Abstract
Immunotherapy emerged as a promising strategy cancer treatment, revolutionizing the field by harnessing the body's own immune system to target and destroy cancer cells. Unlike traditional treatments like chemotherapy and radiation therapy, which directly target cancer cells, immunotherapy works by enhancing the immune response to recognize and eliminating cancer cells more effectively! Several types of immunotherapies have been developed, including immune checkpoint inhibitors, CAR-T cell therapy, tumor-infiltrating lymphocytes (TILs), cytokine therapy, TCR-T, ICIs. These therapies have demonstrated remarkable success in some types of cancer, leading to durable responses and improved survival rates for patients! However, challenges remain, including understanding why some patients respond better than others, managing immune-related adverse events, and overcoming resistance to immunotherapy. Ongoing research efforts aim to address these challenges and further optimizing immunotherapy approaches. In conclusion, immunotherapy represents a paradigm shift in cancer treatment offering new hope to patients, paving the way for continued advancements in the field! With further research and development, immunotherapy holds the potential to transform cancer care and improve outcomes for patients worldwide.
Keywords
Cancer, Treatment, Immunotherapy CAR-T, TCR-T, ICIs, Cytokine therapy.
Introduction
Cancer is a genomic disease with a lot of somatic point mutations that accumulate, leading to structural changes its development, resulting in genomic instability [1, 2]. Cancer tissues, which are mainly composed of a substantial quantity of both neoplastic and nonneoplastic cells, modify the extracellular matrix and thus generate a unique tumour microenvironment (TME) [3, 4]. The TME is defined as an intricate and dense multicellular environment for tumorigenesis that consists of a substantial number of different cells and cellular components, including multiple types of immune cells, endothelial cells, tumour-associated macrophages (TAMs), and a variety of other tissue-resident cell types [3, 5–7]!!! These cells act synergistically in tumour progression, invasion, metastasis, and response to immunotherapies [8]. Consequently, cancer treatment has transitioned from a model centred solely on the cancer itself to one focused on the tumour microenvironment, and cancer immunotherapy has consequently come onto the stage to revolutionise cancer treatment. However, its efficacy is still limited in most clinical settings [9, 10]. In recent years, cancer immunotherapy has been pursued through a myriad of approaches, including molecular targeted therapy, immune checkpoint inhibitors (such as PD-1/L1 and CTLA-4 inhibitors), adoptive cell immunotherapy (such as TIL, NK, CAR-T, CIK/DC-CIK), cytokine therapy, and tumour vaccines [11– 13]!!.Macrophages, as heterogeneous and multifunctional immune cells, play crucial roles in various biological processes, such as maintaining tissue homoeostasis, regulating cancer progression. And defending against pathogens. Their phenotype and functionality are intricately governed by the ambient microenvironment, and macrophages can demonstrate dual antitumour and tumour-promoting effects within the context of cancer [14, 15]. Polarised macrophages can be classified into two distinct subtypes, M1 macrophages and M2 macrophages, both of which are closely associated with tumour immunity [16]. Classically activated M1 macrophages are primarily involved in proinflammatory responses. Their activation is driven by factors such as lipopolysaccharide (LPS), interferon-? (IFN-?), granulocyte macrophage colony-stimulating factor (GM-CSF), or other pathogen-associated molecular patterns [17–19]!! Upon activation, proinflammatory factors, including IL-6, IL-12 and tumour necrosis factor (TNF), are produced!!! They possess the ability to identify and engulf tumour cells, thereby impeding tumour growth and metastasis!!!Furthermore, they can present tumour antigens to T cells, thus triggering specific immune responses and exerting antitumour effects [20–23]. Cancer cells are mainly suppressed by the complicated networks in the immune system, but tumors develop several mechanisms to evade anti-cancer immunity [24]!!! Hence, cancer immunotherapy has been introduced as a new mainstay to utilize the patient’s own immune system in cancer cell eradication. The cancer immunotherapy concept can be categorized into immune checkpoints- targeted therapy and the adoptive transfer of manipulated immune cells. Both of these strategies contribute to improving the immune system’s function in the identification and eradication of cancer cells [25]. Immunotherapy utilizing the body’s immune system to battle different tumors has achieved great success in recent years [26]. However, low response rates and immune-related drug side effects hamper the clinical application of this promising therapy [27]!! In addition to the well-known immune check-point blockade (ICB) therapies, such as PD1/PD-L1 axis block mediated by monoclonal programmed cell death protein 1 (PD1) or programmed death ligand 1 (PD-L1) antibodies to activate exhausted T cells [28], modulating the intratumorally Balance of cytotoxic T lymphocytes (CTLs) and regulatory T cells (Tregs) is another effective strategy to enhance cancer immunotherapy since the tumour cell killing function of in-filtrating CTLs is usually inhibited by up- regulated Tregs, leading to immune home-stasis and tumour progression [29]!!!. Thus, efficient cancer immunotherapy can be realized by regulating these T cell receptors me-dilated positive or negative signals via delivering agonistic or blocking immunotherapeutic agents to the tumour [30]!!! future development of cancer immunotherapy.

Figure no.1- T-cell Immunotherapy approaches which include, 1. cytokinesis T-Cell specific activation, 2. Checkpoint inhibition causing immune checkpoint inhibition, 3. Cancer vaccines targeting on tumour specific antigen peptide ,4adapotive transfer of modified, 5. Chemokine’s cause T -cell recruitment ,6. Bi -specific T- cell engagers
CANCER IMMUNOTHERAPY
- CAR-T
The chimeric antigen receptor (CAR) is a genetically modified and synthesized chimeric antigen receptor. It is a membrane protein composed of different protein domains in series. It is flexible and offers specific antigen recognition. Patient-derived T cells modified by CAR in vitro can recognize tumour antigens and exert antitumor effects without MHC restrictions in vivo (31). CAR-T therapy is a revolutionary approach to cancer therapy. CAR-T therapy has made breakthroughs in lymphomas, mainly targeting CD19. In 2017, the FDA approved two CAR-T products targeting CD19 (Kymriah and Yescarta, (32,33). The first generation of CAR contains CD3x, and the second generation adds a costimulatory domain CD28, or 4-1BB based on CD3x. Through March 2022, the FDA has approved five CAR-T products, all of which are second-generation CARs with indications focused on lymphoma (34,35). The third generation CAR uses a lentivirus as a transfection vector, and the intracellular segment of the CAR can have two or more costimulatory signals. However, some studies have shown that the killing activity of the third-generation CAR-T cells is not significantly improved. This may be because the activation signal generated by one co-stimulatory molecule of ITAM already reaches the threshold of T lymphocyte activation signal. Simply increasing the number of ITAM will not further enhance the activation effect of CAR-T. New ideas for CAR design are now emerging to improve efficacy. Dual-target CAR-T cells can independently identify target antigens and address the off-target effect. CD19/CD22 CAR-T and CD123/CLL1 CAR-T have shown significant antitumor activity and are currently in clinical studies, some of which have entered phase II/III (36,37). According to EXUMA Biotech, targeting CD3 T cells by subcutaneous injection of a self-inactivating lentiviral vector encoding a CAR targeting CD19 resulted in the successful generation of corresponding CAR-T cells in vivo and showed significant effects in mice (AACR 2022 Abstract. This provides a new opportunity to overcome the challenges of production time, scale, and cost of adoptive cell therapies. For solid tumors, Hegde et al. constructed Tan CAR-T that could enhance T cell function and reduce antigen escape by facilitating crosstalk between HER2-ScFv and IL-13Ra2, thus increasing CD28 expression. The data of Tan CAR-T showed good efficacy in a mouse glioblastoma model (38). In 2022, Groskopf et al. published a delivery method for hydrogel that can improve the efficacy of treatment of solid tumors by injection into areas near the tumour (39). BioNTech announced the results of the first human clinical trial (NCT04503278) of BNT211—a new generation of CAR-T therapy targeting solid tumors. The combination of CAR-T targeting CLDN6 and mRNA vaccine CARVac for CLDN6 can effectively enhance the efficacy and provide new ideas for the treatment of solid tumors (AACR 2022, Abstract #CT002). In addition, combination therapy with immune checkpoint inhibitors may also enhance the efficacy of CAR-T for solid tumors (40). However, there are several limitations to the application of this technology. Firstly, the expression of CAR mediated by retroviral or lentiviral vectors may have an impact on the gene expression of T cells, which may produce unpredictable results. So, a comprehensive safety assessment of CAR-T cells is required before application. Secondly, the proliferation of CAR-T cells can only be achieved after induction and activation. Therefore, whether the large-scale expansion of T cells in vitro can maintain immune activity is an important factor. Thirdly, necessary technical processes are required for different patients, which may take high costs and long periods. In addition, immunosuppressive TME and efficiency of delivery to the tumor site are also major barriers to a successful CAR-T therapy. In the future, innovations in CAR design, transduction methodologies, and allogeneic CAR-T are bound to lead to improved responses and transform the treatment of patients with cancer.
- TCR-T
Various new methods have been developed to enhancing the antitumor efficacy of the immune system, including targeting new antigens, using new engineering or modifying TCR, and create safety switches for internal suicide genes. By transferred the exogenous TCR gene that specifically recognizing TAAs into T cells, TCR-T can be constructed to improve the affinity to TAAs and exert an MHC-dependent antitumor effect (41). Compared with CAR-T therapy, TCR-T therapy has a better safety profile due to its MHC restriction, which can alleviate adverse reactions such as cytokine storms. The TCR-T category currently in clinical trials is mainly targeting NY-ESO-1. NY-ESO-1 TCR produced by Adapt immune Therapeutics is currently in phase I/II clinical trials. MART TCR-T, gp100 TCR-T, and TCR-T targeting MAGEA3 or MAGE-A4 have achieved positive results in clinical trials. However, safe use in the clinic should consider the type of antigen and TCR affinity (42,43). In a clinical trial of nine patients treated with TCR-T, 56% (5/9) of patients experienced an OR, one of which was a CR. However, three of nine (44%) patients experienced severe neurologic toxicities, including two deaths. The cause of death, in part, may be a cross-reaction of TCR-T with a similar epitope of MAGE-A12 in brain. While targeting NY-ESO-1, MAGEA3, and other TAAs is an attractive strategy for the application of ACT for the treatment of solid cancers, caution must be taken to ensure a lack of cross reactivity with vital normal tissues. In addition, modification of the CDR region of TCR must be performed with caution. Because the modified receptors, similar to those produced after immunization in HLA-transgenic mice, are not negatively selected in the thymus and may be potential reactive to unrelated normal host proteins. There is a need to develop better screening methods to avoid such toxicity in the future. As more antigen-specific TCRs are identified, more data will become available to better understand how to use TCR-T to treat patients. Immunosuppressive TME also limits the efficacy of TCR-T theory. Combination therapy targeting TME may be a potential strategy to improve the efficacy of TCRT immunotherapy.
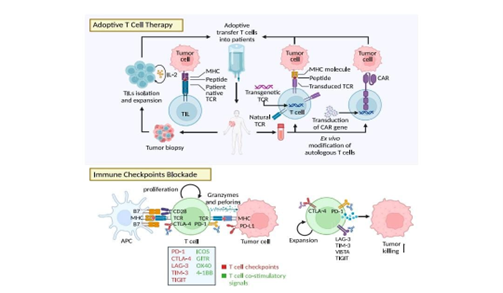
Figure no 2- adoptive T-Cell therapy and Immune checkpoint blockage
- ICIs
ICIs exert effects by braking inhibitory signals that impede T cell activation, thereby reinvigorating antitumour T cell responses. CTLA-4, the first discovered immune checkpoint, was identified as a negative influencer of antigen-presenting cells (APCs)-induced T cell responses. Since ipilimumab was approved for the treatment of unresectable or metastatic melanoma, ICIs have provided efficient and durable responses in various cancers including renal cell carcinoma (RCC), NSCLC, colorectal cancer, and cutaneous squamous cell carcinoma. While several immune checkpoints including LAG-3, TIM-3, and TIGIT are still in preclinical stages, targeting these checkpoints alone or in combination have shown remarkable efficacy in tumour eradication. These checkpoints have different action of mechanisms to brake T cell activation and heterogenous expression in T cell subtypes, which have been systematically summarised in excellent reviews [44 45]. Combining therapies targeting checkpoints could further increase clinical responses. Blocking TIM-3 combined with PD-1 blockade overcomes the resistance to PD-1 blockade in head and neck cancer [46], and nivolumab plus ipilimumab reached enhanced anti-tumour responses in B16 melanoma, RCC, NSCLC, mesothelioma, gastro-oesophageal cancer, and hepatocellular carcinoma (HCC) [47-51]. Moreover, ICIs have synergistic effects with surgery, radiotherapy, chemotherapy, targeted therapies [52 55], and ACTs including CAR-T cell therapy and TILs therapy [56 59]. ICIs display elevated efficacy in immunologically “hot” tumours with large amounts of pre-existing CD8+ TILs [60]. The effectiveness of ICIs is also associated with the functional status of T cells, with precursor exhausted T cells in the TME often considered as a key factor contributing to ICI efficacy [61]. Meanwhile, different T cell status (activation, memory, exhaustion and anergy) presents various metabolism patterns, and investigations concerning how T cell metabolism can regulate the efficacy of ICIs in solid tumours are of mounting interest for researchers [62].
- Cytokine therapy
Cytokines are versatile messengers within the complex immune network and are pivotal in modulating immune responses [63]. Of these, IL-2 is a critical cytokine, originally regarded as a T-cell growth factor [64]. IL-2 possesses a remarkable capacity for T-cell expansion in vitro and in vivo, manifesting potent immunostimulatory properties [65]. Furthermore, the clinical administration of high IL-2 doses has demonstrated compelling evidence of cancer regression in patients with metastatic malignancies [66,67]. Another prominent therapeutic cytokine in cancer treatment is interferon-alpha (IFN-?) [68]. This multifaceted type I IFN has a dual role in tumour control. The first role consists of the direct elimination of tumor cells via the induction of senescence and apoptosis, whereas the second one includes enhancing the effectiveness of anti-tumor immune responses by stimulating DC maturation and augmenting T-cell cytotoxicity [69]. Clinical investigations have also underscored the therapeutic efficacy of high-dose IFN-? in conditions such as chronic myeloid leukaemia and melanoma [70,71]. Moreover, chemokine networks are often dysregulated in cancer, with chemokines being significantly involved in the neovascularization processes. Malignant cells also regularly exploit the chemotactic activity of chemokines [72] C-X-C chemokine receptor type 4 (CXCR4), a chemokine receptor overexpressed in >75% of cancers, is crucial for tumor cell proliferation, dissemination, and angiogenesis [73]. CXCR4 antagonists have demonstrated efficacy in restricting tumor growth in various experimental murine models. Plerixafor is one of the most common CXCR4 antagonists used in clinical applications. It has received approval for mobilizing hematopoietic stem cells, particularly in patients with non-Hodgkin lymphoma or multiple myeloma [74].
CONCLUSION AND RESULT-
Cancer occurrence and development is really a complex process. Various immune-evasion mechanisms can counteract the body’s immune response, which becomes more complex as cancer progresses. Cancer immunotherapy can kill and eliminate tumor cells through the immune system, thus becoming another revolutionary treatment after surgical resection, radiotherapy, chemotherapy, and targeted therapy. Various cancer immunotherapies have shown promising clinical efficacy. However, cancer immunotherapy still faces many problems and challenges. MAbs therapy is a very promising treatment for immunotherapy, which has been repeatedly demonstrated in clinical use. However, due to the immunogenicity, mAbs can cause irAEs, which requires strict monitoring in clinical use. The production process of mAbs is time-consuming and costly, and new purification strategies are needed for higher purity of mAbs. These problems are determined by the nature of the antibody itself, and we believe these problems will partly be solved with new design strategies and further optimization. The overall immune response rate of patients treated with ICB is not high, and there is a need to find reliable and effective biomarkers for precise and personalized immunotherapy. In combination with chemotherapy, mAbs have generated success against advanced-stage cancers, which previously had poor outcomes. In addition, combinations with different mAbs also showed a strong anti-tumor effect. Combination therapy may provide new new opportunities for mAbs to reduce the side effects and improve the therapeutic effect in the future. Conjugation of cytotoxic agents to mAb allows for specific delivery of payloads to tumors, while multipiece antibodies grant novel mechanisms that increase specificity and facilitate delivery to historically intractable compartments. Besides, Fc- engineering mAbs can endow mAbs with stronger antitumor and immune activation ability through the incorporation of amino acid and glycan changes. With an increased understanding of immunobiology and the continued development of molecular biological methods, the possibilities for mAbs therapy are bounded only by the scope of human ingenuity. Small molecule inhibitors for cancer immunotherapy always occupy an important position, although the sales of mAbs are far ahead. Small molecule inhibitors have mature R&D pipelines and the production process of small molecule inhibitors is more controllable than mAbs, which can help reduce costs The controllable pharmacokinetic properties can help reduce the impact of side effects, and the good tissue permeability makes small molecule inhibitors useful for solid tumor immunotherapy. Small molecule inhibitors will always be an effective replacement and supplement for mAbs. Currently, a new form of small molecule inhibitor, proteolysis targeting chimeras (PROTAC) is tested in (pre-)clinical, such as IDO1 PROTACs. But many issues need to be addressed especially on whether it is a safe approach or whether there is a saturation in the degradation of proteins that may limit their effectiveness [75,76]. ACT can be a potent new addition to the toolbox for cancer immunotherapy. However, many TCR-T/CAR-T clinical trials have been hampered by off-target effects and safety concerns [77,78]. While timely intervention is effective in most adverse events, side-effect management of ACT must be held in the whole process of ACT treatment. If tumor antigens are blocked by the self-secretion of tumor cells, they cannot be recognized by the immune system. Rationally designed strategies to identify candidate neoantigens and evaluate their immunogenicity are valuable for boosting the safety and efficacy of ACT. At present, the successful ACT therapy is mainly used in the treatment of haematological tumors. In solid tumors, getting CAR-T cells to infiltrate the tumor is a challenge, which can be compounded by the immunosuppressive TME. ACT combined with small molecule immunomodulator targeting immunosuppressive TIME may be effective for solid tumors. The major challenge in oncolytic virus therapy is the targeted delivery of the virus into the tumor. In most cases, systemic administration does not work well because of pre-existing immunity. Some novel approaches involve the use of nanoparticles, complex viral particle ligands, and immunomodulatory agents to deliver OVs into the tumor. Alternatively, delivery of OVs via a nanoparticles using technologically complex image-guided delivery system has also been considered Immune response after OVs infection suppresses the replication of the virus thereby posing a hindrance to the effective functioning of OVs therapy. Therefore, increasing anticancer treatments and consequently patient prognosis through contributions from molecular biology, immunology, genomics, and bioinformatics will provide a strong foundation for OVs’ potential clinical success in the future.
REFERENCE
- Martínez-Jiménez F, Muiños F, Sentís I, Deu-Pons J, Reyes-Salazar I, Arnedo-PacC, et al.compendium of mutational cancer driver genes. Nat Rev Cancer.2020;20:555–72.
- Zhang Y, Zhang Z. The history and advances in cancer immunotherapy:understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17:807–21.
- de Visser KE, Joyce JA. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41:374–403.
- Barkley D, Moncada R, Pour M, Liberman DA, Dryg I, Werba G, et al. Cancer cell states recur across tumor types and form specific interactions with the tumor microenvironment. Nat Genet. 2022;54:1192–201.
- Bejarano L, Jord?o MJC, Joyce JA. Therapeutic targeting of the tumor micro-environment. Cancer Discov. 2021;11:933–59.
- Casey SC, Amedei A, Aquilano K, Azmi AS, Benencia F, Bhakta D, et al. Cancer prevention and therapy through the modulation of the tumor microenvironment. Semin Cancer Biol. 2015;35:S199–S223.
- Elhanani O, Ben-Uri R, Keren L. Spatial profiling technologies illuminate the tumor micro-Environment. Cancer Cell. 2023;41:404–20.
- Jin MZ, Jin WL. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther. 2020;5:166.
- Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. 2021;21:345–59.
- Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. 2020;52:17–35.
- Lin MJ, Svensson-Arvelund J, Lubitz GS, Marabelle A, Melero I, Brown BD, et al.Cancer vaccines: the next immunotherapy frontier. Nat Cancer. 2022;3:911–26.
- Laskowski TJ, Biederstädt A, Rezvani K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat Rev Cancer. 2022;22:557–75.
- Peng M, Mo Y, Wang Y, Wu P, Zhang Y, Xiong F, et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer. 2019;18:128.
- Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. 2020;15:123–47.
- Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–40.
- Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J
Pharmacol. 2020;877:173090.
- Anderson NR, Minutolo NG, Gill S, Klichinsky M. Macrophage-based approaches for cancer immunotherapy. Cancer Res. 2021;81:1201–8.
- Jaguin M, Houlbert N, Fardel O, Lecureur V. Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell Immunol.2013;281:51–61.
- Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–11.
- Gunassekaran GR, Poongkavithai Vadevoo SM, Baek MC, Lee B. M1 macrophageexosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials. 2021;278:121137.
- Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs.functional differentiation. Front Immunol. 2014;5:514.
- Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, et al. The immune landscape of cancer. Immunity. 2019;51:411–2.
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation:time for reassessment. F1000prime Rep. 2014;6:13.
- Liu Y, et al. Emerging phagocytosis checkpoints in cancer immunotherapy. Signal Transduct Target Ther. 2023;8(1):104.
- Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70(2):86–104.
- Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest (2007) 117:1137–46. doi: 10.1172/JCI31405
- Tan AC, Bagley SJ, Wen PY, Lim M, Platten M, Colman H, et al. Systematic review of combinations of targeted or immunotherapy in advanced solid tumors. J Immunother Cancer (2021) 9:e002459. doi: 10.1136/jitc-2021-002459
- a) D. M. Pardoll, Nat. Rev. Cancer 2012, 12, 252; b) R. D. Schreiber, L. J. Old, M. J. Smyth, Science 2011, 331, 1565; c) M. Luo, H. Wang, Z. Wang, H. Cai, Z. Lu, Y. Li, M. Du, G. Huang, C. Wang, X. Chen, M. R. Porembka, J. Lea, A. E. Frankel, Y.-X. Fu, Z. J. Chen, J. Gao, Nat. Nanotechnol. 2017, 12, 648.
- a) D. J. Irvine, E. L. Dane, Nat. Rev. Immunol. 2020, 20, 321; b) J. D.Martin, H. Cabral, T. Stylianopoulos, R. K. Jain, Nat. Rev. Clin. Oncol. 2020, 17, 251.
- a) P. C. Tumeh, C. L. Harview, J. H. Yearley, I. P. Shintaku, E. J. M. Taylor, L. Robert, B. Chmielowski, M. Spasic, G. Henry, V. Ciobanu, A. N. West, M. Carmona, C. Kivork, E. Seja, G. Cherry, A. J. Gutierrez, T. R. Grogan, C. Mateus, G. Tomasic, J. A. Glaspy, R. O. Emerson, H. Robins, R. H. Pierce, D. A. Elashoff, C. Robert, A. Ribas, Nature 2014,515, 568; b) R. S. Herbst, J.-C. Soria, M. Kowanetz, G. D. Fine, O.Hamid, M. S. Gordon, J. A. Sosman, D. F. McDermott, J. D. Powderly,
- S. N. Gettinger, H. E. K. Kohrt, L. Horn, D. P. Lawrence, S. Rost, M. Leabman, Y. Xiao, A. Mokatrin, H. Koeppen, P. S. Hegde, I. Mellman, D. S. Chen, F. S. Hodi, Nature 2014, 515, 563.CAR T
- Huang X, Yang Y. Driving an improved CAR for cancer immunotherapy. J Clin Invest (2016) 126:2795–8. doi: 10.1172/JCI88959
- Bach PB, Giralt SA, Saltz LB. FDA Approval of tisagenlecleucel: Promise and complexities of a $475 000 cancer drug. Jama (2017) 318:1861–2. doi: 10.1001/ jama.2017.15218
- FDA Approves second CAR T-cell therapy. Cancer Discovery (2018) 8:5–6. doi: 10.1158/2159-8290.CD-NB2017-155
- Abreu TR, Fonseca NA, Gonc? alves N, Moreira JN. Current challenges and emerging opportunities of CAR-T cell therapies. J Control Release (2020) 319:246– 61. doi: 10.1016/j.jconrel.2019.12.047
- Tang J, Hubbard-Lucey VM, Pearce L, O'Donnell-Tormey J, Shalabi A. The global landscape of cancer cell therapy. Nat Rev Drug Discovery (2018) 17:465–6. doi: 10.1038/nrd.2018.74
- Dai H, Wu Z, Jia H, Tong C, Guo Y, Ti D, et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory b cell acute lymphoblastic leukemia. J Hematol Oncol (2020) 13:30. doi: 10.1186/ s13045-020-00856-8
- Zhao J, Song Y, Liu D. Clinical trials of dual-target CAR T cells, donorderived CAR T cells, and universal CAR T cells for acute lymphoid leukemia. J Hematol Oncol (2019) 12:17. doi: 10.1186/s13045-019-0705-x
- Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, et al. Tandem CAR T cells targeting HER2 and IL13Ra2 mitigate tumor antigen escape. J Clin Invest (2016) 126:3036–52. doi: 10.1172/JCI83416
- Grosskopf AK, Labanieh L, Klysz DD, Roth GA, Xu P, Adebowale O, et al. Delivery of CAR-T cells in a transient injectable stimulatory hydrogel niche improves treatment of solid tumors. Sci Adv (2022) 8:eabn8264. doi: 10.1126/ sciadv.abn8264
- Gargett T, Yu W, Dotti G, Yvon ES, Christo SN, Hayball JD, et al. GD2- specific CAR T cells undergo potent activation and deletion following antigen encounter but can be protected from activation-induced cell death by PD-1 blockade. Mol Ther (2016) 24:1135–49. doi: 10.1038/mt.2016.63
- Ping Y, Liu C, Zhang Y. T-Cell receptor-engineered T cells for cancer treatment: current status and future directions. Protein Cell (2018) 9:254–66. doi: 10.1007/s13238-016-0367-1
- June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science (2018) 359:1361–5. doi: 10.1126/ science.aar6711
- Shafer P, Kelly LM, Hoyos V. Cancer therapy with TCR-engineered T cells: Current strategies. Challenges Prospects Front Immunol (2022) 13:835762. doi: 10.3389/fimmu.2022.835762
- Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol. 2021;16:223–49.
- Marin-Acevedo JA, Kimbrough EO, Lou Y. Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol. 2021;14:45.
- Nandini A, Catherine S-P, Ana CA. Tim-3 fnds its place in the cancer immunotherapy landscape. J Immunother Cancer. 2020;8:e000911.
- Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–80.
- Shitara K, Ajani J, Moehler M, Garrido M, Gallardo C, Shen L, Yamaguchi K, Wyrwicz L, Skoczylas T, Bragagnoli A, et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature. 2022;603:942–8.
- Baas P, Scherpereel A, Nowak A, Fujimoto N, Peters S, Tsao A, Mansfeld A, Popat S, Jahan T, Antonia S, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet (London, England). 2021;397:375–86.
- Paz-Ares L, Ramalingam S, Ciuleanu T, Lee J, Urban L, Caro R, Park K, Sakai H, Ohe Y, Nishio M, et al. First-Line Nivolumab Plus Ipilimumab in Advanced NSCLC: 4-Year Outcomes From the Randomized, Open-Label, Phase 3 CheckMate 227 Part 1 Trial. J Thorac Oncol. 2022;17:289–308.
- Yau T, Kang Y, Kim T, El-Khoueiry A, Santoro A, Sangro B, Melero I, Kudo M, Hou M, Matilla A, et al. Efcacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6:e204564.
- Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, Ogata T, Kawakami H, Hsu C-H, Adenis A, et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med. 2022;386:449–62.
- Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, Oyervides Juárez VM, Hsieh JJ, Basso U, Shah AY, et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2021;384:829–41.
- Huober J, Barrios CH, Niikura N, Jarz?b M, Chang Y-C, Huggins-Puhalla SL, Pedrini J, Zhukova L, Graupner V, Eiger D, et al. Atezolizumab With Neoadjuvant Anti-Human Epidermal Growth Factor Receptor 2 Therapy and Chemotherapy in Human Epidermal Growth Factor Receptor 2– Positive Early Breast Cancer: Primary Results of the Randomized Phase III IMpassion050 Trial. J Clin Oncol. 2022;40:2946–56.
- Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer. N Engl J Med. 2012;366:2455–65.
- Adusumilli PS, Zauderer MG, Rivière I, Solomon SB, Rusch VW, O’Cearbhaill RE, Zhu A, Cheema W, Chintala NK, Halton E, et al. A Phase I Trial of Regional Mesothelin-Targeted CAR T-cell Therapy in Patients with Malignant Pleural Disease, in Combination with the Anti-PD-1 Agent Pembrolizumab. Cancer Discov. 2021;11:2748–63.
- Gargett T, Yu W, Dotti G, Yvon ES, Christo SN, Hayball JD, Lewis ID, Brenner MK, Brown MP. GD2-specifc CAR T Cells Undergo Potent Activation and Deletion Following Antigen Encounter but can be Protected From Activation-induced Cell Death by PD-1 Blockade. Mol Ther. 2016;24:1135–49.
- John LB, Devaud C, Duong CP, Yong CS, Beavis PA, Haynes NM, Chow MT, Smyth MJ, Kershaw MH, Darcy PK. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by genemodifed T cells. Clin Cancer Res. 2013;19:5636–46.
- Chong EA, Melenhorst JJ, Lacey SF, Ambrose DE, Gonzalez V, Levine BL, June CH, Schuster SJ. PD-1 blockade modulates chimeric antigen receptor (CAR)-modifed T cells: refueling the CAR. Blood. 2017;129:1039–41.
- Liu YT, Sun ZJ. Turning cold tumors into hot tumors by improving T-cell infltration. Theranostics. 2021;11:5365–86.
- Chow A, Perica K, Klebanof CA, Wolchok JD. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat Rev Clin Oncol. 2022;19:775–90.
- Madden MZ, Rathmell JC. The Complex Integration of T-cell Metabolism and Immunotherapy. Cancer Discov. 2021;11:1636–43.
- Propper DJ, Balkwill FR. Harnessing cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol. 2022; 19: 237–53.
- Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976; 193: 1007–8.
- Hernandez R, Põder J, LaPorte KM, Malek TR. Engineering IL-2 for immunotherapy of autoimmunity and cancer. Nat Rev Immunol. 2022; 22: 614–28.
- Majidpoor J, Mortezaee K. Interleukin-2 therapy of cancer-clinical perspectives. Int Immunopharmacol. 2021; 98: 107836.
- Wrangle JM, Patterson A, Johnson CB, et al. IL-2 and Beyond in Cancer Immunotherapy. J Interferon Cytokine Res. 2018; 38: 45–68.
- Yu R, Zhu B, Chen D. Type I interferon-mediated tumor immunity and its role in immunotherapy. Cell Mol Life Sci. 2022; 79: 191.
- Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nat Rev Immunol. 2015; 15: 405–14.
- Magenau JM, Peltier D, Riwes M, et al. Type 1 interferon to prevent leukemia relapse after allogeneic transplantation. Blood Adv. 2021; 5: 5047–56
- Eigentler TK, Gutzmer R, Hauschild A, et al. Adjuvant treatment with pegylated interferon ?-2a versus low-dose interferon ?-2a in patients with high-risk melanoma: a randomized phase III DeCOG trial. Ann Oncol. 2016; 27: 1625–32.
- Graham GJ, Handel TM, Proudfoot AEI. Leukocyte Adhesion: Reconceptualizing Chemokine Presentation by Glycosaminoglycans. Trends Immunol. 2019; 40: 472–81.
- Domanska UM, Kruizinga RC, Nagengast WB, et al. A review on CXCR4/CXCL12 axis in oncology: no place to hide. Eur J Cancer. 2013; 49: 219–30.
- Lee EQ, Duda DG, Muzikansky A, et al. Phase I and Biomarker Study of Plerixafor and Bevacizumab in Recurrent High-Grade Glioma. Clin Cancer Res. 2018; 24: 4643–9.
- Hu B, Zhou Y, Sun D, Yang Y, Liu Y, Li X, et al. PROTACs: New method to degrade transcription regulating proteins. Eur J Med Chem (2020) 207:112698. doi: 10.1016/j.ejmech.2020.112698
- Hu M, Zhou W, Wang Y, Yao D, Ye T, Yao Y, et al. Discovery of the first potent proteolysis targeting chimera (PROTAC) degrader of indoleamine 2,3- dioxygenase 1. Acta Pharm Sin B (2020) 10:1943–53. doi: 10.1016/j.apsb.2020.02.010
- Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother (2013) 36:133–51. doi: 10.1097/CJI.0b013e3182829747
- van den Berg JH, Gomez-Eerland R, van de Wiel B, Hulshoff L, van den Broek D, Bins A, et al. Case report of a fatal serious adverse event upon administration of T cells transduced with a MART-1-specific T-cell receptor. Mol Ther (2015) 23:1541–50. doi: 10.1038/mt.2015.60


 Mayuri N Jagtap*
Mayuri N Jagtap*
 Vishesh Nitin Raundal
Vishesh Nitin Raundal
 Avinash B. Darekar
Avinash B. Darekar


 10.5281/zenodo.11080372
10.5281/zenodo.11080372