential of lifestyle interventions and anti-diabetic medications in cancer prevention and management.
CONCLUSION
Te occurrence of diabetes is also increasing at an alarming rate. Diabetes also increases the risk of certain types of cancers and induces diferent co-morbidities. Hence, efectiveness of the antidiabetic drugs in treating cancer, depends on the co-morbidities, patient’s conditions, stages of the co-morbid diseases, and on the conjunction of other drugs. Te role of antidiabetic drugs within tumor microenvironment needs more studies. Involvement of such diverse factors produces contradictory results of using any drug in diferent patients. Hence, the successful repurposed use of drugs requires disease management with a comprehensive personalized approach. the increasing occurrence of cancer is a concern to healthcare management across the globe. the concern is not only for the loss of lives but also for the affordability of the treatment cost. The complexity of the disease itself requires a variety of drugs. Te increasing price, and availability of cancer drugs are limitations in maintaining a minimalist quality of life for cancer patients. In this context, several known drugs are repurposed to provide benefits to cancer patients at a reduced cost of money and time. Antidiabetic drugs regulating metabolic mechanisms are considered frst-line choices for repurposed use in treating cancer as both diseases have wide overlap in the underlying biological pathways.
ACKNOWLEDGEMENT: -
Authors are thankful to the “first and foremost, I would like to praise and thank God, the Almighty, who has granted countless blessings, knowledge, and opportunity to the writer. The Authors are thankful to the Management & Principal of Satyajeet College Of Pharmacy, Mehkar for Providing facilities to carry out the work. The authors are thankful to Project Guide Prof. Satish G. Lodhe. The authors are also thankful to Prof. Tejas J. Sharma Providing the ideas about the publication.
REFERENCES
- Vincent EE, Yaghootkar H. Using Genetics to Decipher the Link Between Type 2 Diabetes and Cancer: Shared Aetiology or Downstream Consequence? Diabetologia (2020) 63(9):1706–17. doi:10.1007/s00125-020-05228-y
- Zhao Z, Wen W, Michailidou K, Bolla MK, Wang Q, Zhang B, et al. Association of Genetic Susceptibility Variants for Type 2 Diabetes With Breast Cancer Risk in Women of European Ancestry. Cancer Causes Control (2016) 27(5):679–93. doi:10.1007/s10552-016-0741-6
- Sainz J, Rudolph A, Hoffmeister M, Frank B, Brenner H, Chang-Claude J, et al. Effect of Type 2 Diabetes Predisposing Genetic Variants on Colorectal Cancer Risk. J Clin Endocrinol Metab (2012) 97(5):E845–51. doi:10.1210/jc. 2011-2565
- Pierce BL, Ahsan H. Genetic Susceptibility to Type 2 Diabetes Is Associated With Reduced Prostate Cancer Risk. Hum Hered (2010) 69(3):193–201. doi:10.1159/000289594
- Azar M, Lyons TJ. Diabetes, Insulin Treatment, and Cancer Risk: What Is the Evidence? F1000. F1000 Med Rep (2010) 2(4):1–4. doi:10.3410/M2-4
- Shafiei-Irannejad V, Samadi N, Salehi R, Yousefi B, Zarghami N. New Insights Into Antidiabetic Drugs: Possible Applications in Cancer Treatment. Chem Biol Drug Des (2017) 90(6):1056–66. doi:10.1111/cbdd. 13013
- Renehan AG, Howell A. Preventing Cancer, Cardiovascular Disease, and Diabetes. The Lancet (2005) 365(9469):1449–51. doi:10.1016/S0140-6736(05) 66399-4
- Pati S, Irfan W, Jameel A, Ahmed S, Shahid RK. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers (Basel) (2023) 15(2):485. doi:10.3390/ cancers15020485
- Garcia-Jimenez C, Gutierrez-Salmeron M, Chocarro-Calvo A, GarciaMartinez JM, Castano A, De la Vieja A. From Obesity to Diabetes and Cancer: Epidemiological Links and Role of Therapies. Br J Cancer (2016) 114(7):716–22. doi:10.1038/bjc.2016.37
- Nam SY, Lee EJ, Kim KR, Cha BS, Song YD, Lim SK, et al. Effect of Obesity on Total and Free Insulin-Like Growth Factor (IGF)-1, and Their Relationship to IGF-Binding Protein (BP)-1, IGFBP-2, IGFBP-3, Insulin, and Growth Hormone. Int J Obes (1997) 21(5):355–9. doi:10.1038/sj.ijo.0800412
- García-Jiménez C, Garcia-Martinez JM, Chocarro-Calvo A, De la Vieja A. A New Link Between Diabetes and Cancer: Enhanced WNT/?-Catenin Signaling by High Glucose. J Mol Endocrinol (2014) 52(1):R51–66. doi:10. 1530/JME-13-0152
- Rahmoon MA, Elghaish RA, Ibrahim AA, Alaswad Z, Gad MZ, El-Khamisy SF, et al. High Glucose Increases DNA Damage and Elevates the Expression of Multiple DDR Genes. Genes (2023) 14:144. doi:10.3390/genes14010144
- Ramteke P, Deb A, Shepal V, Bhat MK. Hyperglycemia Associated Metabolic and Molecular Alterations in Cancer Risk, Progression, Treatment, and Mortality. Cancers (2019) 11:1402. doi:10.3390/cancers11091402
- Tao H, O’Neil A, Choi Y, Wang W, Wang J, Wang Y, et al. Pre- and PostDiagnosis Diabetes as a Risk Factor for All-Cause and Cancer-Specific Mortality in Breast, Prostate, and Colorectal Cancer Survivors: A Prospective Cohort Study. Front Endocrinol (Lausanne) (2020) 11:60. doi:10.3389/fendo.2020.00060
- Barua R, Templeton AJ, Seruga B, Ocana A, Amir E, Ethier JL. Hyperglycaemia and Survival in Solid Tumours: A Systematic Review and Meta-Analysis. Clin Oncol (2018) 30:215–24. doi:10.1016/j.clon.2018.01.003
- Flier JS, Underhill LH, Dvorak HF. Tumors: Wounds That Do Not Heal. N Engl J Med (1986) 315:1650–9. doi:10.1056/nejm198612253152606
- Yang S, Chintapalli J, Sodagum L, Baskin S, Malhotra A, Reiss K, et al. Activated IGF-1R Inhibits Hyperglycemia-Induced DNA Damage andPromotes DNA Repair by Homologous Recombination. Am J PhysiologyRenal Physiol (2005) 289:F1144–F1152. doi:10.1152/ajprenal.00094.2005
- Rezvani HR, Kim AL, Rossignol R, Ali N, Daly M, Mahfouf W, et al. XPC Silencing in Normal Human Keratinocytes Triggers Metabolic Alterations That Drive the Formation of Squamous Cell Carcinomas. J Clin Invest (2011) 121:195–211. doi:10.1172/JCI40087
- Ciminera AK, Shuck SC, Termini J. Elevated Glucose Increases Genomic Instability by Inhibiting Nucleotide Excision Repair. Life Sci Alliance(2021) 4: e202101159. doi:10.26508/lsa.202101159
- Lee SC, Chan JCN. Evidence for DNA Damage as a Biological Link Between Diabetes and Cancer. Chin Med J (2015) 128:1543–8. doi:10.4103/0366-6999. 157693


 Sumit Chankhore *
Sumit Chankhore *
 Sachin Chalge
Sachin Chalge
 Madan Chankhore
Madan Chankhore




 10.5281/zenodo.14779377
10.5281/zenodo.14779377