Abstract
A derivative of indole, Isatin (2,3-dioxindole) belongs to the heterocyclic chemical class, which is an important class of compounds. In the field of synthetic chemistry, isatin derivatives play a particularly important role since they serve as both a raw material for the synthesis of medications and a substrate for the synthesis of a wide variety of heterocyclic compounds. In the process of developing new drugs, isatin has been utilised to a significant degree. There are a vast number of compounds that can be discovered in nature that include the ring, and it is also a building block for synthetic molecules that can have a wide variety of impacts on biological systems. The strong biological and pharmacological effects of isatin derivatives have made them a hot topic in medicinal and organic chemistry circles as of late. The worldwide healthcare system's mounting expenditures due to increased life spans and the increasing prevalence of inflammatory diseases have put these disorders in the spotlight. In most cases, long-term treatment of chronic diseases requires the use of corticosteroids and non-steroidal anti-inflammatory drugs, which are associated with a number of side effects and toxicity risks. Isatin can exert its effects in a wide range of ways due to its remarkable molecular flexibility. Isatin influences precursors that cause inflammation and pain, like COX1 and COX2, among its many biological roles. Beyond its anticonvulsant and antibacterial effects, it has been found to have anti-neoplastic, anti-hypertensive, anti-tuberculosis, anti-HIV, antiulcer, and antiviral characteristics. Several isatin compounds have shown promise as anti-inflammatory and analgesic medications; we examined them in this paper.
Keywords
Anti-inflammatory activity, Isatin, Mannich base, Schiff base
Introduction
The study of heterocyclic compounds, which are both necessary for life and abundant in nature, is the primary focus of heterocyclic chemistry. Both photochemistry, biochemistry, medicinal chemistry, and dyestuffs are examples of the many scientific fields that make considerable use of heterocyclic compounds[1]. Heterocyclic compounds make up the great bulk of all molecules. Heterocyclic compounds are belongs to the class of chemicals known as isatins. Other sources of isatin have been found, including those derived from plants, animals, and fungi. As a component of coal tar, it is present in the substance. In order to bring about the cyclization of the chloral hydrate, aniline, and hydroxylamine condensation product, sulfuric acid was utilised. Based on a straightforward formula, the Sandmeyer isonitrosoacetanilide isatin synthesis provides a description of this process[2]. The body has both the lactam and lactim forms of insulin. Both of these forms are present. Indole is the chemical that acts as the parent to both of them. Presented here is an illustration of the amido-imidol tautomeric system[3]. The condensation product of chloral hydrate, aniline, and hydroxylamine can be cyclized to produce this compound, which contains sulfuric acid. The chemical process that is being described here is called the Sandmeyer isonitrosoacetanilide isatin synthesis. Isatin is an interesting molecule that has been demonstrated to be effective in a wide variety of biological activities. This review will concentrate on the pharmacological effects of isatin and its derivatives as its primary topic of discussion[4].
Any number of potentially harmful items (such as bacteria, chemicals, physical trauma, or any other event) might trigger the body's inflammatory response. Its primary purpose is to reduce the amount of damage that occurs and to facilitate the recovery of tissues[5]. The surveillance of the immune system, the efficient repair of injury, and the regeneration of damaged tissue all require inflammatory activity. By releasing cells and mediators that fend against intruders and infections, the inflammatory process safeguards our body from harm. Our bodily defences are maintained by this procedure[6]. But excessive, unwarranted inflammation is the root cause of many diseases and disorders, such as psoriasis, inflammatory bowel disease, and rheumatoid arthritis. Inflammation is one of the main signs of autoimmune diseases. Atherosclerosis, cancer, diabetes, heart disease, RA, Alzheimer's disease, and a host of other diseases and conditions fall within this category. Mediators that are released into the bloodstream from the site of inflammation can be the cause of fever[7]. The severity of fever is dependent on the intensity of the process that is causing the inflammation. The inflammatory process can be broken down into two distinct stages: acute and chronic. It is well recognised that different chemical mediators, including as prostaglandins, leukotrienes, platelet-activating factor, and others, are responsible for the generation of acute and chronic inflammations, which are recognised as being complex processes. Medications that reduce inflammation can exert their effects through a wide range of different methods. In the course of an acute inflammatory reaction, which is characterised by an increase in vascular permeability and cellular infiltration, oedema is brought about by the extravasation of fluid and proteins, as well as the transient accumulation of leukocytes at the site of inflammation[8]. It is possible for chronic inflammation to develop when the acute reaction of the body is unable to entirely eliminate chemicals that promote inflammation. The characteristics that define chronic inflammation are the development of fibroblasts, the infiltration of neutrophils, and the exudation of fluid. The process takes place as a consequence of the proliferation of proliferative cells, which might result in the formation of granulomas or the dissemination of such granulomas. Inflammatory illnesses are responsible for a disproportionately high number of sick days claimed by the working population all over the world. The "King of Human Miseries" category was one of the descriptions that was given for them. Pain is an unpleasant sensory and emotional experience that occurs when there is actual or potential inflammation in the tissues. Pain is a manifestation of inflammation or inflammation[9]. It is possible for pyrexia or fever to develop as a consequence of inflammation. On the other hand, inflammation, pain, and fever are all associated with higher amounts of prostaglandins. Because of this, it is reasonable to assume that the majority of anti-inflammatory medications should contain both analgesic and antipyretic qualities. The first successful separation of isatin (1H-indole-2,3-dione, Figure 1) from indigo occurred in 1841 when nitric and chromic acids oxidised indigo. Erdman and Laurent were the pioneers in this process. Isatin has made great strides in organic synthesis due to its adaptability in terms of synthetic characteristics. Sumpter published the first chemistry study of isatin in 1954, Popp published the second review in 1975, and the third review concentrated on isatin's potential as a building block for other heterocyclic compounds[10].
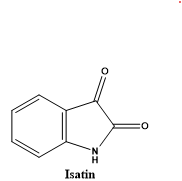
Fig 1: Isatin (1H-indole-2,3-dione)
A ring-containing indole derivative with keto groups at positions 2 and 3, isatin is also known as indoline-2,3-dione or indole-1H-2,3-dione. The structure of the isatin ring is characterised by the combination of one pyrrole ring and one benzene ring[11]. The synthetic versatility of isatin has rekindled curiosity in its bioactivity and chemistry, leading to the creation of stereoselective methods and the improvement of processes for several known reactions. Because of its extremely reactive C3-keto group, which is especially vulnerable to nucleophilic attack, isatin is undeniably fascinating in the area of organic synthesis. It has been found that some isatin compounds have pharmacological effects after reviewing the relevant literature. These actions include those against cancer, epilepsy, inflammation, and pain, as well as against viruses and bacteria. As seen in Figure 2, the isatin moiety can be found in a number of different drugs[12].

Figure 2: Isatin moiety in drugs are shown
Chemistry and classification of NSAIDS:
A huge class of medications with remarkable structural and functional diversity is the class of NSAIDs, or nonsteroidal anti-inflammatory medicines. A significant number of these molecules are weak organic acids, which are characterised by the presence of both an acidic and an aromatic functional group together. Isoform-specific selectivity for PGHS inhibition is one method that can be utilised to discriminate across compounds in terms of their functional variety. It is also possible to categorise nonsteroidal anti-inflammatory drugs (NSAIDs) according to their serum bioavailability, which takes into account the pharmacokinetic aspects of their systemic activity. The next section explores the categorization of NSAIDs, which stands for nonsteroidal anti-inflammatory drugs[13].
NSAIDS classification based on chemical structure:
In terms of their chemical composition, nonsteroidal anti-inflammatory medications (NSAIDs) can be classified into many primary categories. These categories include salicylates, indole/indene acetic acid derivatives, oxicams, anthranilates, and aryl and heteroarylacetic acid derivatives[14].
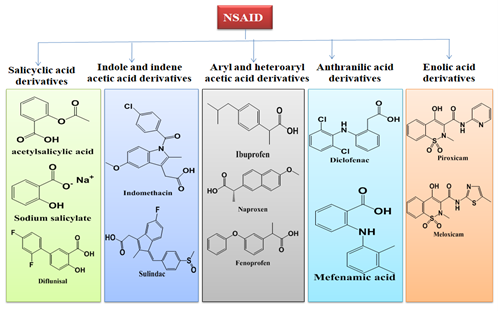
Figure 3: Classification of NSAIDS
Mode of action of NSAIDs :
The use of natural salicylates and salicyclic acid in medical applications dates back a significant amount of time. It wasn't until 1860 that salicylic acid, which has antibacterial, antipyretic, and antirheumatic characteristics, was synthesised using chemical mechanisms. With the development of aspirin approximately forty years later, a salicylate that was more tolerable was created. The "aspirin-like drugs" were a class of treatments that arose immediately after aspirin due to the fact that they exhibited the same mechanisms of action as aspirin. These medications are now known as NSAIDs, or nonsteroidal anti-inflammatory drugs. Nonsteroidal anti-inflammatory drugs (NSAIDs) were believed to work by blocking the production of prostaglandins, according to a theory put forth twenty-five years ago. The existence of two isoforms of the enzyme cyclo-oxygenase (COX)—COX-1, the constitutive isoform, and COX-2, the inducible isoform is now well known[15].
Since then, the prevalent view that these drugs act by suppressing COX has grown. The functions of COX-1 from a physiological standpoint are well-defined. As an illustration, when it is active, it creates prostacyclin, which, when produced by the endothelium and the stomach mucosa, respectively, possesses qualities that are antithrombogenic and cytoprotective. COX-2, which was discovered in migratory and other cell types six years ago, is stimulated by cytokines and stimulating stimuli that are associated with inflammation [16]. Nonsteroidal anti-inflammatory medicines (NSAIDs) are believed to have their anti-inflammatory benefits through inhibition of COX-2, while their undesired side effects, like stomach lining irritation, are believed to be caused by inhibition of COX-1. There will be fewer adverse effects and a stronger anti-inflammatory benefit from medications with a greater COX-2 activity and a better COX-2:COX-1 activity ratio compared to those with a lower ratio. This is due to the fact that the activity ratio of COX-2 relative to COX-1 is greater. Research into specific COX-2 inhibitors will, thus, pave the way for new medicinal developments [17].
Source of data:
We collect all the information from different online sources NCBI, Pubmed, Scopus, International and others indexed journals and this all give us valuable information for this successfully studies of Anti inflammatory activities of Isatin.
Synthesis of Isatin derivatives:
Isatin and various derivatives of it are still manufactured using the Sandmeyer synthesis, which has been around for a very long time and is still utilised there today. With that being said, it is restricted to the most fundamental analogies. We have already covered metalation of anilide derivatives and other alternative synthetic methods like the Gassman, Martinet, and Stolle procedures[18]. Producing inseparable mixes of regioisomeric products on substrates that contain m-substituted or electron-withdrawing substituents might result in low yields. There may be issues with this. The graphic shows the synthesis of many derivatives that are involved in biological activities. It shows the substitution of an aryl ring (A), alkylation of a nitrogen atom in the moiety (B), and changes at its C2 and/or C3 carbonyl functionalities. Because of this, we have discussed a variety of various approaches to the synthesis of these derivatives[19].

Figure 4: Various substitutions on the Isatin scaffold
N-Substituted Isatin derivatives:
In the field of biology, N-substituted isatin compounds have numerous potential uses. One way they do this is by blocking the action of carbonic anhydrase isoform IX (CA IX), an overexpressed variant of the enzyme found in many solid tumours[20]. Several new synthetic methods have been developed for the purpose of producing N-substituted isatins. With I2-DMSO as a catalyst, one can achieve a metal-free synthesis. There have been many such efforts like this one. According to the plan, 2-amino acetophenones will undergo internal cyclization before being activated in order to produce N-alkylated and N-arylated isatins[21].
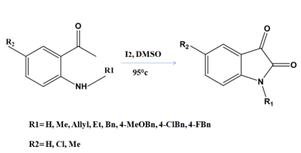
Figure 5: Synthesis of N-alkylated /arylated isatin derivatives from 2-amino acetophenones
C2-Substituted Isatin derivatives :
An essential group of C2-substituted isatin derivatives are 2-hydroxy-2-substituted indol-3-ones with a C2-quaternary centre[22]. As well as being essential building blocks for the synthesis of numerous bioactive and naturally occurring compounds, these derivatives are also constituents of these compounds. Using ceric ammonium nitrate (CAN) and 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) as oxidants, 2-hydroxy-2-substituted indol-3-ones with a C2-quaternary centre have been recently synthesized[23].
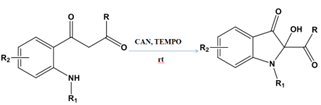
Figure 6: Synthetic routes to 2-hydroxy-2-substituted –indol-3-ones
Anti inflammatory activities of Isatin derivatives :
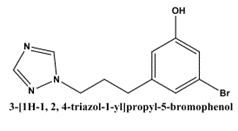
Pramod kumar Sharma et al(2016)[24] showed the synthesis and anti-inflammation activity evaluation of novel triazolyl-isatin hybrids. These hybrids were created and tested to see if they could inhibit the production of Intercellular Adhesion Molecule-1 (ICAM-1) on human endothelial cells, a molecule activated by TNF-?. The two compounds were derived from isatin and triazole. Results from structure-activity relationship (SAR) tests showed that the synthesised compounds' anti-inflammatory potential was significantly increased after the addition of the electron-attracting bromo substituent at position-5 of the isatin moiety. (3)[1H-1, 2, 4-triazol-1-yl] is its chemical formula.5-bromophenol was the name of the compound. In chemical terms, the substance 3-[2-(4-methoxyphenyl)hydrazono]In terms of effectiveness, indolin-2-one (19) stood out among the synthesised molecules. The compound showed an 89% reduction of ICAM-1 at a minimal inhibitory concentration (MTD) of 200 µM and an IC50 value of 20 µM. When a 1, 2, 4-triazole ring and an electron-donating methoxy group were added to the phenylhydrazone moiety, the anti-inflammatory activity of the composition was quadrupled.
Dantas et al(2020) showed the study of anti inflammatory and antinociceptive effects of the isatin derivative. Researchers are interested in several of these synthetic chemicals because of the powerful biological activity that isatin derivatives have. In light of this, the present work set out to examine, in a mouse model, the anti-inflammatory and antinociceptive properties of COPHCT, an isatin derivative. We checked the outcomes at1.2,2.5, and 5.0 mg/kg of this drug. We used two models to find out how well the anti-inflammatory medication worked: one that used carrageenan to make paw edoema and another that used zymosan. The antinociceptive efficacy of the drug was evaluated using both formalin and the acetic acid-induced abdominal writhing test. The paw edoema test showed that all of the chemical doses significantly reduced the edoema by the second hour, but the reaction was substantially stronger in the fourth hour. Results showed that at all doses tested, zymosan significantly reduced leukocyte migration and total protein concentration levels in the zymosan-induced air pouch model. Phase one of the formalin test revealed that dosages of1.0,2.5, and 5.0 mg/kg of COPHCT had no effect. Conversely, they reduced the length of time that paw pain lasted by 73.61%, 79.56%, and 73.15% in the second phase, respectively. There was a dose-dependent response, however at 5.0 mg/kg COPHCT, there was a notable reaction that reduced the frequency of abdominal writhings by 24.88%. These studies proved that this chemical can reduce edoema's anti-inflammatory effects and its associated vascular permeability and cell migration. The substance's potential antinociceptive effects may also be affected by the dosage[25].
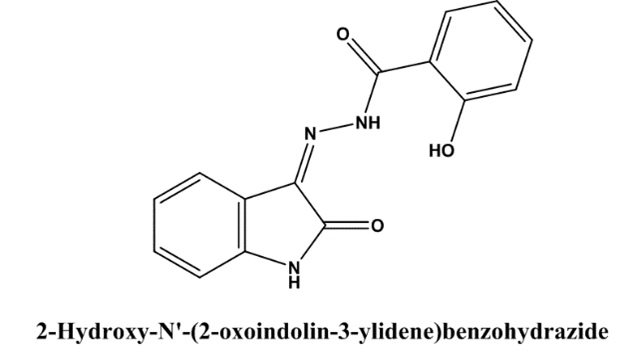
Ravi jarapula et al(2016) showed the study of In vivo anti inflammatory activity and molecular docking studies of New Isatin derivatives. Condensation of 2-hydroxybenzohydrazide with substituted isatins allowed for the creation of a novel class of 2-hydroxy-N?-(2-oxoindolin-3-ylidene) benzohydrazide derivatives. The synthesised molecules underwent chemical examination using methods like mass spectrometry, 1H-NMR, and Fourier transform infrared spectroscopy. The chemicals were also tested for their ability to decrease inflammation in living beings using the carrageenan-induced paw edoema technique. From modest to substantial, the anti-inflammatory effects of the medicines studied were all over the map. The 65% and 63% reductions in paw edoema, respectively, were attributed to compounds VIIc and VIId. Molecular docking studies were conducted using VLife MDS 4.3 with the active sites of COX-1 and COX-2 as targets (PDB IDs: 3N8Y and 3LN1, respectively). Compounds VIIc, VIId, and VIIf showed good docking scores of -57.27, -62.02, and -58.18 into the active site of COX-2, which is comparable to the conventional COX-2 inhibitor celecoxib. Additionally, COX-1 enzymes had the lowest dock values of -8.03, -9.17, and -8.94, respectively. A strong correlation was discovered between in silico and in vivo investigations. The synthetic chemicals VIIc, VIId, and VIIf may possess therapeutic promise, as indicated by docking and anti-inflammatory studies[26].
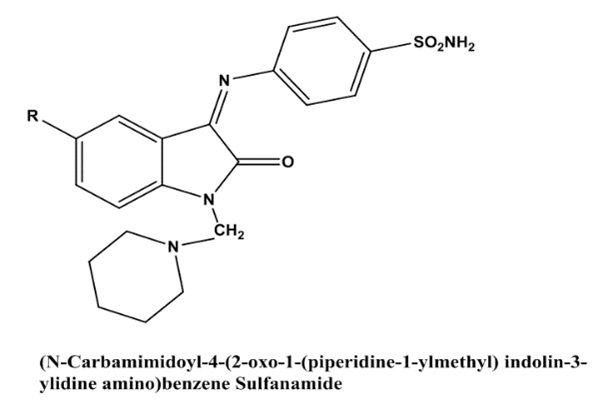
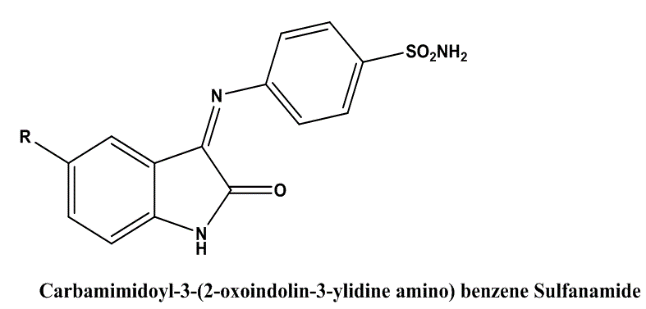
B Durga Prasad et al(2012) showed the study of synthesis, characterization and anti inflammatory activity of Isatin derivatives. A low amount of fresh isatin and other p-substitute anilines were used to produce (N-carbamimidoyl 1-4-(2-oxo-1-(piperidine-1-ylmethyl) indolin-3-ylidineamino) benzene sulphonamide. The procedure for treating glacial acetic acid reflux with isatin and sulfanilamide is outlined in the following sections (a-h). Creating carbamimidoyl-3-(2-oxo-indalin-3-yl)indene amino) benzene sulphonamide (a-h) using organic means The final step in making the (Ncarbamimidoyl 1-4-(2-oxo-1-(piperidine-1-ylmethyl) indolin-3-ylidineamino) benzene sulphonamide (a-h) was a Mannich reaction using piperidine and formaldehyde. The amount of microwave energy used to conduct this reaction was 140 watts. The old approach took a very long period, but microwave irradiation produced much higher yields in much less time. The newly synthesised compounds were characterised using a battery of analytical tools, including elemental analysis, mass spectra, infrared spectroscopy, and hydrogen nuclear magnetic resonance. We tested the anti-inflammatory properties of all the synthesised isatin derivatives in this work[27].
Ajmer Singh Grewal et al(2014) showed the Isatin derivatives with several biological activities. A derivative of indole, isatin (2,3-dioxindole) belongs to the heterocyclic chemical class, which is an important class of compounds. In the field of synthetic chemistry, isatin derivatives play a particularly important role since they serve as both a raw material for the synthesis of medications and a substrate for the synthesis of a wide variety of heterocyclic compounds. Isatin derivatives have recently attracted a lot of attention in the fields of medical and organic chemistry due to the potent biological and pharmacological effects that they possess. A wide variety of pharmacological actions are exhibited by isatin and its derivatives. These effects include anticonvulsant, antiviral, antihelmintic, anti-HIV, antioxidant, anti-inflammatory, anticancer, antimycobacterial, and depressive effects on the central nervous system. In this overview, we will discuss some of the most common methods for producing isatin and its derivatives, as well as the significant progress that scientists have made in terms of harnessing the biological and pharmacological effects of these substances[28].
Kosaraju Lahari et al(2020) showed the study of design and synthesis of novel Isatin derivatives as potent anti inflammatory agents. By synthesising a number of distinct indole-2,3-dione derivatives, a potent analgesic, anti-inflammatory, and antibacterial medication could be developed. By utilising elemental and spectral investigations, the synthetic derivatives' molecular structures were confirmed. Use of the tail flick method, the carrageenan-induced foot paw edoema technique, and the agar streak dilution method were employed, respectively, to assess the analgesic, anti-inflammatory, and antibacterial properties of different drugs in vitro. Furthermore, the potential for ulcerogenicity of strong compounds was assessed and approximated. Furthermore, the synthesised compounds exhibited mild to good analgesic, anti-inflammatory, and antibacterial potencies, and the ulcer index was low to reasonable. We now understand the structural anisotropy relationships (SARs) between the biological activity of the substances in the title and their functional group changes. the chemical formula Here is the chemical formula for 1-(4-chlorobenzylidene)-4-(4-(1-((dimethylamino)methyl))A highly effective variation of this series, 5-nitro-2-oxoindolin-3-ylideneamino)phenyl)thiosemicarbazide VIc, has been identified[29].
M. Chaitanya et al (2019) revealed the results of docking and in silico investigations into potential anti-inflammatory effects of new isatin compounds. The goal of this effort is to synthesise several new isatin derivatives using the results of the in silico studies as a foundation. In order to produce the compounds in issue, p-dimethyl amino benzaldehyde and substituted isatins were utilised in the scientific process. In order to obtain substituted isatin-3-hydrazones, the first step is to treat substituted isatins with hydrazine hydrate at the appropriate concentration. Through the use of P-Dimethyl amino benzaldehyde, the subsequent step involves the production of new isatin derivatives. This is accomplished by treating isatin-3-hydrazone. Every single one of these isatin derivatives has undergone molecular docking tests to see how well they work as anti-inflammatory drugs. The COX-2 enzyme's active site (PDB ID: 5F19) was the focus of the molecular docking studies. With the highest binding energy and docking score among all the newly synthesised derivatives, the 5,6 dichloro substituted chemical (IIIe) stood out. As a result, the anti-inflammatory activity has been established, which is a positive development. A number of compounds, such as IIId, IIIh, III, and IIIe, have activity levels that are considered to be moderate. The data obtained from these in silico and docking investigations were initially intended to be used in the manufacture of compounds that have anti-inflammatory properties[30].

Da Fonseca et al(2021) found that Swiss mice treated with an Isatin derivative had anti-inflammatory and anti-nociceptive properties. Since pain and inflammation are signs of many different diseases and can be treated in many ways, it is crucial to study the therapeutic effects of new compounds. Among the biological features of isatin thiosemicarbazone that have been shown in previous study are its anticancer, antifungal, and antibacterial activity. This study set out to examine the anti-inflammatory and anti-nociceptive properties of (Z) 2 (5 nitro 2-oxoindolin-3 ilidene) N hydroazinecarbothioamide (PA Int5) and its effects on the central nervous system (CNS) of mice. This was done because these chemicals have complex biological impacts. Using nociceptive and inflammatory animal models, researchers examined three distinct oral doses of PA Int5. One, two, and five milligrammes per kilogramme were the doses. The sedative effects of PA Int5 (5 mg/kg, oral gavage) were studied using rotarod and open field testing. This was done to further eliminate the potential of nonspecific effects caused by the nociceptive tests. Two models, one produced by carrageenan and the other by zymosan, were used to assess the efficacy of anti-inflammatory drugs. In contrast, the efficacy of anti-noceptive drugs was investigated using the formalin and acetic acid-induced belly contortion tests. The abdominal contortion paradigm was used to test PA Int5's antinoceptive efficacy at a dosage of 5 mg/kg. The second-phase nociception in the formalin test was observed to be diminished when 2.5 and 5 mg/kg of PA Int5 were delivered. Neither the patients' motor coordination nor their ability to move on their own will were affected by the highest dose of PA Int5 that was studied. Results showed that carrageenan considerably decreased paw edoema levels after injection at all doses studied. The zymosan-induced air pouch model showed that PA Int5 significantly decreased inflammation-related leukocyte migration and protein levels. Taken together, these findings show that PA Int5 has anti-inflammatory and anti-nociceptive properties, which could make it (and its derivatives) a new and helpful tool in the fight against pain[31].
Alejandro cenalmor et al(2023) showed the evaluation of Anti-neuroinflammatory activity of Isatin derivatives. Many neurodegenerative diseases, including Alzheimer's, proceed due to inflammation in the neurological system. Among the many symptoms seen in neurological diseases caused by microglia that have become excessively active are neurotoxicity and a prolonged inflammatory response. In this study, lipopolysaccharide-triggered microglia are utilised as a cell model. We assess the possible anti-neuroinflammatory effects of a range of isatin derivatives that we synthesise. We tested four distinct isatin moiety substitutions on BV2 microglia cells to see whether any of them had anti-inflammatory properties. The strongest favourable effects were observed at a concentration of 25 µM for the chlorinated compound 20 and the N1-alkylated compound 10. Low cytotoxicity and the ability to decrease microglial cell release of nitric oxide, pro-inflammatory interleukin 6, and tumour necrosis factor ? were indicators of this. The results show that compounds 10 and 20 could be great lead compounds in the search for new neuroprotective medications given their combined potential[32].
Venkateshwarlu E et al(2012) One of the many naturally occurring chemicals with pharmacological properties is isatin. The goal of this animal study was to examine the anti-inflammatory, analgesic, and antipyretic properties of several isatin derivatives, such as Isatin-3-[N2-(2-benzalaminothiazol-4-yl)]hydrazones (IA-IJ). Indomethacin, at a dose of 10 mg/kg, served as the standard for this study. Results were presented for both the 10 mg/kg and 100 mg/kg doses. To assess carrageenan's anti-inflammatory efficacy, the rat paw edoema model was employed. Using the plethysmometer, we were able to measure the average rise in paw volume and the percentage inhibition of paw volume at different time intervals. Utilising a Brewer's yeast-induced pyresis model and a decrease in rectal temperature, the efficacy of antipyretics was assessed. The analgesic effect was achieved by employing Eddy's hot plate technique. There was a dose-dependent effect (p<0>
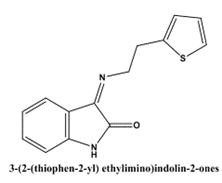
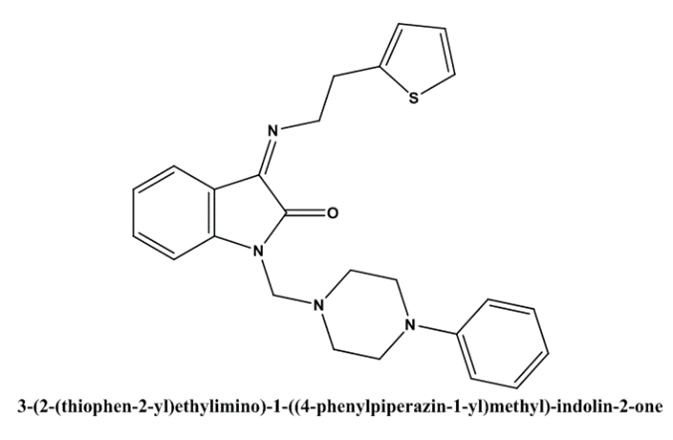
Bhushan D Varpe et al(2022) showed Docking studies and in vitro inflammation reduction efficacy assessments were part of the research on Isatin's schiff base with 2-thiopheneethylamine and its mannich bases. Antitubercular and anti-inflammatory effects were investigated in vitro using Schiff base conjugates of isatin with 2-thiopheneethylamine and its Mannich bases 3 (2-(thiophen-2-yl) ethylimino)indolin-2-ones. A acceptable yield was achieved during the synthesis of compounds I through VIII. A percentage inhibition of denaturation of bovine serum albumin (BSA) was used to test the drugs' anti-inflammatory efficacy in vitro. The compounds' antitubercular activity was also assessed by testing them against Mycobacterium tuberculosis (MTB H37Rv strain, ATCC 27294) using the Almar blue assay. Among the compounds that were synthesised, 3-(2-(thiophen-2-yl)ethylimino)-1-((4-phenylpiperazin-1-yl)methyl)-indolin-2-one (VII) exhibited the most effective antitubercular and anti-inflammatory properties, with MIC values of 3.12 µg/mL and 81.85% inhibition of denaturation of BSA at 100 µg/mL, respectively. The results of the docking investigation show that compounds with anti-inflammatory characteristics may be able to attach to the receptor protein. To investigate the possible pharmacokinetic properties, analyses of ADME and in silico drug-likeness were performed[34].
Maharaj pogula et al(2012) published research on the anti-inflammatory effects of newly synthesised isatin derivatives. The synthesis of N1-(2-oxo2, 3-dihydro-1H-indol-3-yl) benzohydrazide derivatives was achieved by coupling benzohydrazide with a number of substituted isatins.Techniques such as chromatography, infrared spectroscopy, nuclear magnetic resonance (1HNMR), and mass spectrometry were utilised in order to investigate the chemicals that were synthesised. In order to determine whether or not the chemicals that were synthesised were effective in reducing inflammation in living organisms, the induced rat paw edoema method was utilised. According to the results of acute toxicity tests conducted on rats, the compounds were not harmful at doses of up to 500 mg/kg (b.w.) when they were supplied intraperitoneally. Our results show that VI a(R=H), VI d(R=5-Cl), Vie(R=5-F), and VIh(R=6-Br) are reasonably active compounds[35].
Farshid et al(2021) showed the study of synthesis and anti-inflammatory effects of 1,3 substituted isatin derivatives. Inflammation can be triggered by pathophysiological factors such as infections and physical injuries, which results in the accumulation of plasma fluid and blood cells in particular regions of the body. It is fascinating to consider the biological profile of isatin, which has the potential to be a heterocyclic molecule. In this work, many isatin derivatives that contained Schiff and mannich base fragments were synthesised by a procedure that consisted of three stepping steps. The formation of benzohydrazide compounds was accomplished through the reaction of benzoat derivatives with hydrazine hydrate substance. There was a production of Schiff bases through the process of submitting isatin to the benzo hydrazide derivatives. In order to generate the required mannich bases, the Schiff bases were coupled with amine derivatives through a process that involved formaldehyde and trifluoroacetic acid. In order to obtain the final product in the case of 2-hydroxybenzohydrazide, the corresponding mannich base derivative was subjected to condensation with 2-hydroxybenzohydrazide. The croton oil-induced ear edoema test was utilised in order to evaluate the anti-inflammatory capabilities of the compounds that were ultimately finalised. Among the medications that were investigated, compound b3, which was made up entirely of the mannich base fragment, was the one that was shown to be especially effective in lowering inflammation. Compounds comprising C2 and C3, which include Schiff and mannich base groups, have a moderate level of anti-inflammatory activity. The result is that the existence of substituents at the 1 position of the isatin nucleus, as well as the type of substituents that are present, are believed to have a substantial impact on the anti-inflammatory actions of these compounds[36].
Chinnasamy rajaram et al(2014) Isatin derivatives with new Schiff and Mannich bases have been synthesised and investigated for their potential to alleviate pain, reduce inflammation, and inhibit the growth of microorganisms. Inventive and original there is a compound known as 3-(4-(2-(substitutedbenzylideneamino)thiazol-4-yl)phenylimino((dimethylamino)methyl'. Reactions between a number of aromatic aldehydes and isatin derivatives resulted in the production of Schiff and Mannich base derivatives of -5-fluoroindolin-2-one. In order to verify the chemical structures of all of the compounds that were synthesised, various techniques such as infrared, 1H-NMR, mass spectra, and elemental analysis were utilised. We carried out tests to determine whether or not each synthetic compound possessed analgesic, anti-inflammatory, and antibacterial characteristics. Several of the compounds that were synthesised showed promising results in terms of their ability to alleviate pain, reduce inflammation, and inhibit the growth of bacteria when tested. The chemicals that were found to be effective analgesics, anti-inflammatory agents, and antibacterial agents during this examination will be the subject of subsequent research that will be carried out in our laboratory[37].
Elham jafari et al(2022) There are a number of disorders that are linked to inflammation, which is the body's defence system. The cyclooxygenase enzymes are responsible for the production of prostaglandins, which are an important component of the inflammatory responses. Through the use of inhibitors of these enzymes, inflammation can be efficiently treated effectively. Isatin derivatives have been found in a few studies to be capable of inhibiting these enzymes, which can contribute to a reduction in inflammation. Scientific investigations in the field of medicinal chemistry have demonstrated that isatin and its derivatives possess a remarkable biological profile, particularly with regard to the anti-inflammatory effects that they possess. An example of a heterocyclic molecule is isatin. The purpose of this study was to investigate the interaction of isatin derivatives with cyclooxygenase enzymes in order to give a plausible mechanism. The results of the experiments were confirmed by scientific theory. In this study, a docking programme was employed to investigate the possible interactions that could occur between specific isatin-based derivatives and the cyclooxygenase 1 and 2 enzymes. These enzymes have been shown to have anti-inflammatory properties in in vitro testing. Research was also conducted on the pharmacokinetic properties of the chemicals that were synthesised, as well as investigation into whether or not they adhered to the Lipinski law. It has been discovered that the two varieties of cyclooxygenase enzymes have the lowest binding energies to the molecules I2 and I3, which are the lowest binding energies. All of the compounds that were examined were found to be in compliance with Lipinski's rule. In accordance with the findings of docking research, in vitro anti-inflammatory testing indicated that compounds I2 and I3 had a modest level of anti-inflammatory impact. By inhibiting this enzyme, it is possible to obtain evidence regarding the possible mechanism of action of these drugs. It is necessary to do additional confirmation through the use of diagnostic kits that contain these chemicals and tests that involve enzyme inhibition[38].
Shobhit et al(2023) The worldwide healthcare system's mounting expenditures due to increased life spans and the increasing prevalence of inflammatory diseases have put these disorders in the spotlight. In most cases, long-term treatment of chronic diseases requires the use of corticosteroids and non-steroidal anti-inflammatory drugs, which are associated with a number of side effects and toxicity risks. Finding and developing anti-inflammatory drugs with few side effects has been a leading research priority for many scientists. Isatin can exert its effects in a wide range of ways due to its remarkable molecular flexibility. Isatin influences precursors that cause inflammation and pain, like COX1 and COX2, among its many biological roles. Beyond its anticonvulsant and antibacterial effects, it has been found to have anti-neoplastic, anti-hypertensive, anti-tuberculosis, anti-HIV, antiulcer, and antiviral characteristics. Here we investigated several of the isatin-containing compounds that have previously shown promise as anti-inflammatory and analgesic drugs. The goal of this review was to compile useful information that might aid in the creation of safe and effective anti-inflammatory and analgesic drugs[39].
Perumal et al(2010) Condensation of 5-substituted imesatin with various substituted aromatic aldehydes allowed for the creation of novel schiff bases in this study. We used infrared, 1H-NMR, 13C-NMR, mass spectrometry, and elemental analysis to validate the chemical structures of the compounds that were generated. To study the analgesic, anti-inflammatory, and antibacterial effects of these synthetic compounds, the researchers used the Tailimmersion method, the carrageenan-induced paw oedema method, and the paper disc diffusion strategy. We also calculated the MICs of each drug by using the Agar streak dilution method. It was found that the majority of the compounds that were synthesised possessed significant anti-bacterial and anti-fungal effects. Among all of the compounds that were synthesised, compounds 5b, 5h, and 5i had the most remarkable anti-inflammatory and analgesic properties[40].
K Swathi et al(2014) The results of this investigation present a number of unique 5-[2(3)-dialkylamino alkoxy] compounds. 4-thiosemicarbazone 2-ones and 5-[2(3)-dialkylamino alkoxy] are examples of indole compounds. The synthesis of indole 3-hydrazone 2-ones consisted of the production of 5-hydroxy isatin. Analysis techniques such as infrared, nuclear magnetic resonance, and mass spectrometry were utilised in order to characterise the product structures at hand. Additional research was conducted on the anti-inflammatory impact of the target compounds that were synthesised and characterised. The research was conducted using a rat model of carrageenan-induced paw edoema. The anti-inflammatory effect of the newly synthesised derivatives was low, whereas that of compounds IIIc-e and Vc-e was equally moderate. Compounds IIIa-b and Vab showed the most promise in terms of anti-inflammatory activity, leading to a notable decrease in inflammation[41].
Prajakta et al(2021) An anti-inflammatory and anticonvulsant derivative of substituted coumarin acetohydrazide is to be designed and produced.The anticonvulsant and anti-inflammatory characteristics are the primary areas of experimental exploration in this work.We performed experiments to find out how7-hydroxy-4-methyl-coumarin and substituted isatin in glacial acetic acid interacted so that we could study the effects of coumarin acetohydrazide derivatives on the anti-inflammatory and anticonvulsant characteristics of these substances. Through the use of molecular docking, we examined the COX and carbonic anhydrase II enzymes for potential anticonvulsant and anti-inflammatory effects, respectively. The anti-inflammatory activity in rats was evaluated using the carrageenan-induced paw edoema technique. A dosage of 200 milligrammes per kilogramme of body weight was prescribed. The anticonvulsant effects in rats were investigated using the maximum electroshock (MES) model at a dosage of 200 milligrammes per kilogramme of body weight. in combination with (Z)-2-(4-methyl-2-oxo-2H-chromen-7yl)oxy)-N-(5-nitro-2-oxoindolin-3-ylidine Interaction levels between celecoxib and aceto-hydrazide-N-(1-methyl-2-oxoindolin-3-ylidene) acetohydrazide ranged from 71.94% to 77.06?ter five hours, indicating that the former was more effective at reducing inflammation. The majority of the compounds showed anticonvulsant action when given at a dosage of 200 mg/kg, according to the MES screen. The efficacy of twelve different drugs in preventing MES-induced convulsions was assessed. Acetohydrazide and another molecule with the same molecular formula but a different ring configuration were the most active. These two compounds offered the highest level of protection, which was 67%. A substantial amount of pharmacophoric information was obtained from the structure-activity relationship, which demonstrated that the isatin ring swap considerably reduced the development of seizures and inflammation. The biological activity and molecular docking analysis show that all of the coumarin acetohydrazide derivatives that were synthesised are more effective at reducing inflammation than Celecoxib and more effective at reducing seizures than phenytoin[42].
Rajarshi et al(2020) In the process of developing new drugs, isatin has been utilised to a significant degree. There are a vast number of compounds that can be discovered in nature that include the ring, and it is also a building block for synthetic molecules that can have a wide variety of impacts on biological systems. qualities such as anticancer, anti-inflammatory, antiviral, anticonvulsant, antitubercular, antidiabetic, and antibacterial have been established by these heterocycles that include isatin. There are numerous other qualities that have also been demonstrated. Isatin derivatives can be synthesised using a number of different methods, two of which being the Sandmeyer and Stolle processes. N-pyrazoloyl hydrazone of isatin and N-thiopheneacetyl hydrazone of isatin shows good inflammatory activities. Numerous researchers have taken use of the isatin moiety by using the NH at the first position, as well as the C2 and C3 carbonyl locations, in order to synthesise derivatives that exhibit a wide range of biological activity. Furthermore, it provides vital information regarding the structure-activity relationships and docking studies of isatin derivatives, both of which contribute to the potent pharmacological profile of these compounds, in addition to providing structural insights. In addition to this, the cataloguing of patents and medications that include isatin is related to this[43].


CONCLUSION
Isatin is a scaffold that is present in a wide variety of pharmaceutically significant compounds, both naturally occurring and synthesised. The chemicals that it contains, particularly isatin, have been shown to have anti-inflammatory properties. There is a two-way cycle in which inflammation causes certain diseases; it is possible for inflammation to help in the treatment of certain diseases, and vice versa; hence, inflammation can be caused by certain diseases. Due to the various unfavourable side effects that are associated with synthetic anti-inflammatory drugs, it is not recommended to use these medications for a lengthy period of time. Inflammation is typically treated with these drugs, which are regularly used. Therefore, anti-inflammatory medications that are both effective and safe are required. A significant amount of interest among researchers is directed towards the discovery of anti-inflammatory medications that are both effective and have minimal or no negative side effects. The discovery of isatin represents a big step forward in our efforts to find novel analgesic and anti-inflammatory chemicals that have powerful biological effects and a lower propensity for ulcerogenicity.
REFERENCE
- Mondal P, Jana S, Bose A, Banerjee M. Synthesis and evaluation of 1, 3 Di-substituted schiff, mannich bases and spiro isatin derivatives. Journal of Young Pharmacists. 2010 Apr 1;2(2):169-72.
- Singh GS, Desta ZY. Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks. Chemical Reviews. 2012 Nov 14;112(11):6104-55.
- Ibrahim MM, Elsaman T, Al-Nour MY. Synthesis, Anti?Inflammatory Activity, and In Silico Study of Novel Diclofenac and Isatin Conjugates. International journal of medicinal chemistry. 2018;2018(1):9139786.
- Pandurangan K, Krishnappan V, Subramanian V, Subramanyan R. Antinociceptive effect of certain dimethoxy flavones in mice. European Journal of Pharmacology. 2014 Mar 15;727:148-57.
- Singh GS, Desta ZY. Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks. Chemical Reviews. 2012 Nov 14;112(11):6104-55.
- Jabbar SS, Najim SM, Fadhil AA. Evaluation of anti-inflammatory activities of newly synthesized isatin derivatives in rats. Int Res J Pharm. 2019;10(2):75-80.
- Mohsin NU, Irfan M. Selective cyclooxygenase-2 inhibitors: A review of recent chemical scaffolds with promising anti-inflammatory and COX-2 inhibitory activities. Medicinal Chemistry Research. 2020 May;29:809-30.
- Prakash CR, Raja S, Saravanan G. Design and synthesis of 4-(1-(4-chlorobenzyl)-2, 3-dioxoindolin-5-yl)-1-(4-substituted/unsubstituted benzylidene) semicarbazide: Novel agents with analgesic, anti-inflammatory and ulcerogenic properties. Chinese Chemical Letters. 2012 May 1;23(5):541-4.
- Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proceedings of the society for experimental biology and medicine. 1962 Dec;111(3):544-7.
- Pakravan P, Kashanian S, Khodaei MM, Harding FJ. Biochemical and pharmacological characterization of isatin and its derivatives: from structure to activity. Pharmacological Reports. 2013 Mar;65(2):313-35.
- Bekircan O, Bektas H. Synthesis of Schiff and Mannich bases of isatin derivatives with 4-amino-4, 5-dihydro-1H-1, 2, 4-triazole-5-ones. Molecules. 2008 Sep 10;13(9):2126-35.
- Kumar PR, Raju S, Goud PS, Sailaja M, Sarma MR, Reddy GO, Kumar MP, Reddy VK, Suresh T, Hegde P. Synthesis and biological evaluation of thiophene [3, 2-b] pyrrole derivatives as potential anti-inflammatory agents. Bioorganic & medicinal chemistry. 2004 Mar 1;12(5):1221-30.
- Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochemical pharmacology. 2020 Oct 1;180:114147.
- Ong CK, Lirk P, Tan CH, Seymour RA. An evidence-based update on nonsteroidal anti-inflammatory drugs. Clinical medicine & research. 2007 Mar 1;5(1):19-34.
- Dhalla IA, Gomes T, Mamdani MM, Juurlink DN. Opioids versus nonsteroidal anti-inflammatory drugs in noncancer pain. Canadian Family Physician. 2012 Jan 1;58(1):30-.
- Arfè A, Scotti L, Varas-Lorenzo C, Nicotra F, Zambon A, Kollhorst B, Schink T, Garbe E, Herings R, Straatman H, Schade R. Non-steroidal anti-inflammatory drugs and risk of heart failure in four European countries: nested case-control study. bmj. 2016 Sep 28;354.
- Matsui H, Shimokawa O, Kaneko T, Nagano Y, Rai K, Hyodo I. The pathophysiology of non-steroidal anti-inflammatory drug (NSAID)-induced mucosal injuries in stomach and small intestine. Journal of clinical biochemistry and nutrition. 2011;48(2):107-11.
- Sandmeyer T. Über isonitrosoacetanilide und deren Kondensation zu Isatinen. Helvetica Chimica Acta. 1919;2(1):234-42.
- Eldehna WM, Abo-Ashour MF, Nocentini A, El-Haggar RS, Bua S, Bonardi A, Al-Rashood ST, Hassan GS, Gratteri P, Abdel-Aziz HA, Supuran CT. Enhancement of the tail hydrophobic interactions within the carbonic anhydrase IX active site via structural extension: Design and synthesis of novel N-substituted isatins-SLC-0111 hybrids as carbonic anhydrase inhibitors and antitumor agents. European journal of medicinal chemistry. 2019 Jan 15;162:147-60.
- Reddy MR, Rao NN, Ramakrishna K, Meshram HM. I2–DMSO promoted intramolecular oxidative cyclization of 2-(aryl or alkyl amino)-acetophenones for the synthesis of isatins. Tetrahedron Letters. 2014 Aug 20;55(34):4758-62.
- Zhang C, Li S, Bures? F, Lee R, Ye X, Jiang Z. Visible light photocatalytic aerobic oxygenation of indoles and pH as a chemoselective switch. ACS Catalysis. 2016 Oct 7;6(10):6853-60.
- Satish G, Polu A, Ramar T, Ilangovan A. Iodine-mediated C–H functionalization of sp, sp2, and sp3 carbon: a unified multisubstrate domino approach for isatin synthesis. The Journal of Organic Chemistry. 2015 May 15;80(10):5167-75.
- Wen SS, Zhou ZF, Xiao JA, Li J, Xiang H, Yang H. Facile oxidative cyclization to access C2-quaternary 2-hydroxy-indolin-3-ones: synthetic studies towards matemone. New Journal of Chemistry. 2017;41(20):11503-6.
- Sharma PK, Balwani S, Mathur D, Malhotra S, Singh BK, Prasad AK, Len C, Van der Eycken EV, Ghosh B, Richards NG, Parmar VS. Synthesis and anti-inflammatory activity evaluation of novel triazolyl-isatin hybrids. Journal of Enzyme Inhibition and Medicinal Chemistry. 2016 Nov 1;31(6):1520-6.
- Dantas LL, Fonseca AG, Pereira JR, Furtado AA, Gomes PA, Fernandes-Pedrosa MF, Leite AC, Rêgo MJ, Pitta MG, Lemos TM. Anti-inflammatory and antinociceptive effects of the isatin derivative (Z)-2-(5-chloro-2-oxoindolin-3-ylidene)-N-phenyl-hydrazinecarbothioamide in mice. Brazilian Journal of Medical and Biological Research. 2020 Sep 7;53(10):e10204.
- Jarapula R, Gangarapu K, Manda S, Rekulapally S. Synthesis, in vivo anti?inflammatory activity, and molecular docking studies of new isatin derivatives. International journal of medicinal chemistry. 2016;2016(1):2181027.
- Prasad BD, Vasanthi R, Kanth BC, Prabhakar D, Mohan MR. Synthesis, characterization and anti-inflammatory activity of isatin derivatives. Int J Bio Pharm Res. 2012;3:182-7.
- Grewal AS. Isatin derivatives with several biological activities. International Journal of Pharmaceutical Research. 2014 Jan;6(1):1-7.
- Lahari K, Sundararajan R. Design and synthesis of novel isatin derivatives as potent analgesic, anti-inflammatory and antimicrobial agents. Journal of Chemical Sciences. 2020 Dec;132:1-5.
- Chaitanya M, Swathi K. In Silico and Docking Studies of Novel Isatin Derivatives for Anti-Inflammatory Activity. Int. J. Pharm. Biolog. Sci.-IJPBSTM. 2019;9(1):1342-8.
- Da Fonseca AG, Fernandes Ribeiro Dantas LL, Rodrigues JP, Alencar Filho MP, De Melo Rêgo MJ, Da Rocha Pitta MG, De Moraes Gomes PA, De Melo Silva VG, Lima Leite AC, Furtado AA, Fernandes Pedrosa MD. PA Int5: An isatin thiosemicarbazone derivative that exhibits anti nociceptive and anti inflammatory effects in Swiss mice. Biomedical Reports. 2021 Jul 1;15(1):1-9.
- Cenalmor A, Pascual E, Gil-Manso S, Correa-Rocha R, Suárez JR, García-Álvarez I. Evaluation of anti-neuroinflammatory activity of isatin derivatives in activated microglia. Molecules. 2023 Jun 20;28(12):4882.
- Venkateshwarlu E, Venkateshwar RJ, Umasankar K, Dheeraj G. Study of anti-inflammatory, analgesic and antipyretic activity of novel isatin derivatives. Asian J Pharm Clin Res. 2012 Aug;5(4):187-90.
- Varpe BD, Jadhav SB. Schiff base of isatin with 2-thiopheneethylamine and its Mannich bases: Synthesis, docking, and in vitro anti-inflammatory and antitubercular activity. Russian Journal of Bioorganic Chemistry. 2022 Apr;48(2):372-9.
- Pogula M, Priyanka KB, Suresh R, Shobarani B, Sammaiah G. Synthesis and characterization of new Isatin derivatives for anti-inflammatory activity. Int. J. Pharm. Pharm. Sci. 2012;4:248-51.
- Hassanzadeh F, Jafari E, Khayambashi N, Hajhashemi V. Synthesis and anti-inflammatory effects evaluation of 1, 3 substituted isatin derivatives. The Thai Journal of Pharmaceutical Sciences. 2021;45(4):248-52.
- Prakash CR, Raja SU, Saravanan G. Synthesis, Analgesic, Anti-Inflammatory and In Vitro Antimicrobial Studies of Some Novel Schiff and Mannich Base of 5-Substituted Isatin Derivatives. International Journal of Pharmacy and Pharmaceutical Sciences. 2014;6(10):160-6.
- Jafari E, Hassanzadeh F, Khayambashi N, Hajhashemi V. Interaction Study of 1, 3 Substituted Isatin Derivatives with Anti Inflammatory Properties with Cyclooxygenase 1 and 2 Enzymes by Molecular Docking Method. Journal of Shahid Sadoughi University of Medical Sciences. 2022 Mar 15.
- Shrivastava S, Ahuja D. Analgesic and Anti-inflammatory Activities of Isatin Derivatives-A Review. Pakistan Heart Journal. 2023 May 9;56(2):1497-512.
- Panneerselvam P, Reddy RS, Murali K, Kumar NR. Synthesis, analgesic, anti-inflammatory and antimicrobial activities of some novel Schiff’s bases of 5-subsituted Isatin. Der Pharma Chemica. 2010;2(1):28-37.
- Swathi K, Sarangapani M. Synthesis and anti-inflammatory activity of a novel series of isatin hydrazone & isatin thiosemicarbazone derivatives. World Journal of Pharmacy and Pharmaceutical sciences. 2014;3(2):2070-8.
- Adsule PV, Chabukswar AR, Nanaware R. Design, Synthesis, Anti-inflammatory & Anticonvulsant Activity of Substituted Heterocyclic Compounds. Journal of Pharmaceutical Research International. 2021 Nov 1;33(47B):96-111.
- Nath R, Pathania S, Grover G, Akhtar MJ. Isatin containing heterocycles for different biological activities: Analysis of structure activity relationship. Journal of Molecular Structure. 2020 Dec 15;1222:128900


 Anita Singh*
Anita Singh*
 Dona Adak
Dona Adak
 Kumud Kumari
Kumud Kumari
 Kajal Kumari
Kajal Kumari
 Shweta Pandey
Shweta Pandey
















 10.5281/zenodo.12905488
10.5281/zenodo.12905488